To identify the main clinical manifestations, triggers, and treatment of severe allergic reactions (SAR) in children and adolescents (n=191, up to 18 years of age) seen by allergologists and registered in the Online Latin American Survey of Anaphylaxis (OLASA).
Results53.0% of the patients were males and the aetiological agent was identified in 85.5% of them as follows: foods (36.1%), drugs (27.7%), and insect stings (26.2%). The most common symptoms during an acute episode were cutaneous (94.2%), and respiratory (78.5%). Most patients were treated in emergency setting, yet only 34.6% received parenteral epinephrine and 14.3% had to be hospitalised.
ConclusionCutaneous symptoms ranked the order of clinical presentation of SAR. Food was the main triggering agent in the younger cases and insect sting and drugs in the adolescents. Treatment provided for SAR was not appropriate. It is necessary to improve educational programmes in order to enhance the knowledge on this potentially fatal emergency.
Although anaphylaxis is considered a disease of modern times, the first report that is known about anaphylaxis is the death of Pharaoh Menes from a wasp sting, in 2600 BC, but nowadays this information has been questioned.1 Portier and Richet, in the early 1900s were the first to describe anaphylactic reactions in dogs as consequence of repeated injections of sea anemone toxin.2,3 Thereafter, sporadic cases were reported in the following centuries, and after the years 1950 the first case series of anaphylaxis from individuals with medications, diagnostic agents, insect venoms, and foods were published and knowledge about anaphylaxis improved and it was better characterised and investigated.4According to The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network, anaphylaxis has been defined as “a serious allergic reaction that is rapid in onset and may cause death” and is likely to be diagnosed when there is involvement of skin or mucosal tissue (e.g. hives, angio-oedema) and airway compromise (wheezing, dyspnoea) and/or reduced blood pressure or associated symptoms (hypotonia, syncope), along with a temporal relationship (minutes to several hours) to a potential causative agent.2,3,5–7
The lifetime prevalence of anaphylaxis from all triggers is estimated to be from 0.05% to 2%.6 Data on anaphylaxis prevalence and incidence are sparse, often inaccurate and certainly underestimate the true incidence of anaphylaxis.8Triggers of anaphylaxis are represented by these three main agents: foods, medications, and insect stings. They range in contribution according to the age of studied population, study design, and geographic area. Foods are the most common cause of anaphylaxis in childhood. Other less common causes in both children and adults include latex, immunotherapy-related reactions, exercise, cold or are idiopathic.7–19
Data of anaphylaxis in children and adolescents are scarce and most of them are limited to hospitalised patients or specific provoking agents (e.g. foods).20–25 In a recent study, Hompes et al. published data from the anaphylaxis registry of German-speaking countries in children and adolescents (aged from 3 months to 17 years). In this study they reported the main clinical picture, provoking agents, accompanying factors and treatment received during the acute episode. Food allergens accounted for 58% of the cases, mainly due to legumes, insect sting for 24%, and drugs for 8%.26
Data on anaphylaxis in Latin America are scant. The Online Latin American Survey of Anaphylaxis (OLASA) was tailored to evaluate the main clinical manifestations, triggers and treatment of patients with severe allergic reactions seen by allergists in Latin America and Portugal.27 In this study we evaluated the data of children and adolescents obtained with OLASA.
Materials and methodsThe data presented in this paper are part of the previously published OLASA (Encuesta/Denúncia de Anafilaxia en Iberoamérica online) developed by the Latin American Society of Allergy, Asthma and Immmunology (SLAAI).27 These data refer to patients aged up to 18 years old seen by allergists and presenting severe allergic reactions, from July 2008 to June 2010. Attending allergists filled in the standardised OLASA questionnaire which was composed by 45 questions regarding the current episode and past episodes (triggering agent, clinical features, place of reaction, treatment received, place where reaction occurred, evolution of the current episode after treatment, frequency of episodes, etc.). This questionnaire, originally developed and validated in Portuguese was translated into Spanish and was available online at SLAAI website.28
From a total of 634 cases registered we analysed those aged 18 years old or younger (n=191). These patients were registered from 14 countries: Brazil (45.0%); Argentina and Venezuela (15.7% each); Mexico (5.8%); Portugal (3.7%); Equator (3.1%); Colombia and Uruguay (2.6% each); Chile and Bolivia (1.6% each); Cuba (1.0%); and Nicaragua, Paraguay and Peru (0.5% each). Results were presented as simple frequency of positive answers in relation to the total of valid responses.
