Lipoinflamation is the inflammation generated in the adipose tissue. It can contribute to the development of insulin resistance. The lipoinflammation-associated mechanisms are related to the function of adipocytes and macrophages present in the adipose tissue. In this regard, the level of nucleoside adenosine is increased in individuals with obesity. Causes or consequences of this increase are unknown. Although, adenosine activating its receptors (A1, A2A, A2B and A3) is able to differentially modulate the function of adipocytes and macrophages, in order to avoid the reduction of insulin sensitivity and generate an anti-inflammatory state in subject with obesity. In this review we propose that adenosine could be a key element in the development of new strategies for limit lipoinflammation and regulate metabolic homeostasis through modulation of adipocyte-macrophage dialog.
La inflamación generada en el tejido adiposo o lipoinflamación, puede contribuir al desarrollo de la resistencia a la insulina. Los mecanismos asociados a la lipoinflamación están relacionados con la función de los adipocitos y los macrófagos presentes en el tejido adiposo. En este contexto, el nivel del nucleósido adenosina está aumentado en individuos con obesidad. Las causas o consecuencias de este aumento no se conocen. Aunque, adenosina al activar a sus receptores (A1, A2A, A2B y A3) es capaz de modular diferencialmente la función de adipocitos y macrófagos, con el fin de evitar la reducción de la sensibilidad a la insulina y generar un estado antiinflamatorio en el individuo con obesidad. En esta revisión proponemos que adenosina podría ser un elemento clave en el desarrollo de nuevas estrategias para el control de la lipoinflamación y homeostasis metabólica a través de la regulación del diálogo adipocito-macrófago.
Obesity, regarded as an abnormal or excessive accumulation of body fat which has a harmful effect upon the health of the individual, is defined by the World Health Organization (WHO) as a body mass index (BMI) of ≥30kg/m2.1 The prevalence of obesity has increased rapidly throughout the world, becoming an epidemic.2,3
Much research has focused on clarifying the physiopathology of obesity.4 In this context, adipocytes are specialized cells with functions that extend beyond simple lipid uptake and accumulation. Indeed, they are currently regarded as endocrine cells. In turn, adipose tissue macrophages constitute a complex family of cells that recognize the metabolic signals released from the adipose tissue as an activation signal, resulting in the generation of a chronic inflammatory state. Lipoinflammation is therefore a result of an interaction between adipocytes and macrophages. The mechanisms of communication between these two cell types are not fully known.
Adenosine (ADO) is a nucleoside derived from the metabolism of adenosine triphosphate (ATP), which is very widely produced within the body. It exerts a series of homeostatic effects through the activation of four receptors coupled to protein G: A1, A2A, A2B and A3.5,6 The functions of ADO include an increase or decrease in blood flow, the inhibition of the aggregation of platelets and macrophages, the reduction of inflammatory states (to the point of causing immune deficiency situations in extreme cases), the reduction of lipid metabolism, and the facilitation of insulin sensitivity and its consequent metabolic effects, among other functions.7 Despite the available information on ADO and its relation to adipose tissue, the role of this molecule in communication between adipocytes and macrophages has not been fully clarified.
The present review postulates that ADO could constitute a key element in the development of new strategies for the control of lipoinflammation and metabolic homeostasis through regulation of the communication between adipocytes and macrophages.
Adipose tissue and lipoinflammationAdipose tissue is more than simply a fat storing tissue; it has a number of physiological functions, depending on the histological type of the tissue. Thus, white adipose tissue is mainly in charge of storing excess energy, while brown adipose tissue specializes in dissipating energy in the form of heat, in response to situations of hypothermia or excess energy. Beige adipose tissue, which is mainly found in the subcutaneous tissue derived from white adipose tissue, possesses functions similar to those of brown adipose tissue.8
Adipose tissue in general is composed of adipocytes, preadipocytes, endothelial cells, fibroblasts and immune cells–fundamentally macrophages and T lymphocytes. Adipocytes are able to exert their influence upon the rest of the tissues by releasing interleukins, chemokines, growth factors and hormones.9 In fact, white adipose tissue is currently recognized as a secretory organ with an effect upon the physiology of the entire body, since it is able to regulate systemic functions such as insulin sensitivity, immune response, cardiovascular function and autocrine and paracrine processes. This tissue therefore not only plays a passive role as an energy store but also acts as a modulator in metabolic homeostasis, thermoregulation, hormone regulation, blood pressure and coagulation.8
However, in the context of an excessive energy intake through the diet, together with a deficit in accumulated energy expenditure, body fat is susceptible to adipose tissue remodeling.10 In effect, 70–80% of all obese individuals suffer from such remodeling, which affects both the structure and normal function of the tissue, generating a subclinical and chronic inflammatory process. The mechanisms underlying the lipoinflammatory state associated with obesity are still the subject of research. However, animal and human models have shown that hypoxic zones are generated in the early stages of adipose tissue hypertrophy, i.e., areas of adipose tissue with low oxygen exposure, which in turn exhibit an increased secretion of proinflammatory interleukins, growth factors (see Table S1 of the supplementary material in the annex)10–12 and inflammatory modulators such as leptin, adiponectin and resistin.13
Furthermore, it has been seen that hypoxia in adipose tissue reduces the expression of two proteins that are crucial for tissue recovery, namely peroxisome proliferator-activated receptor-gamma (PPARy) and adiponectin, which is in charge of reducing the inflammatory state in this tissue.11 Thus, while such inflammation is initially of an acute nature, it becomes chronic, systemic and of low grade when not correctly resolved: a situation known as lipoinflammation.14,15
Proinflammatory interleukins derived from adipose tissue macrophagesMacrophages form part of the immune system and are derived from monocytes generated in the bone marrow. In turn, there are two macrophage activation pathways. The cells that are activated via the classical pathway (M1 macrophages) play a proinflammatory role, while those cells that are activated via the alternative pathway (M2 macrophages) are related to the resolution of inflammation.
