To determine the seroprevalence of SARS-CoV-2 in patients with immune-mediated inflammatory diseases (IMID) treated with biologic (bDMARDs) or synthetic targeted disease-modifying antirheumatic drugs (tsDMARDs).
MethodsAn observational, descriptive, prospective and cross-sectional study of analytical prevalence analysis was conducted in patients with IMID with bDMARDs or tsDMARDs. Seroprevalence was compared by measuring immunoglobulin G (IgG) against SARS-CoV-2 between October/2020 and May/2021.
ResultsA total of 550 IMID`s patients were studied, all of them on treatment with bDMARDs or tsDMARDs. The seroprevalence of the total patient group was 16% (88/550). Patients receiving therapy with tumor necrosis factor alpha inhibitors (TNFi) had a higher seroprevalence compared to other biologic and synthetic targeted therapies (OR 1.792, [95% CI 1.088−2.951]; p = 0.021). The influence on seroprevalence of concomitant use with b/tsDMARDs of conventional synthetic DMARDs (csDMARDs) was also analyzed. A lower seroprevalence was demonstrated in the group of patients treated with TNFi and methotrexate together, compared with those on TNFi monotherapy, 10.1 vs. 24.1%, (OR 0.355, [95% CI 0.165−0.764]; p = 0.006). No significant differences were found with the other DMARDs. Regarding IMIDs, no differences in seroprevalence were identified between the different disease groups.
ConclusionPatients on treatment with TNFα inhibitors have better humoral response compared to the other b/tsDMARDs, however, when associated with methotrexate the seroprevalence decreases significantly.
Determinar la seroprevalencia del SARS-CoV-2 en pacientes con enfermedades inflamatorias inmunomediadas (IMID) tratados con fármacos antirreumáticos modificadores de la enfermedad biológica (FAMEb) o sintéticos dirigidos (FAMEsd).
MétodosSe realizó un estudio observacional, descriptivo, prospectivo y transversal de análisis de prevalencia analítica en pacientes con IMID tratados con FAMEb o FAMEsd. Se comparó la seroprevalencia midiendo la inmunoglobulina G (IgG) frente al SARS-CoV-2 entre octubre/2020 y mayo/2021.
ResultadosSe estudiaron un total de 550 pacientes con IMID, todos ellos en tratamientos con FAMEb o FAMEsd. La seroprevalencia del grupo total de pacientes fue del 16% (88/550). Los pacientes que recibieron terapia con inhibidores del factor de necrosis tumoral alfa (inhib. de TNFα) presentaron mayor seroprevalencia frente al resto de terapias biológicas y sintéticas dirigidas (OR 1,792, [IC 95% 1,088−2,951]; p = 0,021). También se analizó la influencia en la seroprevalencia del uso concomitante con los FAMEb/sd de los FAME convencionales sintéticos (FAMEcs). Se demostró una menor seroprevalencia en el grupo de pacientes tratados con inhib. de TNFα y metotrexato juntos, comparando con los que se encontraron con inhib. de TNFα en monoterapia, 10,1 vs. 24,1%, (OR 0,355, [IC 95% 0,165−0,764]; p = 0,006). Con el resto de FAMEcs no se encontraron diferencias significativas. En cuanto a las IMID, no se identifican diferencias en la seroprevalencia entre los diferentes grupos de enfermedades.
ConclusiónLos pacientes en tratamiento con inhibidores de TNFα tienen mejor respuesta humoral en comparación con los demás FAMEb/sd, sin embargo, cuando se los asocia con metotrexato la seroprevalencia disminuye de forma significativa.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an infectious disease that was first isolated in Wuhan, China towards the end of 2019. It subsequently spread globally, causing a pandemic.1,2
This virus infects human cells by binding a 'spike' protein on its surface (protein S) to the angiotensin-converting enzyme 2 (ACE2) within the host. Therefore, the organs involved are preferentially those expressing this ACE2 (the lungs, nasal and oral mucosa, blood vessels, kidneys, heart, gastrointestinal tract, pancreas and brain).3,4
Patients with immune-mediated inflammatory diseases (IMIDs) are more susceptible to infections due to both their underlying disease and the treatments they receive. Previous studies evaluated the seroprevalence of SARS-CoV-2 in IMID patients compared to the general population, showing similar seroprevalence in several of them. No significant differences were found in the severity of SARS-CoV-2 infection compared to the rest of the population.5–9
In this study we aim to assess the seroprevalence of SARS-CoV-2 in patients with IMID treated with biological or synthetic targeted disease-modifying antirheumatic drugs (bDMARDs or tsDMARDs), as well as to determine potential changes in seroprevalence with different concomitant treatments.