The study protocol was approved by the regional Ethics Committee.
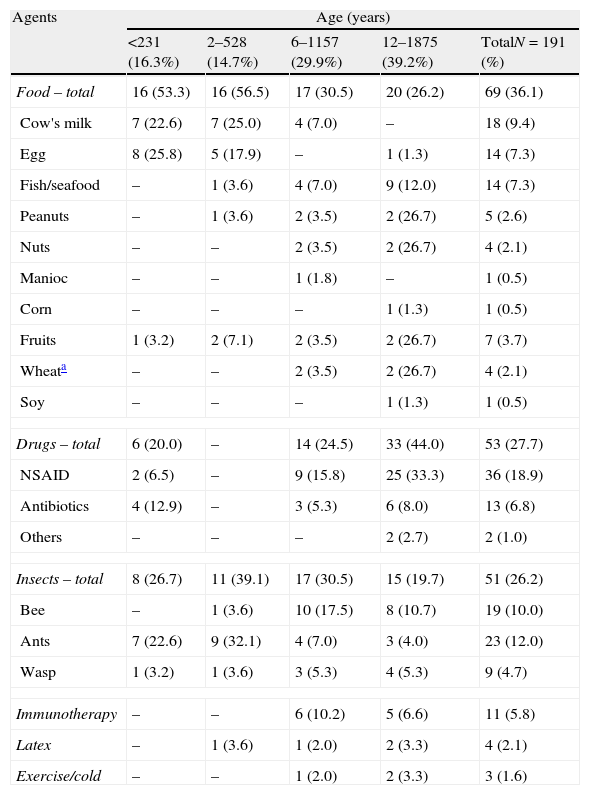
ResultsDemographics showed a slight predominance of male gender (53.0%), and ages ranged from 1 to 18 years stratified as follows: 16.3% were younger than 2 years; 14.7% from 2 to 5 years; 29.9% from 6 to 11 years; and 39.2% from 12 to 18 years. The acute episode occurred at home in 62.2%. Table 1 shows the triggering agents assumed by patients/caregivers for the current episode. 85.5% identified as triggers: foods (36.1%), drugs (27.7%), and insect stings (26.2%).
Main triggering agents for severe allergic reactions according to the age of patients registered in the Online Latin American Survey on Anaphylaxis.