Similarly to adipocytes, macrophages found in adipose tissue play a fundamental role in the development of lipoinflammation. In this regard, an increase in macrophage presence in adipose tissue is positively correlated to an increase in the BMI.16 Furthermore, an increase in the number of macrophages in adipose tissue has been observed in obese mice, with no quantitative changes in liver or muscle. This reflects a selective effect induced in adipose tissue.17,18
In order to clarify the role of macrophages in adipose tissue, Xu et al.17 measured the genetic transcription profile of white and brown adipose tissue, muscle and liver isolated from obese mice. From among the multiple genes analyzed, the authors recorded a significant increase in proinflammatory cytokines.17 In searching for the cellular origin of these cytokines, they separated two cell groups: mature adipocytes and a vascular-stromal fraction including macrophages and preadipocytes. The increase in these proinflammatory cytokines was seen to be predominantly attributable to the vascular-stromal fraction, thus suggesting that they were produced by macrophages and/or preadipocytes.
Apart from the number of macrophages present in adipose tissue, another important consideration is the function of these cells, since the adipose tissue of lean mice was seen to fundamentally contain macrophages of an antiinflammatory (type M2) phenotype.19 By contrast, obese mice showed a decrease in the expression of antiinflammatory proteins, with an increase in the gene expression of tumor necrosis factor-alpha (TNF-α) and nitric oxide synthase, both associated with the presence of type M1 macrophages (i.e., with a proinflammatory phenotype).19 Congruent with these findings, increased type M1 cell mobilization toward adipose tissue was observed in obese individuals,20 the type M1 cells in turn being responsible for an increased release of proinflammatory cytokines, thereby reinforcing the inflammatory circuit.21 These results were confirmed by other investigators who found the macrophages present in the adipose tissue of normal weight rodents to express M2 phenotype genes. By contrast, the macrophages present in the adipose tissue of obese rodents expressed classical M1 phenotype genes.22
In order to strengthen the idea of the heterogeneity of the macrophages present in adipose tissue, Bassaganya-Riera et al.23 characterized glycoprotein F4/80 expression in mouse macrophages, followed by differentiation between those cells showing high or low expression of the protein. The experiment showed the macrophages with low F4/80 expression to predominate in the adipose tissue of lean mice, while both phenotypes were seen to accumulate in obese mice. An accumulation of macrophages with intense F4/80 expression was moreover seen to be related to an increase in insulin resistance and to greater amounts of TNF-α and monocyte chemoattractant protein (MCP-1) compared with macrophages showing low F4/80 expression. Likewise, cells with intense F4/80 expression were seen to generate greater amounts of PPARγ/δ and toll-like receptor 4 (TLR-4). In turn, macrophages with low F4/80 expression synthesized lesser amounts of antiinflammatory interleukin-4 (IL-4).23 Therefore, an antiinflammatory state predominates in mice expressing less glycoprotein F4/80 while, by contrast, macrophages with increased F4/80 expression predominate in obese mice. These results strengthen the theory regarding the phenotypic changes experienced by macrophages in obesity, as well as the important role of these cells in the lipoinflammatory process.
In this regard, it has been suggested that the groups of macrophages present in adipose tissue could have different origins. Some could be derived from the preadipocytes present in this tissue, and under favorable conditions could acquire functions typical of macrophages, such as the phagocytosis of bacteria.24 Another source of macrophages in adipose tissue could be a hematogenous supply via the blood vessels. This phenomenon has been demonstrated in vivo models using fluorescent markers specific to monocytes in the bloodstream.25