Patients and methodsStudy design and participantsAn observational, descriptive, prospective and cross-sectional study was designed to analyse analytical prevalence. Patients with IMIDs on treatment with bDMARDs or tsDMARDs from the Rheumatology, Gastroenterology and Ophthalmology departments of the Hospital Universitario Infanta Sofía and the Rheumatology department of the Hospital Universitario Infanta Leonor who attended regular follow-up visits between October 2020 and May 2021 were included. The inclusion criteria are the same as those of the predefined BIOCOVID study9: age ≥18 years with a previous diagnosis of IMID, in treatment with bDMARDs or tsDMARDs uninterruptedly since at least 3 months before the start of the pandemic (1 December 2019). Regarding rituximab-treated patients, those whose last treatment cycle was after 1 September 2019 were included. None of the patients included in this study had previously received vaccination against SARS-CoV-2.
Clinical variablesPatients were grouped according to treatment received, type of immune-mediated disease and comorbidities.
Biological DMARDs were classified into 7 groups: (1) TNFα inhibitors (infliximab, etanercept, adalimumab [and its biosimilars], golimumab, certolizumab); (2) interleukin 6 (IL-6) inhibitors (tocilizumab and sarilumab); (3) interleukin 17 and 12/23 inhibitors (including secukinumab, ixekizumab, ustekinumab); (4) the CD20 inhibitor (rituximab); (5) the T cell co-stimulator inhibitor (abatacept); (6) the α4β7 integrin inhibitor (vedolizumab); and (7) others (anakinra [IL-1 inhibitor] and belimumab [B-lymphocyte stimulator (BLyS) inhibitor]).
Synthetic targeted DMARDs were classified into Janus kinase (JAK) inhibitors (tofacitinib, baricitinib) and phosphodiesterase 4 (PDE4) inhibitor (apremilast). Data were also collected on concomitant treatment to bDMARDs and tsDMARDs with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs): methotrexate, leflunomide, sulfasalazine, hydroxychloroquine, azathioprine. The remaining csDMARDs, such as mycophenolate mofetil, cyclosporine A, tacrolimus and mercaptopurine, were associated with the same group.
Within the disease group, patients with rheumatoid arthritis and other inflammatory arthropathies (chronic seronegative polyarthritis and juvenile idiopathic arthritis, adult Still's disease) were classified as group 1; as group 2, patients with spondyloarthritis (both axial and peripheral) and psoriatic arthritis; as group 3, patients with organ-specific autoimmune diseases (ulcerative colitis, Crohn's disease, uveitis); as group 4, patients with connective tissue diseases (systemic lupus erythematosus, Sjögren's syndrome, systemic sclerosis, mixed connective tissue disease, myositis or dermatomyositis), and as group 5, vasculitides (giant cell arteritis [GCA], polymyalgia rheumatica, vasculitides other than GCA, Behçet's disease).
The presence and type of comorbidities were divided into 5 categories: (1) patients with no comorbidities; (2) patients with cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidaemia, ischaemic heart disease, stroke); (3) patients with pulmonary diseases (bronchial asthma, chronic obstructive pulmonary disease [COPD], sleep apnoea-hypopnoea syndrome [SAHS]); (4) patients with a history of cancer (breast, prostate, lung), and (5) patients with other comorbidity to those mentioned in the previous groups (thyroid pathology, gastro-oesophageal reflux, chronic gastritis, pancreatitis, diverticulosis, celiac disease, liver disease, renal lithiasis, benign prostatic hyperplasia, malnutrition, anaemia, gout, dementia, epilepsy, psychiatric pathology).
COVID-19 symptom data were also collected from patients with positive serology and grouped into asymptomatic, mild symptoms and severe symptoms (patients who have required hospital admission).
Serological testHumoral response based on IgG antibody levels against SARS-CoV-2 S1 protein was measured using the two-step sandwich immunoassay technique by indirect chemiluminescence (CLIA) on the Advia Centaur XP platform (Siemens). The system reports the results, as suggested by the manufacturers, using index values: "non-reactive" if index <1.00, and "reactive" if index ≥1.0, classified as negative and positive, respectively. Conversion formula: 1.00 index value = 1.00 U/mL.
Sample size and statistical analysisThe study by Favalli et al.10 reported a prevalence of SARS-CoV-2 antibodies of 15.2%. Based on this, it was estimated that a minimum of 545 IMID patients needed to be included to determine the analytical prevalence with an estimation error of 3% and a confidence level of 95%.
For the descriptive statistics of the quantitative study variables, the mean ± standard deviation or median [interquartile range] was used once the distribution had been verified. To describe the qualitative variables, the absolute (n) and relative (%) frequencies were used and their 95% confidence interval (95% CI 95%) was calculated.
Chi-square tests (or Fisher's exact tests) were used to study differences in seroprevalence in IMID patients with different bDMARD or tsDMARD treatments. Binary logistic regression models were performed to determine the odds ratio (OR) of seroprevalence and its 95% CI for each of the treatments compared to the rest. Similarly, the existence of differences in seroprevalence in patients receiving or not concomitant treatment with csDMARD and/or associated glucocorticoids and according to the type of IMID and the presence/absence of the different comorbidities was analysed (if necessary, the Haldane correction was applied to calculate the effect when a value was null).