| Agents | Age (years) | ||||
| <231 (16.3%) | 2–528 (14.7%) | 6–1157 (29.9%) | 12–1875 (39.2%) | TotalN=191 (%) | |
| Food – total | 16 (53.3) | 16 (56.5) | 17 (30.5) | 20 (26.2) | 69 (36.1) |
| Cow's milk | 7 (22.6) | 7 (25.0) | 4 (7.0) | – | 18 (9.4) |
| Egg | 8 (25.8) | 5 (17.9) | – | 1 (1.3) | 14 (7.3) |
| Fish/seafood | – | 1 (3.6) | 4 (7.0) | 9 (12.0) | 14 (7.3) |
| Peanuts | – | 1 (3.6) | 2 (3.5) | 2 (26.7) | 5 (2.6) |
| Nuts | – | – | 2 (3.5) | 2 (26.7) | 4 (2.1) |
| Manioc | – | – | 1 (1.8) | – | 1 (0.5) |
| Corn | – | – | – | 1 (1.3) | 1 (0.5) |
| Fruits | 1 (3.2) | 2 (7.1) | 2 (3.5) | 2 (26.7) | 7 (3.7) |
| Wheata | – | – | 2 (3.5) | 2 (26.7) | 4 (2.1) |
| Soy | – | – | – | 1 (1.3) | 1 (0.5) |
| Drugs – total | 6 (20.0) | – | 14 (24.5) | 33 (44.0) | 53 (27.7) |
| NSAID | 2 (6.5) | – | 9 (15.8) | 25 (33.3) | 36 (18.9) |
| Antibiotics | 4 (12.9) | – | 3 (5.3) | 6 (8.0) | 13 (6.8) |
| Others | – | – | – | 2 (2.7) | 2 (1.0) |
| Insects – total | 8 (26.7) | 11 (39.1) | 17 (30.5) | 15 (19.7) | 51 (26.2) |
| Bee | – | 1 (3.6) | 10 (17.5) | 8 (10.7) | 19 (10.0) |
| Ants | 7 (22.6) | 9 (32.1) | 4 (7.0) | 3 (4.0) | 23 (12.0) |
| Wasp | 1 (3.2) | 1 (3.6) | 3 (5.3) | 4 (5.3) | 9 (4.7) |
| Immunotherapy | – | – | 6 (10.2) | 5 (6.6) | 11 (5.8) |
| Latex | – | 1 (3.6) | 1 (2.0) | 2 (3.3) | 4 (2.1) |
| Exercise/cold | – | – | 1 (2.0) | 2 (3.3) | 3 (1.6) |
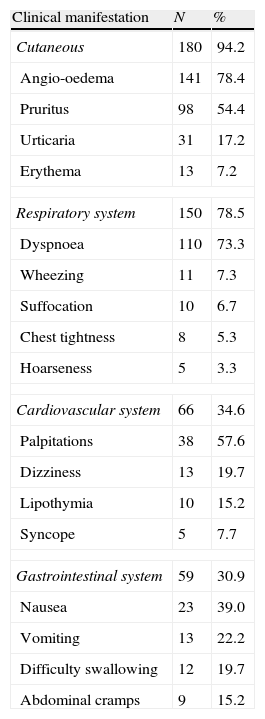
Table 2 shows the major clinical manifestations reported by patients/caregivers. There was a significant predominance of cutaneous symptoms (pruritus and angio-oedema), followed by respiratory (dyspnoea), cardiovascular (tachycardia) and gastrointestinal (nausea and dysphagia) symptoms.
Clinical manifestations during acute severe allergic reaction (% of each system involved).
| Clinical manifestation | N | % |
| Cutaneous | 180 | 94.2 |
| Angio-oedema | 141 | 78.4 |
| Pruritus | 98 | 54.4 |
| Urticaria | 31 | 17.2 |
| Erythema | 13 | 7.2 |
| Respiratory system | 150 | 78.5 |
| Dyspnoea | 110 | 73.3 |
| Wheezing | 11 | 7.3 |
| Suffocation | 10 | 6.7 |
| Chest tightness | 8 | 5.3 |
| Hoarseness | 5 | 3.3 |
| Cardiovascular system | 66 | 34.6 |
| Palpitations | 38 | 57.6 |
| Dizziness | 13 | 19.7 |
| Lipothymia | 10 | 15.2 |
| Syncope | 5 | 7.7 |
| Gastrointestinal system | 59 | 30.9 |
| Nausea | 23 | 39.0 |
| Vomiting | 13 | 22.2 |
| Difficulty swallowing | 12 | 19.7 |
| Abdominal cramps | 9 | 15.2 |
Most patients (73.8%) had the current acute severe allergic episode treated in an emergency setting and 14.1% at the place where the reaction occurred. The time that emergency treatment was administered was variable: up to 60min in 45.4% patients, from 60min to 6h in 44.1%, and after this in the remaining 10.4%. Improvement of symptoms was observed promptly in the first hour after treatment and 66.7% were discharged completely asymptomatic from the emergency room, 21.1% went home with medication and 14.2% were hospitalised. Some patients (6.2%) had to return to the hospital due to clinical impairment which occurred in different times: 10.0% in the first 6h, 50.0% between 6 and 24h, and 40.0% after 24h.