In addition to the above, it should be mentioned that both obese humans and mice have been reported as presenting elevated MCP-1 levels in adipose tissue,26 the function of this molecule being to recruit macrophages.22 In this regard, MCP-1 is a potent chemokine that exerts chemotactic activity through the activation of a membrane receptor called chemokine receptor type 2 (CCR2). In the context of obesity, in vivo studies have demonstrated an increase in the expression of this cytokine in obese mice versus their lean controls.27 The mechanism underlying the increase in MCP-1 synthesis in adipose tissue is the subject of research, but it is known that the MCP-1 gene promoter can be stimulated in the presence of insulin.27 Consequently, a regulatory circuit could be established between insulin and MCP-1.
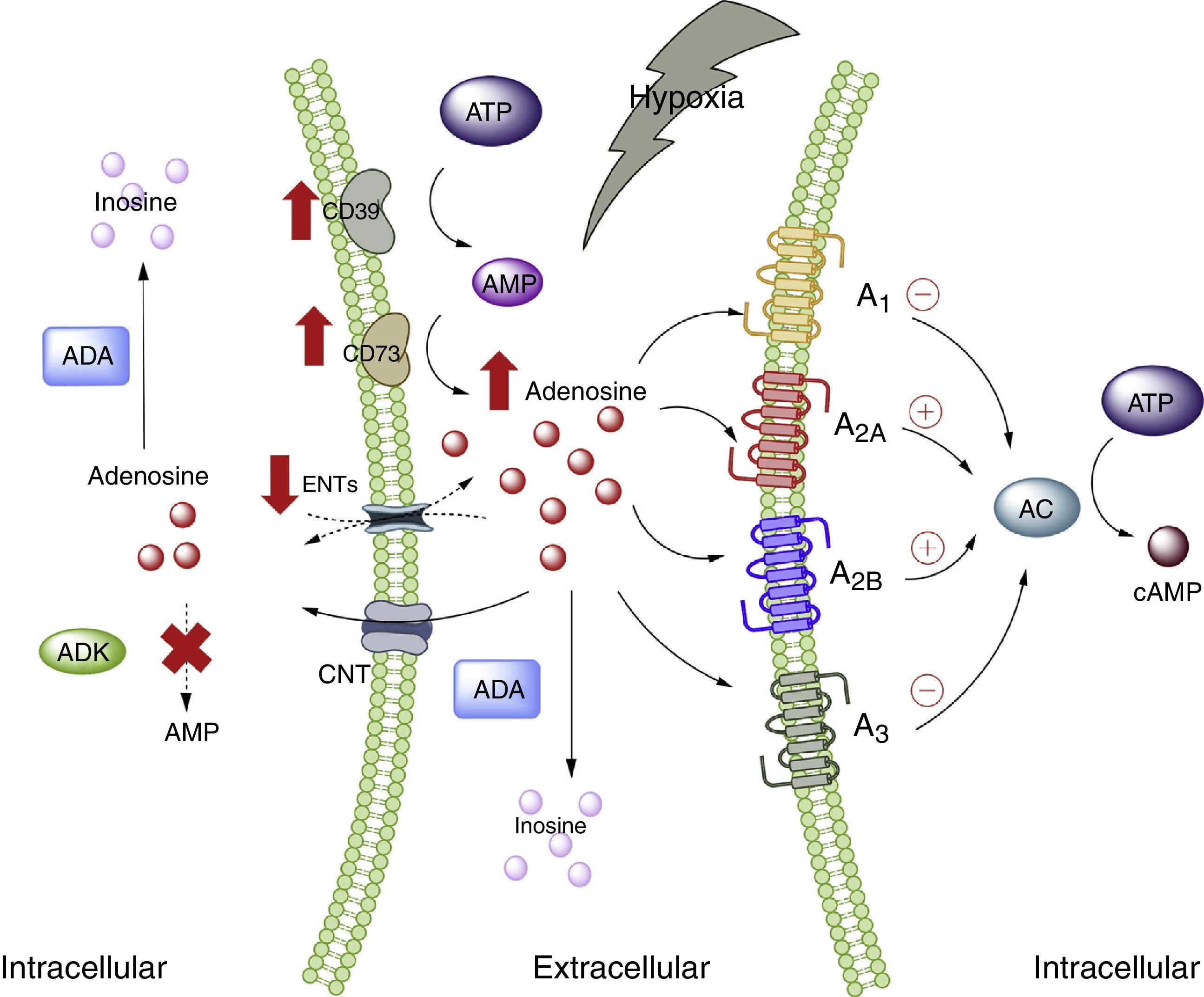
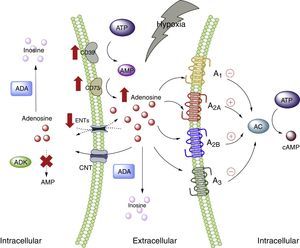
Adenosine and adipose tissueIn the physiological setting, the ADO concentrations are <1μM, which according to the affinity constants of the ADO receptors are able to activate receptors A1, A2A and A3. By contrast, the activation of receptor A2B requires concentrations >1μM.28 Nevertheless, under conditions of cellular stress such as hypoxia, stimulation of all the ADO receptors is to be expected. This is because hypoxia, in the same way as inflammation, is characterized by an increase in the extracellular concentration of ADO, with the purpose of acting as an autocrine and paracrine mediator, inducing mechanisms designed to protect the host against the stressor.5,29 The several pathways involving nucleoside transporter proteins or enzymes that metabolize purines explain the high extracellular concentrations of ADO30 (Fig. 1).
Adenosine and adenosine receptors. Schematic representation of adenosine synthesis and metabolization. Metabolic pathway activation in situations of cellular stress (such as hypoxia) is also summarized. AC: adenyl cyclase; ADA: adenosine deaminase; ADK: adenosine kinase; AMP: adenosine monophosphate; ATP: adenosine triphosphate; CNT: concentrative nucleoside transporter; ENT: equilibrative nucleoside transporter. The solid lines indicate normal pathways. The dashed lines indicate diminished pathways.
Despite the available information on ADO and its relation to adipose tissue, the exact role of this nucleoside in such tissue has not been fully clarified, and there is even less information as to the plasma ADO levels in obese individuals. In this regard, increased ADO concentrations have been reported in the adipose tissue of obese individuals compared with subjects of normal weight.31 In turn, our group has described high plasma ADO levels in obese children.32 Furthermore, in this same study we showed that children with high ADO levels also present increased total cholesterol, triglyceride and LDL-cholesterol levels compared with children showing undetectable ADO levels. In addition, the BMI was seen to be directly correlated to circulating ADO concentrations. On the other hand, preliminary studies in pregnant women also showed increased circulating ADO levels, basically among obese women versus non-pregnant women or pregnant women of normal body weight according to gestational age (data not published). These studies therefore indicate that obesity is associated with elevated circulating ADO levels, though the causes or consequences of this increase are not known.
Adipose tissue has been shown to contain ADO receptors,33–35 which modulate the immune response, particularly in situations of cellular stress such as hypoxemia and ischemia in obese individuals5,6 (Fig. 2). The following section offers a more detailed analysis of the participation of each of the ADO receptors present in adipose tissue within the context of lipoinflammation.