All statistical calculations were performed using SPSS statistical software (version 25.0, IBM Corp., USA). Statistically significant differences were assumed to exist if the p value obtained was less than or equal to 0.05.
ResultsA total of 550 patients with IMID were studied, of whom 339 (61.6%) were female, with a mean age of 52.3 ± 12.4 years.
The seroprevalence of the total patient group was 16% (88/550).
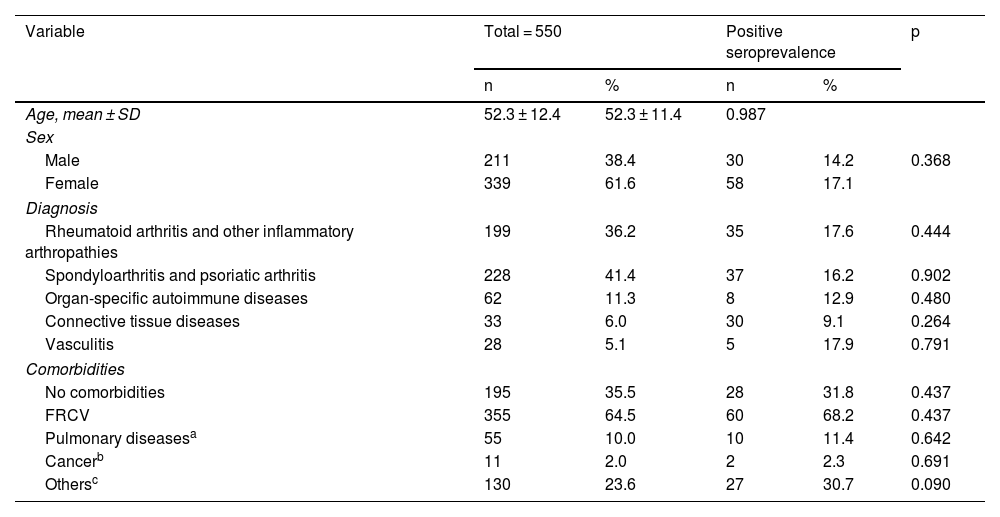
Concerning sex, no significant difference was observed in seroprevalence (14.2% in men vs. 17.1% in women; p = 0.368) nor in relation to age (seropositive: 52.3 ± 11.4 vs. seronegative: 52.3 ± 12.6; p = 0.987) (Table 1).
Socio-demographic characteristics of patients.
| Variable | Total = 550 | Positive seroprevalence | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age, mean ± SD | 52.3 ± 12.4 | 52.3 ± 11.4 | 0.987 | ||
| Sex | |||||
| Male | 211 | 38.4 | 30 | 14.2 | 0.368 |
| Female | 339 | 61.6 | 58 | 17.1 | |
| Diagnosis | |||||
| Rheumatoid arthritis and other inflammatory arthropathies | 199 | 36.2 | 35 | 17.6 | 0.444 |
| Spondyloarthritis and psoriatic arthritis | 228 | 41.4 | 37 | 16.2 | 0.902 |
| Organ-specific autoimmune diseases | 62 | 11.3 | 8 | 12.9 | 0.480 |
| Connective tissue diseases | 33 | 6.0 | 30 | 9.1 | 0.264 |
| Vasculitis | 28 | 5.1 | 5 | 17.9 | 0.791 |
| Comorbidities | |||||
| No comorbidities | 195 | 35.5 | 28 | 31.8 | 0.437 |
| FRCV | 355 | 64.5 | 60 | 68.2 | 0.437 |
| Pulmonary diseasesa | 55 | 10.0 | 10 | 11.4 | 0.642 |
| Cancerb | 11 | 2.0 | 2 | 2.3 | 0.691 |
| Othersc | 130 | 23.6 | 27 | 30.7 | 0.090 |
CVRF: cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidaemia, ischaemic heart disease, stroke).