Single or associate medications used to treat the acute episodes were recognised by the majority of patients and included: systemic corticosteroids (oral or injectable) in 50.8% patients, antihistamines (oral or injectable) in 50.8%, epinephrine (subcutaneous or intramuscular) in 34.6%, and short-acting beta2 agonists in 8.9%. Furthermore, 9.9% required resuscitation while in hospital care.
A history of previous acute severe allergic episode was reported by 42.0% patients; 88.2% had 1–3 episodes, 9.2% had 4–10 episodes and 2.6% had more than 10 episodes. Time interval between these episodes was variable: 15–29 days (12.3%), 30–60 days (8.6%), 2–6 months (9.9%), and 6–12 months (19.8%). According to the severity of the previous episodes, 18.8% reported more intense episodes and 30.9% reported no change. Asthma was the co-morbidity most frequent among these children (41.9%) with a more severe picture. When patients were discharged from the emergency room, only 24.5% received orientation on prevention of future attacks and to seek for specialised treatment.
DiscussionDespite the difficulties and limitations observed in compiling data about severe allergic reactions, it is clear that there is an increase in anaphylaxis prevalence and/or incidence. In a recent review Mulla et al. discussed about anaphylaxis incidence rates and time trends in the United States, pointing out the different sources and selection methods that were applied in obtaining these data.10 Apart from this they showed an increase in the hospitalisation rates due to anaphylaxis in children and adults living in New York city.10
Establishing the prevalence and/or incidence rates was not the aim of OLASA. These indexes are usually based on data collected from Emergency Medical Services systems, emergency department visits, hospital admissions, visits to allergists, medical records obtained from resident population of a specific area, and analysis of epinephrine auto-injectors prescriptions. Each of these has potential limitations.12In this study we evaluated data from Latin American patients registered by their attending allergists on OLASA. Patients had been assisted at the emergency room for a severe acute allergic episode by a non-specialist who characterised it as an anaphylactic reaction. This could be a limitation of this study. According to Mulla et al.10 registries are representative sources of epidemiologic data only if the reporting of the condition is mandatory and the data are validated. All patients included in this study were evaluated by a specialist, had the diagnosis confirmed and thereafter data inserted in OLASA.2,3,5–7
The proportion of registered children under 2 years of age in OLASA was approximately 30% and foods (cow's milk and egg) were the most frequent triggering agents. Rudders et al., reviewing medical records of children who presented to emergency department with a food-related acute allergic reaction, observed cow's milk and peanut as the main provoking agents for children in the same age group.9 In the older one and adolescents, peanut was still an important triggering agent and nuts and shellfish were pointed as frequent.9In general, sensitisation to foods is dependent of child's age, cultural and dietetic feeding patterns. It justifies the different types of sensitisation to food worldwide. Although sensitisation to cow's milk and egg9,21,24,26 predominates in the first months of age, sensitisation to peanut has a special significance in some regions.9,21,24–26After 6 years of age, drugs (non-steroidal anti-inflammatory agents and antibiotics) and insect sting (bee, ants, wasp) were the main triggering agents (Table 1). Similar results were reported by de Silva et al. who observed that the mean age of patients allergic to drugs was 13 years and for inset stings 9 years.25 As observed in other paediatric anaphylaxis reviews, despite the age of patients studied, following foods, drugs and insect stings were the main causes of anaphylaxis.24–26