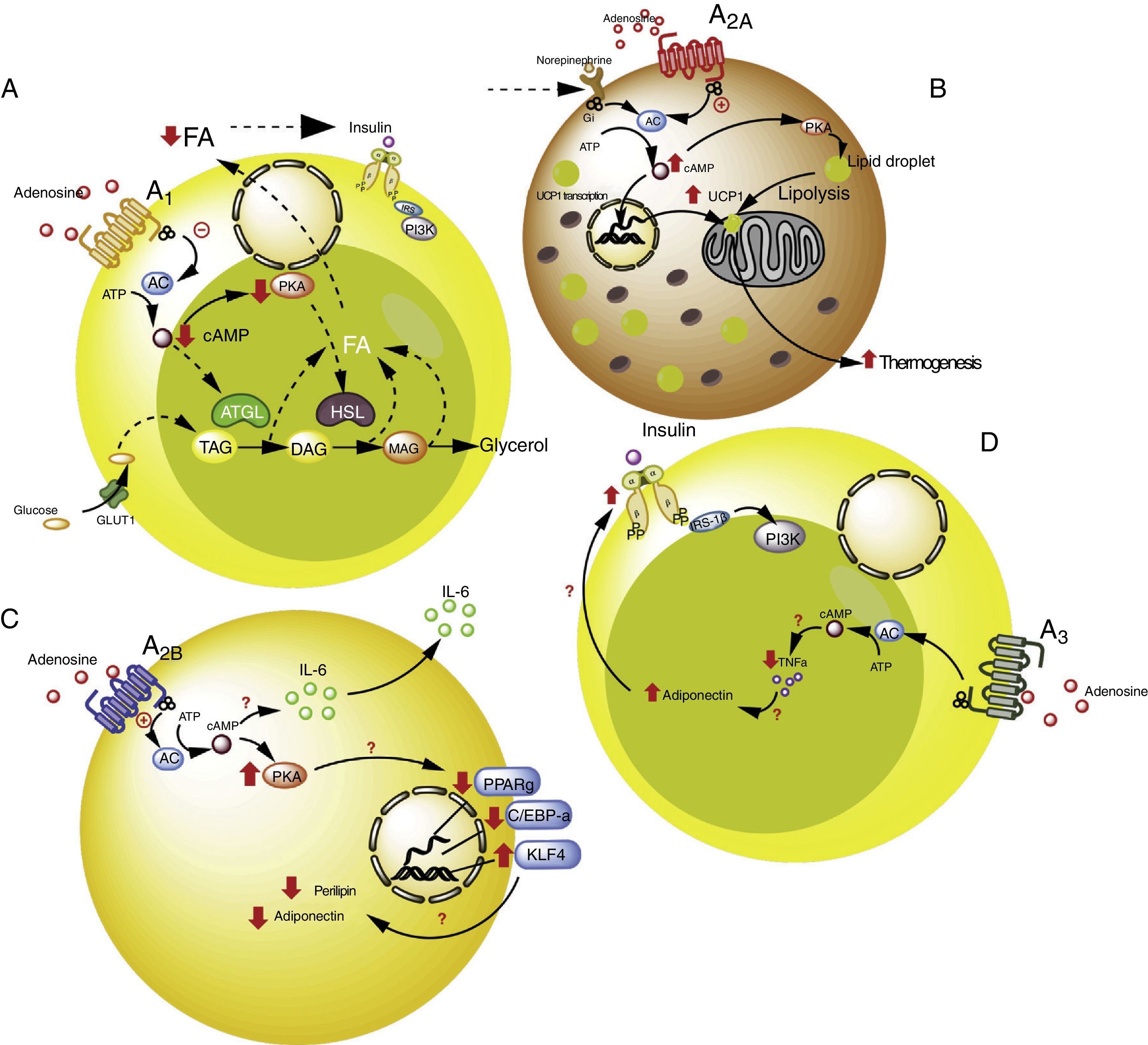
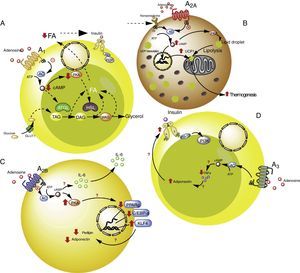
Adenosine receptors in adipose tissue. (A) Intracellular signaling pathways associated with the A1 receptors in white adipose tissue. In this tissue the A1 receptors inhibit lipolysis, which results in an increase in insulin sensitivity in muscle and adipose tissue. (B) Signaling pathways associated with the A2A receptors in brown adipose tissue. In this tissue the A2A receptors stimulate the action of uncoupling protein-1 (UCP1) in mitochondria, promoting thermogenesis and therefore metabolic homeostasis. (C) The A2B receptors in preadipocytes on the one hand inhibit adipogenesis by reducing adiponectin and perilipin, and on the other promote the production of IL-6 and therefore lipoinflammation. (D) The A3 receptors in white adipose tissue reduce the levels of TNF-α, thereby promoting the action of adiponectin, with the stimulation of adipogenesis. See details in the text. AC: adenyl cyclase; ATGL: denutrin (adipose triglyceride lipase); C/EBP-a: enhancer-binding protein (CCAAT-enhancer-binding proteins); DAG: diacylglycerides; GLUT1: glucose transporter 1; HSL: hormone-sensitive lipase; KLF4: Krüppel-like factor 4; MAG: monoacylglycerides; PKA: protein kinase A; PPARg: peroxisome proliferator-activated receptor gamma; TAG: triacylglycerides. The solid lines indicate normal pathways. The dashed lines indicate diminished pathways.
Activation of the A1 receptor exerts an antilipolytic effect in adipocytes, which in turn could modulate lipoinflammation.36 In this regard, Dole et al.,37 in fat samples isolated from the rat epididymis, found the A1 receptor to inhibit lipid decomposition into triglycerides and free fatty acids (FFAs). These findings were subsequently confirmed in vivo models with obese Zucker rats or mice.38–41 Furthermore, in a model in mice, tecadenoson–a selective A1 receptor agonist–was found to reduce FFA levels independently of the dose used.42 Likewise, another selective agonist of this same receptor, known as GR79236, was not only able to inhibit lipolysis, but also promoted insulin sensitivity.43
With regard to the cellular pathways implicated in the metabolic effects of the A1 receptor, the latter is known to reduce cyclic adenosine monophosphate (cAMP) formation and the activation of protein kinase A, which in turn inhibits both hormone-sensitive lipase and triglyceride lipase present in adipose tissue, these being two proteins that actively participate in lipolysis.44 It was therefore concluded that activation of the A1 receptor inhibits lipolysis, with a reduction in circulating FFA levels and the facilitation of FFA accumulation within the adipose tissue, thereby promoting insulin sensitivity in muscle and adipose tissue.45,46 It is thus believed that A1 receptor agonists may prove useful in managing the metabolic complications observed in obese patients (see Table S2 of the supplementary material in the annex).
A2 receptors in adipocytesThe information available regarding the A2 receptors in adipocytes is more limited. In this regard, we will examine the potential role of the A2A receptors in thermogenesis and the role of the A2B receptors in adipogenesis–two metabolic processes of relevance in relation to adipocytes.