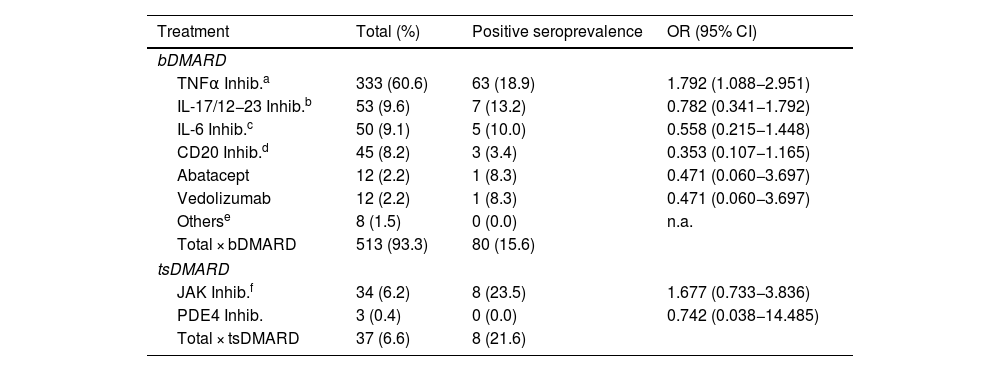
When the different bDMARDs or tsDMARDs were analysed among themselves, it was observed that patients treated with TNFα inhibitors had a higher seroprevalence compared to the rest of the drugs: 18.9% (63/333) vs. 11.5% (25/217) (OR: 1.792 [CI 95%: 1.088−2.951]; p = 0.021). Patients treated with drugs inhibiting IL-17 and IL-12/23 had a seroprevalence of 13.2% (7/53) compared to 16.3% (81/497) for all other biologic and synthetic targeted therapies. With IL-6 inhibitors, a seroprevalence of 10% (5/50) was observed compared to 16.6% (83/500) for the other DMARDs. With rituximab there was a trend towards lower seroprevalence versus the other treatments: 6.7% (3/45) vs. 16.8% (85/505) (OR: 0.353 [CI 95%: 0.107−1.165]; p = 0.075). Patients treated with abatacept and vedolizumab had a seroprevalence of 8.3% (1/12) versus 16.2% (87/538) for the other therapies (Table 2).
Seroprevalence of patients grouped by bDMARD/tsDMARD.
| Treatment | Total (%) | Positive seroprevalence | OR (95% CI) |
|---|---|---|---|
| bDMARD | |||
| TNFα Inhib.a | 333 (60.6) | 63 (18.9) | 1.792 (1.088−2.951) |
| IL-17/12−23 Inhib.b | 53 (9.6) | 7 (13.2) | 0.782 (0.341−1.792) |
| IL-6 Inhib.c | 50 (9.1) | 5 (10.0) | 0.558 (0.215−1.448) |
| CD20 Inhib.d | 45 (8.2) | 3 (3.4) | 0.353 (0.107−1.165) |
| Abatacept | 12 (2.2) | 1 (8.3) | 0.471 (0.060−3.697) |
| Vedolizumab | 12 (2.2) | 1 (8.3) | 0.471 (0.060−3.697) |
| Otherse | 8 (1.5) | 0 (0.0) | n.a. |
| Total × bDMARD | 513 (93.3) | 80 (15.6) | |
| tsDMARD | |||
| JAK Inhib.f | 34 (6.2) | 8 (23.5) | 1.677 (0.733−3.836) |
| PDE4 Inhib. | 3 (0.4) | 0 (0.0) | 0.742 (0.038−14.485) |
| Total × tsDMARD | 37 (6.6) | 8 (21.6) | |
DMARDs: disease-modifying antirheumatic drugs; bDMARD: biologic DMARDs; tsDMARD: targeted synthetic DMARDs; IL-6 inhibitors: interleukin 6 inhibitors. IL-6: interleukin 6 inhibitors; Inhib. IL-17 and 12/23: inhibitors of interleukins 17 and 12/23; Inhib. JAK: JAK kinase inhibitors; Inhib. PDE4: phosphodiesterase 4 inhibitor (apremilast); n.a.: not applicable; OR: odds ratio; TNFα: tumour necrosis factor alpha.
Biological DMARDs in the group "other" (belimumab and anakinra) were not analysed, given the small sample size. Patients treated with tsDMARDs had a seroprevalence of 21.6% (8/37) vs. 15.6% (80/513) of patients with bDMARDs, with no significant difference (OR: 0.670 [95% CI: 0.295–1.518]; p = 0.352). Within this group, JAK inhibitors stand out, with a seroprevalence of 23.5% (8/34) compared to 15.5% (8/34) for PDE4 inhibitors (Table 2).
Seroprevalence according to concomitant treatment with csDMARDsOf the 550 patients, 240 (43.6%) were concomitantly treated with csDMARDs. Of these, a total of 132 (56.4%) were treated with methotrexate, 42 (17.9%) with leflunomide, 24 (10.3%) with azathioprine, 15 (6.4%) with sulfasalazine, 15 (6.4%) with hydroxychloroquine and 12 (5.1%) were treated with other types of csDMARDs (mycophenolate: 6 [2.6%], cyclosporine: 2 [0.8%], tacrolimus: 3 [1.3%] and mercaptopurine: 1 [0.4%]).
Patients concomitantly treated with some type of csDMARDs had a lower seroprevalence compared to patients treated with bDMARD or tsDMARD monotherapy: 10.7% vs. 19.9% (OR: 2.082 [95% CI: 1.265–3.426]; p = 0.003).