Among the older patients other triggering agents such as specific subcutaneous immunotherapy, latex and exercise are pointed as significant agents.As has been reported by several authors, cutaneous symptoms were predominant (94.2%) among patients registered in OLASA, most commonly angio-oedema (78.2%) and pruritus (54.4%).9,24–26 The next most prevalent symptoms were respiratory (78.5%), mainly dyspnoea (73.3%), similar to that observed by Hompes et al.26 and superior to others.9,23–25 Cardiovascular symptoms were the third most often reported among patients with anaphylaxis, mainly the older ones. In OLASA, 34.3% of the patients presented cardiovascular symptoms (palpitations and dizziness) a figure superior to that reported by de Silva et al.25 The clinical picture of patients in OLASA demonstrated that almost all patients presented cutaneous symptoms in addition to other organ involvement, in agreement with the definition of anaphylaxis.2–5
Although most reactions occurred at home, 73.8% of the patients were treated in the hospital, some (45.4%) within the first hour of reaction. However, few of them received appropriate treatment considering that parenteral epinephrine is recommended by international guidelines as the only effective first-aid treatment of anaphylaxis if it is administered soon after the onset of symptoms or after exposure to an offending trigger.7,11,15,17,18,23,24 In this study only 34.6% of the patients received epinephrine (subcutaneous or intramuscular) alone or in association to antihistamines (oral or parenteral) and/or systemic corticosteroids (oral or parenteral).After treatment, patients were kept in the emergency room for observation, 66.7% were discharged completely asymptomatic, and 21.1% went home with medication. Only 14.2% of patients needed hospitalisation after emergency room treatment, higher than the 7% reported by Braganza et al.,24 and in the average previously reported: from 3% to 41%.11,13 The use of different criteria for admission in part justifies the discrepancy of the indexes observed.25–27Biphasic anaphylaxis has been well characterised in adult series of anaphylaxis. It is defined as the recurrence of symptoms in the first 72h after being treated independently of exposition to the triggering agent. In our study, 6.2% patients returned to the hospital due to clinical impairment mainly in the first 24h (56.0%) after been treated and discharged from the emergency room, characterising a biphasic anaphylaxis. It is estimated to occur in between 1% and 20% of cases depending on the study examined.29–31 In paediatric anaphylaxis series there is no information about its incidence.9,24–26
Other alarming evidence is that at least 42.0% of patients had had a previous episode of anaphylaxis, less severe in 50.3%, and only 24.5% had received orientation on prevention of future episodes and referred to allergy clinics for treatment. Co-morbidity asthma was present in 41.9% of patients.
As we have previously observed, our findings are alarming because although anaphylaxis is a potentially fatal medical emergency, its therapeutic and educational approach in Latin America is not appropriate. The improvement in the diffusion of the concepts of anaphylaxis, like its clinical picture, alarming symptoms, triggering agents, and treatment approach is fundamental to set up strategies for the prevention and treatment of these episodes. Educational programmes for general practitioners, paediatricians, and allied health professionals are necessary in order to enhance the knowledge of this emergency condition.
Conflict of interestThe authors have no conflict of interest to declare.
Latin American Anaphylaxis Working Group (Pediatrics):Argentina: Bandin G, Bozzola CM, Bracaccini AM, Copioli JC, Cuello M, De Falco A, De Gennaro MS, Gambarte FL, Imwinkelried MC, Marcipar A, Mindel E, Orellana JC, Pozo RJ, Ramon GD, Sacerdote D, Sasia L, Sayago L, Vazquez OT; Bolivia: Mendonza A, Sea M; Brazil: Britto L, Chong Neto HJ, DiGesu R, Ensina LF, Fernandes F, Guedes H, Kuschnir F, Miyake A, Naspitz CK, Olivier CE, Santos LLJ; Chile: Marinovic MA, Perez T; Colombia: Bissinger I, Jaller Raad, Serrano C; Cuba: Alvarez M, Batista Rojas O; Equator: Cherrez Ojeda I, Viteri ME, Zambrano HJ; Mexico: Celio Murillo R, Enriquez Salazar JR, Gomez M, González-González AY, Hernandez LE, Huerta RE, Muñoz MD, Rodriguez N, Ruiz Dias HM, Segura NH, Soria JE; Nicaragua: Urbina Palacios G; Paraguay: Ratti M; Peru: Farfan R; Portugal: Gomes E; Uruguay: Castro G, Morena G, Schuhl J.