A2A receptors are abundant in the brown adipose tissue of humans and mice, though little is known of their effects upon this cell population. In this regard, it was shown that C57BL/6 mice fed a high-fat diet and treated with the A2A receptor agonist CGS-21680, gained less weight and had better glucose tolerance versus controls.47,48 An increase in the activity of this receptor therefore could protect mice against obesity induced by a high-fat diet. The mechanisms involved in this protective effect have been associated with the increased activity of uncoupling protein-1 (UCP1), which contributes to thermogenesis through the uncoupling of oxidative phosphorylation.47,48 In addition, another study reported that mice fed a high-fat diet and receiving beige adipose tissue implants showed improved glucose utilization, which in turn was related to increased UCP1 expression in adipose tissue.49 Thus, the promoting of A2A receptor activity facilitates the effects of UCP1, contributing to thermogenesis and thus to metabolic homeostasis. Due to these effects, it is believed that this receptor may be of particular interest in individuals with insulin resistance.50
Very little is known about the role of the A2B receptors in relation to adipocyte function. Nevertheless, there is evidence that these receptors may inhibit cell differentiation from preadipocytes51 or mesenchymal cells into adipocytes.52–54 In particular, in 7F2 cells (a preadipocyte cell line), where A2B receptors were over-expressed, cell differentiation toward an adipose phenotype (adipogenesis) was seen to be inhibited.52 Likewise, in adipose tissue from humans and mice, it was seen that the selective A2B receptor agonist BAY 60-6583 inhibited the differentiation of mesenchymal cells into adipocytes, associated with an increase in Krüppel factor 4 (KLF4) expression and a decrease in the activity of the prolipogenic transcription factors PPARγ and C/EBP-α.53,54
By contrast, an important observation was the fact that the A2B receptor antagonist CVT-6883, inhibited the production of interleukin-6 (IL-6) in an animal model (C57BL/6).55 This is significant, since IL-6 in turn is associated with the lipoinflammation and insulin resistance present in obesity.56 Thus, the existing information suggests that activation of the A2B receptors could have a contradictory effect in obesity–inhibiting adipogenesis on one hand but also promoting lipoinflammation on the other. Further studies are needed in order to better understand these phenomena.
A3 receptors in adipocytesWith regard to the participation of the A3 receptors in adipocyte physiology, there is indirect evidence of their implication in metabolic control. As an example, obese individuals exhibit an increase in proinflammatory cytokines such as TNF-α, the blood levels of which have been shown to be controlled by activity of the A357 receptors. Since TNF-α in turn regulates the expression of adiponectin, one would expect a decrease in this proinflammatory cytokine, mediated by the A3 receptors, to be associated with an increase in adiponectin levels, thereby favoring insulin sensitivity.58–60 Although there are no studies which refer to this process, we believe that the stimulation of A3 receptor activity may contribute to lowering the levels of TNF-α, so favoring adiponectin action upon the adipocytes, and contributing to their integrity and normal function.
Adenosine and macrophage-adipocyte interactionIn addressing adenosine and macrophage-adipocyte interaction, it is important to note that a functional relationship between macrophages and adipocytes has been postulated, since there is a correlation between the dysregulation of their metabolic pathways and the inflammatory state in the context of obesity.61 An illustrative example of this is the fact that macrophages and adipocytes express similar proteins such as fatty acid transporter proteins, PPARγ, and pro- and antiinflammatory cytokines.23,62 Also worthy of note is the fact that preadipocytes can exhibit phagocytic activity and undergo differentiation toward macrophages.61 Furthermore, by releasing cytokines such as TNF-α and IL-6, macrophages modify the phosphorylation profile of the insulin receptor, specifically IRS-1, in different cells, including adipocytes.63
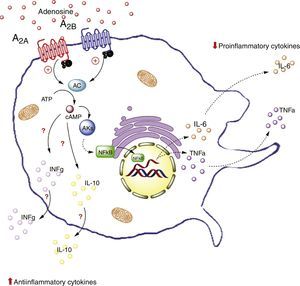