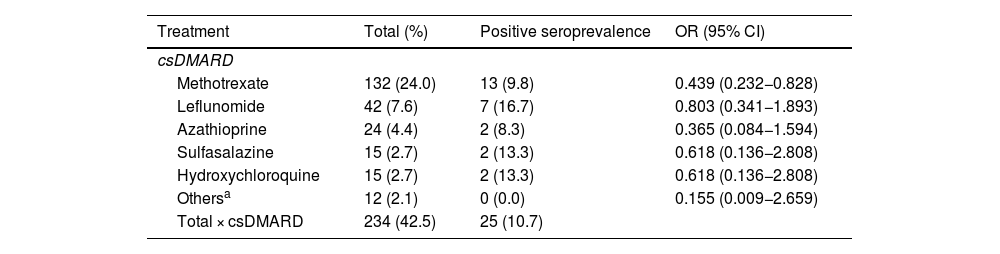
When comparing seroprevalence in patients concomitantly treated with the different types of DMARDs versus patients treated with bDMARD or tsDMARD monotherapy, the only csDMARD that showed a lower seroprevalence was methotrexate: 9.8% vs. 19.9% (OR: 0.439 [95% CI: 0.232−0.828]; p = 0.011). No significant difference in seroprevalence was found with the other csDMARDs between patients concomitantly treated with these csDMARDs versus monotherapy (Table 3).
Odds Ratio of positive SARS-CoV-2 antibody serology of patients on bDMARD with other concomitant csDMARD compared to those on bDMARD monotherapy.
| Treatment | Total (%) | Positive seroprevalence | OR (95% CI) |
|---|---|---|---|
| csDMARD | |||
| Methotrexate | 132 (24.0) | 13 (9.8) | 0.439 (0.232−0.828) |
| Leflunomide | 42 (7.6) | 7 (16.7) | 0.803 (0.341−1.893) |
| Azathioprine | 24 (4.4) | 2 (8.3) | 0.365 (0.084−1.594) |
| Sulfasalazine | 15 (2.7) | 2 (13.3) | 0.618 (0.136−2.808) |
| Hydroxychloroquine | 15 (2.7) | 2 (13.3) | 0.618 (0.136−2.808) |
| Othersa | 12 (2.1) | 0 (0.0) | 0.155 (0.009−2.659) |
| Total × csDMARD | 234 (42.5) | 25 (10.7) | |
csDMARDs: conventional synthetic disease-modifying antirheumatic drugs; OR: odds ratio.
csDMARD + tsDMARD were not analysed due to the very small sample size of this group.
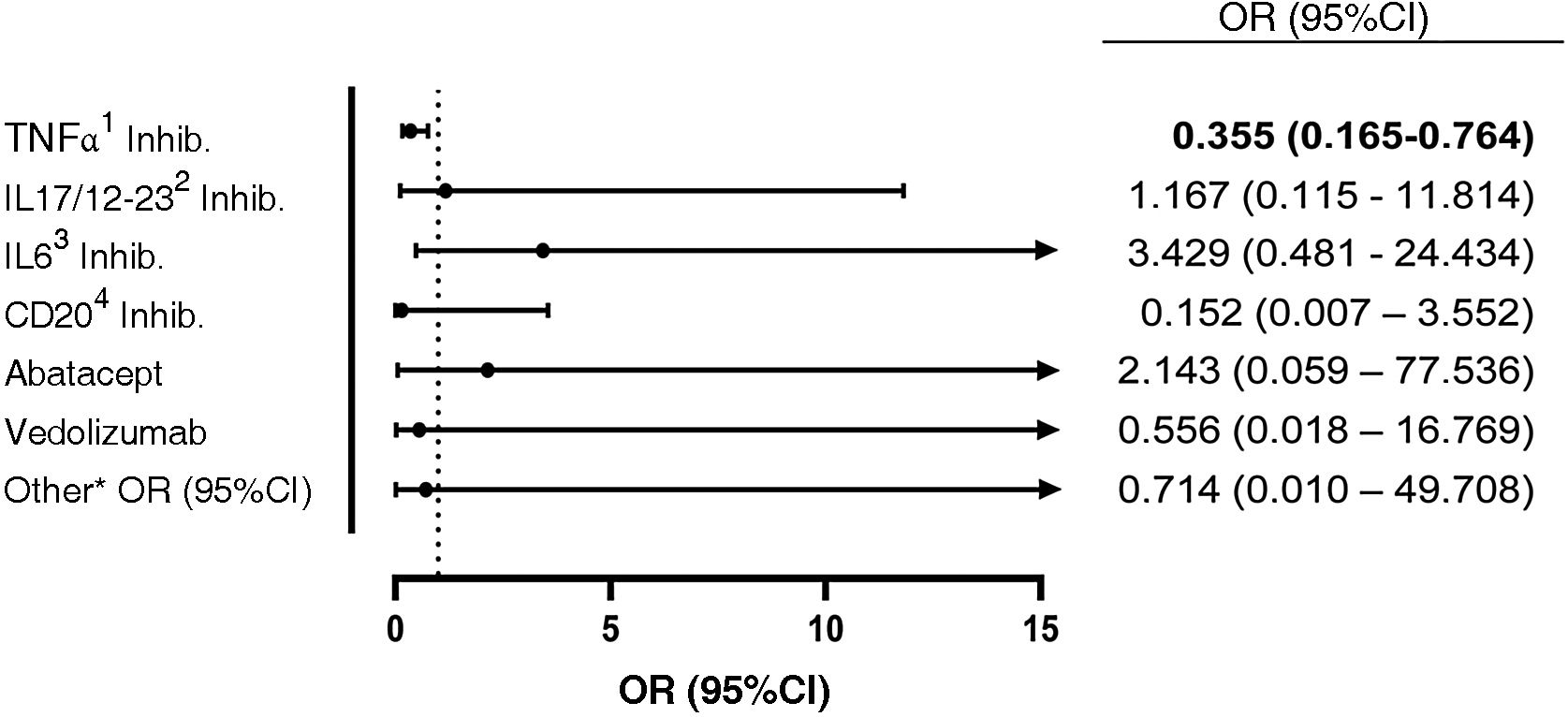
The seroprevalence of each of the drugs in monotherapy versus concomitant treatment with methotrexate was determined within the group of all bDMARDs and tsDMARDs. In the group of patients treated with TNFα inhibitors and methotrexate, a seroprevalence of 10.1% was found, vs. 24.1% in patients treated with TNFα inhibitors in monotherapy (OR: 0.355 [95% CI: 0.165−0.764]; p = 0.006). No differences were observed with the other biologics (Fig. 1).