The potential role of adenosine in this complex cellular dialog between adipocytes and macrophages is addressed below. In particular, we will address this issue from the perspective of the interaction between ADO and its A2A and A2B receptors in macrophages (Fig. 3).
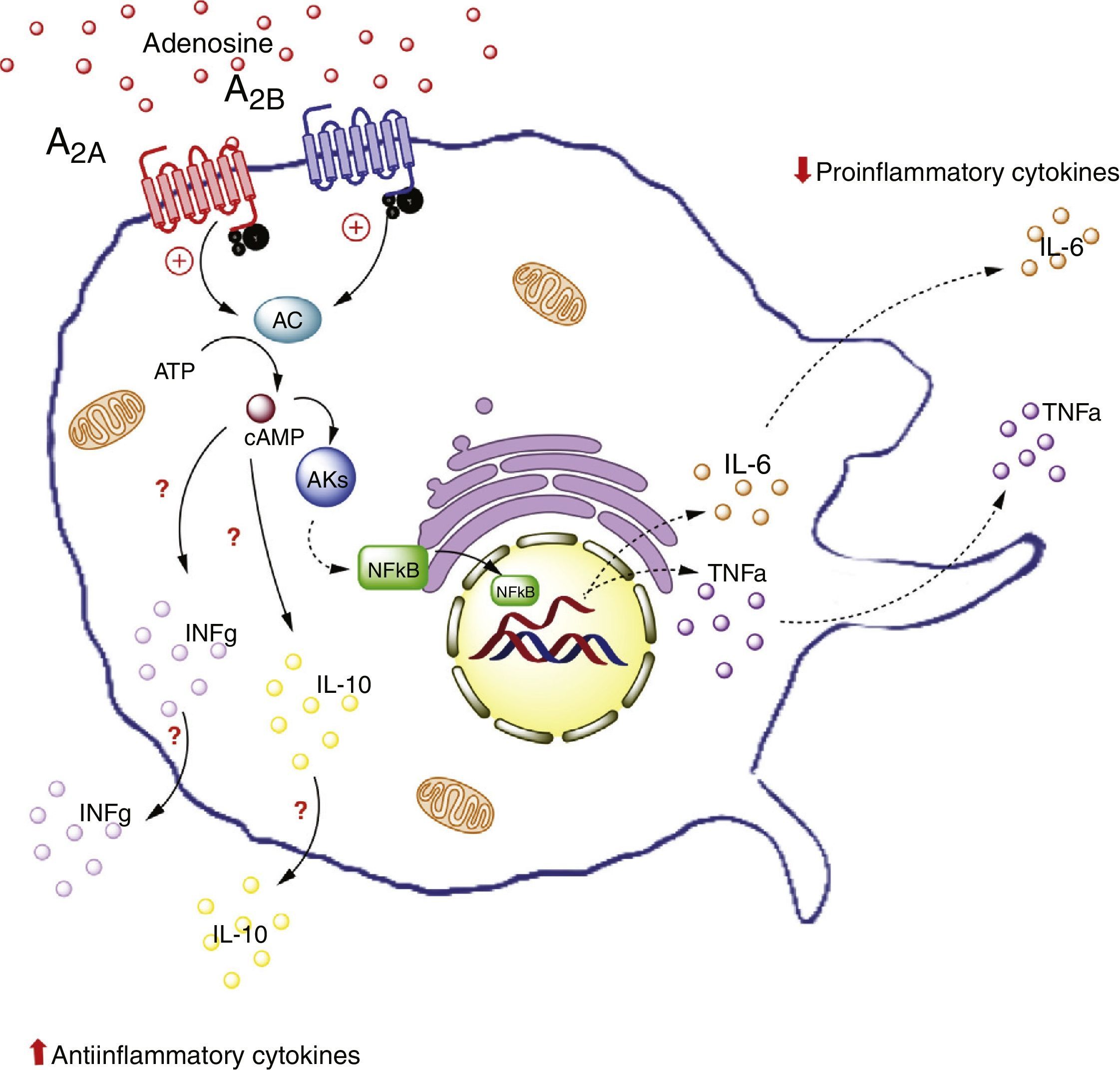
Adenosine receptors in macrophages. Activation of the A2A receptors in macrophages on the one hand lowers the production of proinflammatory cytokines (TNF-α), and on the other increases the production of antiinflammatory interleukins (IL-10), generating an antiinflammatory state. In contrast, activation of the A2B receptors in macrophages promotes the production of IL-10 and INF-γ. AC: adenyl cyclase; INFg: interferon-gamma; NFkB: nuclear factor kappa B; PKs: protein kinases. The solid lines indicate normal pathways. The dashed lines indicate diminished pathways.
Little information is available on the function of ADO receptors in adipose tissue macrophages. We will therefore focus on the effects of ADO and the A2A receptors present in circulating macrophages. Specifically, reference will be made to the relevant data on the expression and activity of these receptors in macrophages found in tissues such as the lungs and stomach, and in endotoxic models, where benefits have been observed related to A2A receptor activation.
For example, an in vivo study in liver tissue from A2A receptor-deficient mice (A2AKO mice) demonstrated an exacerbated systemic inflammatory response secondary to lipopolysaccharide exposure versus the control mice.64 This points to the importance of extracellular ADO and of this receptor type in particular, in reducing systemic inflammation. Furthermore, it is important to note that A2A receptor activation inhibits TNF-α production in macrophages.65 However, this is not the only receptor to generate such inhibitory activity, since there is evidence of modulation of the production of TNF-α in macrophages of A2AKO mice.66
Likewise, it has been seen that A2A receptor activation increases the levels of antiinflammatory interleukin IL-10 in macrophages.67 For example, in a model of mice exposed to Escherichia coli, the macrophages were seen to increase IL-10 expression upon exposure to the pathogen. By contrast, no such effect was recorded in macrophages of A2AKO mice,68 thus confirming the significance of the A2A receptors in the antiinflammatory process.
That is to say, A2A receptor activation in macrophages reduces the production of proinflammatory cytokines (such as TNF-α) and at the same time increases the production of antiinflammatory interleukins (such as IL-10), with the generation of an antiinflammatory state as the final outcome. This effect could take place in adipose tissue macrophages.
A2B receptors in macrophagesThe studies that have contributed most to our understanding of the ADO-mediated interactions between macrophages and adipose tissue are possibly those which refer to the activity of the A2B receptors. In this regard, Csoka et al.7 studied A2B receptor-deficient mice (A2BKO mice) with or without a fat-rich diet. The A2BKO mice fed the normal diet experienced a significant weight increment versus the control group. In contrast, the body weight of the mice fed the fat-rich diet was found to be similar to that of wild mice fed the same fat-rich diet. The investigators therefore decided to evaluate the A2BKO mice fed the normal diet versus their respective controls. From the metabolic perspective, the blood glucose levels were seen to be greater in the A2BKO mice, while the muscle metabolic consumption index (referring to the soleus, vastus and gastrocnemius muscles) was lower, suggesting lesser insulin affinity in these animals. In relation to lipid metabolism, the A2BKO mice presented significantly higher total cholesterol, LDL-cholesterol and triglyceride levels. In an attempt to explain the abovementioned phenomenon, the investigators showed that the A2BKO mice had a profile characteristic of individuals with inflammation (including increased IL-6 and TNF-α levels), as well as a decrease in IL-10 and interferon-gamma (IFγ) concentrations in adipose tissue of the epididymis.7 Based on these results, it was concluded that the A2BKO mice receiving a fat-rich diet for a given period of time suffered alterations in insulin sensitivity, resulting in poor glucose and lipid metabolic control.
In the same study, Csoka et al. analyzed the participation of the adipose tissue macrophages in the metabolic phenomena observed in the A2BKO mice. In this respect, the A2BKO mice were seen to present a greater accumulation of type F4/80 macrophages+, associated with an increase in the levels of proinflammatory interleukins (CCL2, TNF-α, IL-6), but with low levels of antiinflammatory interleukins (IL-10 and INF-γ) in adipose tissue of the epididymis. These findings confirmed the participation of the A2B receptors in the recruitment of macrophages and their antiinflammatory role in adipose tissue. This study provided insight as to how ADO via activation of the A2B receptors could constitute a key factor in metabolic regulation through the maintenance of inflammatory homeostasis.7
To further explore the theory that ADO and its interaction with the A2B receptors present in adipose tissue macrophages is one of the mechanisms regulating glucose and lipid metabolism, Johnston-Cox et al.69 carried out a study in transgenic mice (CD68-Tg) with the A2B receptor recovered in macrophages of A2BKO mice. Three groups were established: control mice, A2BKO mice and CD68-Tg mice administered a high-fat diet for 16 weeks. At the end of this period, percentage body fat was seen to be higher in the A2BKO mice versus the other two groups. These results apparently differed from those published by Csoka,7 who observed no significant changes in weight among the A2BKO mice administered a high-fat diet versus the wild animals fed the same diet. A number of methodological differences could account for these observations, however. For example, the studies7,69 used different diets, with different supplementing times and differences in the strains of the animals, among other aspects. It is also interesting to point out that Johnston-Cox et al.69 reported accumulated fat, not only the weight of the animal as in the other study.7
Furthermore, among the results obtained by Johnston-Cox et al.,69 it should be mentioned that in contrast to the CD68-Tg or control mice, the A2BKO mice showed an increase in fasting blood glucose, with lower glucose clearance and an increase in the two-hour insulin curve. With regard to lipid metabolism, the recovery of A2B receptor expression in macrophages recorded in the CD68-Tg mice prevented the plasma or liver cholesterol and triglyceride increments observed in the A2BKO mice. From the inflammatory perspective, the A2BKO mice showed high TNF-α and IL-6 levels in liver, something which was not seen in the CD68-Tg or control mice. Lastly, an analysis was made of the hepatic expression of insulin receptor substrate-2 (IRS-2). This protein showed significantly lower levels in A2BKO mice versus the other two groups.69 Overall, this study clearly demonstrated the tissue protection resulting from activation of the A2B receptors in macrophages, showing that the mere presence of A2B receptors suffices to allow recovery from the lipoinflammatory and metabolic complications seen in obesity.
Conclusions and future prospectsObesity is characterized by an underlying inflammatory process generated by adipocytes and macrophages. The mechanisms establishing the inflammation-enhancing interactions between these two types of cells are currently the subject of intense research, in view of their pharmacological and preventive implications in the context of the epidemic increase in obesity throughout the world. The present review has analyzed how ADO, an endogenous nucleoside, is able to mediate the dialog between adipocytes and macrophages.
Adipocytes are able to synthesize proteins (such as proinflammatory cytokines) that increase insulin resistance in obese individuals. These cells, and adipose tissue in general, are therefore no longer seen as passive storage elements. Indeed, adipose tissue is currently regarded as an endocrine tissue. The concrete case of obesity is characterized by lipoinflammation, with an increase in the expression and release of proinflammatory cytokines that in turn generate chronic, subclinical systemic inflammation. Although it is clear that adipocytes participate in the production of these cytokines, it is true that the macrophages present in adipocytes also play an active role.
Macrophages form a large and complex family of cells with multiple functions related to the regulation of inflammation and the protection of the host against harmful exogenous agents. In simple terms, adipose tissue shows an increase in the number and functions of macrophages presenting phenotype M1 (proinflammatory cells). These cells have at least two origins: differentiation from preadipocytes on the one hand, and recruitment from circulating macrophages on the other (this being the most extensively studied mechanism). In line with these observations, studies involving models of obese mice have revealed an accumulation of F4/80 macrophages with proinflammatory activity in adipose tissue. This is nevertheless a very simplified view, bearing in mind the complexity of macrophage phenotypes and functions. Further studies of these cells in adipose tissue are needed.
One of the central issues of this review was to illustrate the participation of ADO in the regulation of inflammatory and metabolic function. Information on the participation of this molecule and of its receptors in adipocyte or macrophage function, or on the interactions between these two types of cells in the context of obesity, is limited or only partial. For example, it has been suggested that this nucleoside, on activating the A1 and A2A receptors present in adipocytes, could participate in insulin homeostasis through the reduction of FFAs. Accordingly, the A1 receptors could promote the use of FFAs as an energy substrate, which in turn would reduce the levels of circulating FFAs, facilitating insulin sensitivity. The A2A receptors in turn are known to be present in all types of adipose tissue (white, brown and beige). The most robust evidence indicates that the A2A receptors present in beige adipose tissue are necessary for temperature control in animal models. On the other hand, the activity of the A2B receptors is associated with adipogenesis or preadipocyte differentiation into adipocytes, with a consequent capacity to accumulate fat. The A3 receptors in the same adipocytes intervene in insulin sensitivity mainly though the inhibition of IL-6 and TNF-α, which are proinflammatory cytokines that interfere with insulin metabolism. However, despite the relevance of the conclusions of the analyzed studies, it would be an oversimplification to conclude that these cells and ADO alone participate in these phenomena.
While the ADO-mediated regulation of adipocyte function is complex, the situation can be expected to become even more complicated on analyzing the influence of this molecule upon adipose tissue macrophages. The information available in this field is scarce. Specifically, the circulating A2A receptors (there is a lack of data at adipose tissue level) play an antiinflammatory role, as has been demonstrated by many in vitro and in vivo studies. On the basis of this evidence, it is easy to hypothesize that these receptors exert a protective effect in the context of lipoinflammation, though no evidence has yet been obtained in this regard.
This antiinflammatory role of the A2A receptors is reinforced by the A2B receptors. In particular, the effect of the A2B receptors in the down-regulation of lipoinflammation has been shown to be highly significant, reducing the expression of proinflammatory cytokines (such as TNF-α and IL-6) and promoting the production of antiinflammatory cytokines (such as IL-4, IL-10 and IF-γ). Furthermore, the presence of A2B receptors in macrophages of A2BKO mice resulted in the improvement of lipoinflammation and insulin sensitivity, together with metabolic improvements among the animals administered a high-fat diet. Based on the above, the A2B receptors could become a new therapeutic target in the management of the inflammatory and metabolic complications of obesity.