Odds ratio of positive SARS-CoV-2 antibody serology of patients on bDMARD with concomitant methotrexate compared to patients on bDMARD monotherapy. Methotrexate + tsDMARD was not analysed due to the very small sample size of this group.
1 TNFα inhibitors: infliximab, adalimumab, etanercept (and its biosimilars), certolizumab, golimumab.
2 IL-17 and IL-12/23 inhibitors: secukinumab, ixekizumab, ustekinumab.
3 IL-6 inhibitors: tocilizumab, sarilumab.
4 CD20 inhibitors: rituximab.
* Others: belimumab, anakinra.
Of the 550 patients, 94 (17.1%) were receiving concomitant treatment with glucocorticoids, 82 (87.2%) at doses ≤7.5 mg/day of prednisone, and 12 (12.8%) at doses >7.5 mg/day. There was a trend towards lower seroprevalence in the group of patients treated with glucocorticoids compared to those not taking these drugs (9.6 vs. 17.6%; p = 0.055).
The seroprevalence of patients on glucocorticoid treatment at doses ≤7.5 mg/day of prednisone was 9.8%, vs. 8.3% of patients on doses > 7.5 mg/day, with no significant difference (p = 0.678).
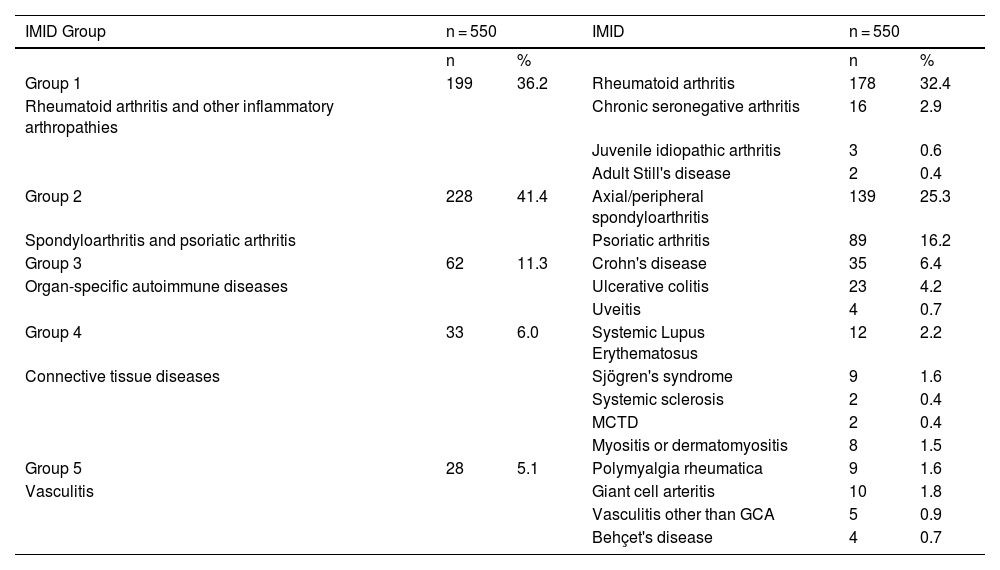
Seroprevalence according to groups of immune-mediated diseasesThe frequency of the different IMIDs is shown in Table 4.
Grouping of patients according to immune-mediated diseases.
| IMID Group | n = 550 | IMID | n = 550 | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Group 1 | 199 | 36.2 | Rheumatoid arthritis | 178 | 32.4 |
| Rheumatoid arthritis and other inflammatory arthropathies | Chronic seronegative arthritis | 16 | 2.9 | ||
| Juvenile idiopathic arthritis | 3 | 0.6 | |||
| Adult Still's disease | 2 | 0.4 | |||
| Group 2 | 228 | 41.4 | Axial/peripheral spondyloarthritis | 139 | 25.3 |
| Spondyloarthritis and psoriatic arthritis | Psoriatic arthritis | 89 | 16.2 | ||
| Group 3 | 62 | 11.3 | Crohn's disease | 35 | 6.4 |
| Organ-specific autoimmune diseases | Ulcerative colitis | 23 | 4.2 | ||
| Uveitis | 4 | 0.7 | |||
| Group 4 | 33 | 6.0 | Systemic Lupus Erythematosus | 12 | 2.2 |
| Connective tissue diseases | Sjögren's syndrome | 9 | 1.6 | ||
| Systemic sclerosis | 2 | 0.4 | |||
| MCTD | 2 | 0.4 | |||
| Myositis or dermatomyositis | 8 | 1.5 | |||
| Group 5 | 28 | 5.1 | Polymyalgia rheumatica | 9 | 1.6 |
| Vasculitis | Giant cell arteritis | 10 | 1.8 | ||
| Vasculitis other than GCA | 5 | 0.9 | |||
| Behçet's disease | 4 | 0.7 | |||
MCTD: mixed connective tissue disease.
When analysed by disease groups, a seroprevalence of 17.6% was observed in patients of group 1 vs. 15.1% in the rest of the patients; 16.2% in group 2 vs. 15.8% in the rest of the patients; 12.9% in group 3 vs. 16.4% in the rest; 9.1% in group 4 vs. 16.4% in the rest; 12.9% in group 3 vs. 16.4% of the rest; 9.1% in group 4 vs. 16.4% of the rest, and 17.9% in group 5 vs. 15.9% of the rest, with no significant differences between disease groups (Table 1).
Seroprevalence according to comorbidity groupsThe seroprevalence of patients with and without comorbidities showed no significant differences (OR: 1.213 [95% CI: 0.745–1.974]; p = 0.437). When analysing the different categories of comorbidities versus patients without comorbidities, no significant differences were found either (Table 1).
Symptoms of COVID-19Of the 88 patients with positive serology for SARS-CoV-2, 28 (31.8%) were asymptomatic, 52 (59.1%) had mild symptoms and 8 (9.1%) had severe symptoms.
DiscussionThis study found a higher seroprevalence of SARS-CoV-2 in patients treated with anti-TNFα compared to the other treatment groups, although without reaching statistical significance. These data are in contrast to the work of Simon et al.,11 who found a lower proportion of seroprevalence in patients on TNFα inhibitors. These differences can probably be explained by variability in the populations studied. Of all the patients with IMID included in their study, only 23% were treated with TNFα inhibitors; however, in our study, up to 60% were treated with TNFα inhibitors. The CD20 inhibitor (rituximab) treatment group showed a trend towards a lower seroprevalence, although the sample size of these patients was small, which is probably why the result was not statistically significant. There are several previously published studies demonstrating more severe COVID-19 infection outcomes in rituximab patients and lack of seroconversion after rituximab.12–15 Nevertheless, despite the impairment of humoral immunity in this cohort of patients following infection, several investigations have demonstrated the development of a robust cellular (T-cell) response. Furthermore, the majority of these patients have been observed to maintain this effect for up to 9–12 months following recovery from COVID-19 infection, and for at least 6 months following mRNA vaccination.16–19
Similarly, studies in patients with inflammatory bowel diseases (IBD) treated with vedolizumab did not demonstrate an increased risk of SARS-CoV-2 infection. Seroprevalences were found to be similar to those in the general population. As in our study, no differences were observed with this treatment.20–22
With regard to combination therapy with csDMARDs, seroprevalence was significantly lower in patients treated with TNFα inhibitors who received concomitant treatment with methotrexate compared to monotherapy with TNFα inhibitors. However, no significant differences were found in this group of patients with the other csDMARDs nor with any of the csDMARDs in the other groups of the different biologic and synthetic targeted therapies.
No significant difference in seroprevalence was observed in a study by Favalli et al.23 among patients receiving bDMARDs in combination with csDMARDs. However, there are published data on the lack of post-vaccination seroconversion in these patients due to the attenuation of humoral immunity caused by methotrexate.24–26
Seroprevalence of IMID patients treated with b/tsDMARDs observed in our study is very similar to the Italian group, perhaps because both Spain and Italy were among the most affected countries in terms of the number of infections in the first waves of the pandemic compared to other European countries.23 Moreover, these studies found that there were no significant differences between seroprevalence in patients treated with biological therapies and healthy subjects.11,23
In this study there was a trend towards lower seroprevalence in patients treated with glucocorticoids. However, no significant differences were found, probably due to the small number of patients receiving these treatments. Published data indicates that seroprevalence decreases significantly with doses of prednisone exceeding 10 mg/day of prednisone (p = 0.0253).23 A number of studies have indicated that the use of steroid therapy is associated with higher rates of COVID-19 infection, increased hospitalisations, intensive care unit admissions and mortality.27–30 Brenner et al.,29 in their study of IBD patients, also observed a higher proportion of severe COVID-19 in those receiving glucocorticoids as part of their disease treatment (OR: 6.87 [CI 95%: 2.30–20.51]), but not in those treated with TNFα inhibitors. To determine the existence of a real influence of glucocorticoids on seroprevalence, it would be necessary to assess not only the daily doses received, but also the cumulative doses of the drug.