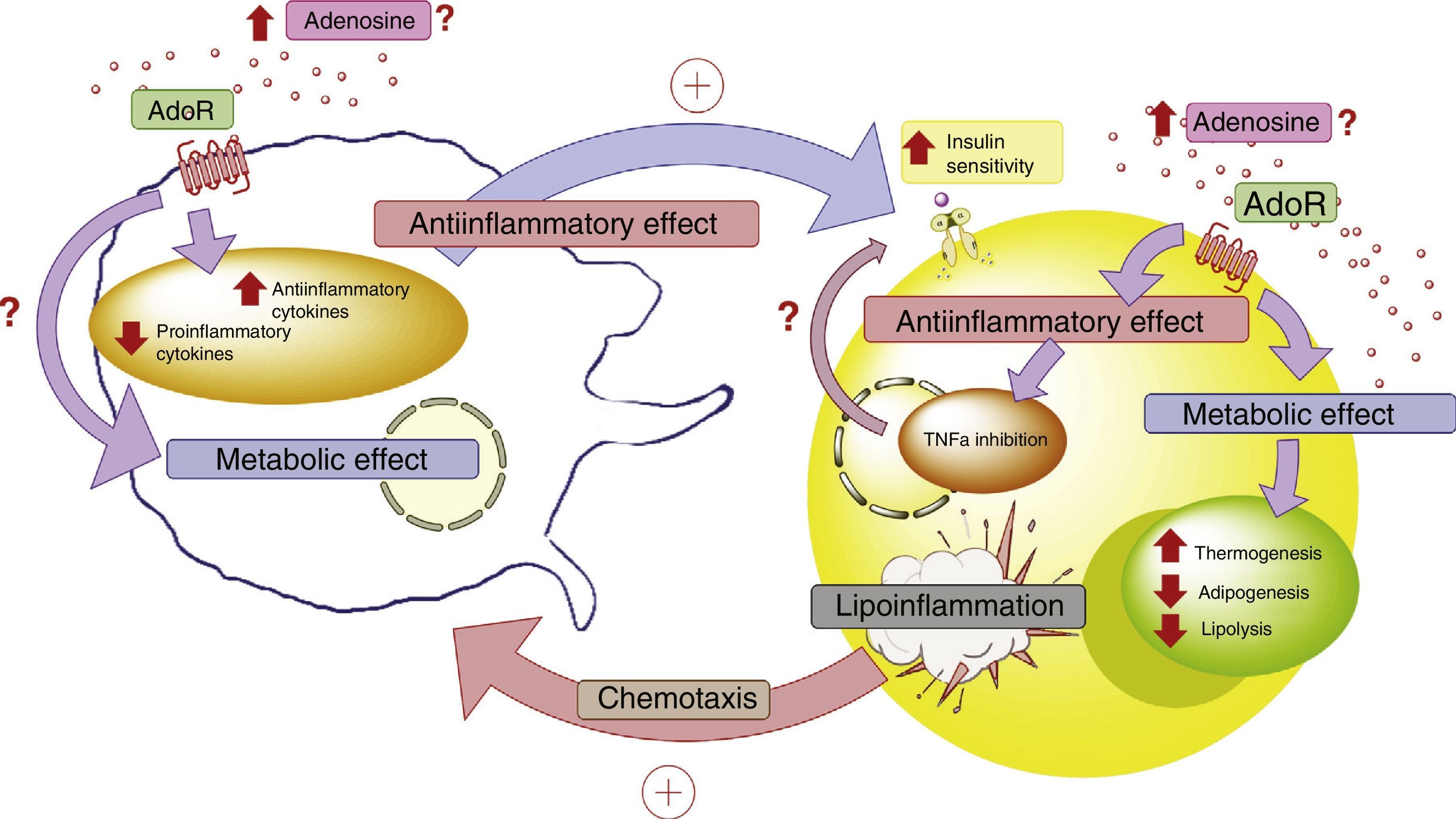
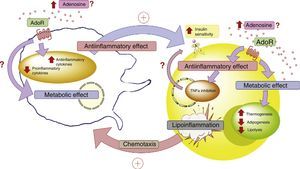
In conclusion, more in-depth knowledge is required of the role of ADO and its receptors in the cellular dialog between adipocytes and macrophages in adipose tissue. This aspect is relevant, since these elements purportedly play a leading role in lipoinflammation and in the metabolic alterations found in obese individuals (Fig. 4).
Adenosine and interaction between adipocytes and macrophages. Disease states such as lipoinflammation are characterized by adipose tissue hypoxia, resulting in an increase in circulating adenosine levels, as well as the production of macrophage chemotactic factors by adipose tissue. The increase in adenosine activates membrane receptors (AdoR) associated with protein G, which reduces the production of proinflammatory cytokines and stimulates the production of antiinflammatory cytokines in both the adipocytes and macrophages. These latter cytokines facilitate the action of the insulin receptors in adipose tissue, incrementing their sensitivity and favoring the corresponding metabolic response. In this context, activation of the adenosine receptors in adipose tissue exerts a metabolic effect, incrementing thermogenesis and reducing both adipogenesis and lipolysis. This is complemented by the antiinflammatory role of the adenosine receptors. All the above generates a compensatory effect that tends to improve the metabolic and lipoinflammatory state in obese individuals. AdoR: adenosine receptors.
The authors state that they have no conflicts of interest.
The authors thank the members of the Laboratory of Vascular Physiology and Tumor Angiogenesis Research Group (Laboratorio de Fisiología Vascular y Grupo de Investigación en Angiogénesis Tumoral [GIANT]) for their discussion and analysis of the present study. Thanks are also due to the Applied Nutrition in Obesity Study Group (Grupo de Estudio en Nutrición Aplicada en Obesidad) of the Universidad del Bio Bio for their continued research support. This study has been financed by the projects Fondecyt Regular 1140586, Fondequip EQM140104, DIUBB GI153109/EF and GI 152920/EF.
Please cite this article as: Meriño M, Briones L, Palma V, Herlitz K, Escudero C. Rol de los receptores de adenosina en la interacción adipocito-macrófago durante la obesidad. Endocrinol Diabetes Nutr. 2017;64:317–327.