As for the immuno-mediated diseases, similar seroprevalences were found in our study between the different disease groups, as in previous published work, where no significant differences in seroprevalence were found between the different IMID groups.11,23
Concerning comorbidities, no influence was observed on seroprevalence compared to patients without comorbidities. Saadoun et al. also found no significant difference in seroprevalence between IMID patients with and without comorbidities.31 However, another study found an increased risk of seropositivity for IgG antibodies in IMID patients with at least one comorbidity (p = 0.003), just as the absence of comorbidities was associated with a low probability of a positive anti-SARS-CoV-2 antibody test (p = 0.004).32 Evidence from several previously published studies has shown that the presence of co-morbidities has contributed to worse outcomes in cases of COVID-19 infection, especially among patients with hypertension, diabetes mellitus, obesity, COPD and neoplasms, who develop symptoms of greater severity, accompanied by comparatively higher mortality rates.33 Our study has analysed the influence of different types of comorbidities on seroprevalence in our group of patients but found no association between comorbidities and seroprevalence.
In terms of strengths, mention should be made of the prospective and cross-sectional approach of the work, thus enabling real-time data acquisition. In addition, binary logistic regression models were used, which adds further statistical support.
The main limitations of this study are the small sample size for some bDMARDs and tsDMARDs. This prevents accurate determination of their real impact on seroprevalence due to the lack of representative data. Also, the lack of sample randomisation may have limited the generalisability of the findings.
On the other hand, regarding concomitant glucocorticoid use, it was not possible to obtain information on cumulative doses for all patients over the years.
As for the immune response, only the humoral response was determined through the measurement of IgG antibodies. The cellular response was not analysed as the necessary means for the procedure were not available.
The present study is clinically relevant as it helps to identify those patients who may experience a poor immune response or worse outcome after COVID-19 infection, depending on the immunosuppressive treatment they have received, allowing for a better understanding of how certain treatments may affect the immune response in this group of patients.
In conclusion, the results of our study indicate that patients treated with TNFα inhibitors exhibit a better humoral response compared to other biologic treatments or tsDMARDs. As for concomitant treatment with csDMARDs, methotrexate has a negative influence on seroprevalence in these patients. Glucocorticoids were not shown to have an impact on seroprevalence in our study.
Ethical considerationsThe study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Hospital Universitario La Paz Medicine Ethics Committee on research involving medicinal products (CEIm), HULP code: PI-4164 on 4 June 2020. Written informed consent was obtained from all study participants.
FundingThe study has received two grants from the Fundación para la Investigación e Innovación Biomédica del Hospital Universitario Infanta Sofía y Hospital Universitario del Henares (FIIB HUIS HHEN): Grant with assigned code FHH20/COVID04, awarded on 12 June 2020, and Grant with assigned code FHH20/COVID07, awarded on 11 December 2020.
Conflict of interestThe authors declare that they have no conflict of interest.
Liz Romero-Bogado. Martina Steiner. Santiago Muñoz-Fernández. Tatiana Cobo-Ibáñez. Alejandro Gómez. Beatriz Paredes-Romero. Ana Esteban-Vázquez. Section of Rheumatology, Hospital Universitario Infanta Sofía, Universidad Europea de Madrid, San Sebastián de los Reyes, Madrid, Spain.
Teresa Navío. Laura Cebrián. Fernando Sánchez. M. Ángeles Matías. Section of Rheumatology, Hospital Universitario Infanta Leonor, Universidad Complutense de Madrid, Madrid, Spain.
Israel John Thuissard Vasallo. Cristina Andreu Vázquez. Ana Isabel Castillo. Faculty of Biomedical and Health Sciences, Universidad Europea de Madrid, Villaviciosa de Odón, Madrid, Spain.
Cristina García-Yubero. Alicia Martínez. Pharmacy Section, Hospital Universitario Infanta Sofía, Universidad Europea de Madrid, San Sebastián de los Reyes, Madrid, Spain.
Mar Esteban. Section of Ophthalmology, Hospital Universitario Infanta Sofía, Universidad Europea de Madrid, San Sebastián de los Reyes, Madrid, Spain.
Noemí Manceñido. Ramón Pajares. M. Rosario Arribas. Section of Gastroenterology, Hospital Universitario Infanta Sofía, Universidad Europea de Madrid, San Sebastián de los Reyes, Madrid, Spain.
Concepción Esteban. Servicio de Farmacia, Hospital Universitario Infanta Leonor, Universidad Complutense de Madrid, San Sebastián de los Reyes, Madrid, Spain.
Raquel Guillén. Irene García. Central Laboratory, Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Madrid, Spain.