Many brain processes that cause death are mediated by intracranial hypertension (ICH). The natural course of this condition inevitably leads to brain death. The objective of this study is to carry out a systematic review of cerebral pathophysiology and intracranial pressure (ICP) monitoring.
DevelopmentStudying, monitoring, and recording ICP waves provide data about the presence of different processes that develop with ICH.
ConclusionsCorrect monitoring of ICP is fundamental for diagnosing ICH, and even more importantly, providing appropriate treatment in a timely manner.
Muchos procesos encefálicos que causan la muerte de los pacientes que los presentan están mediados por hipertensión intracraneal (HIC). La historia natural de la misma conduce inexorablemente a esta muerte encefálica. El objetivo de este trabajo estriba en realizar una revisión de la fisiopatología cerebral y de la monitorización de la presión intracraneal (PIC).
DesarrolloEl estudio de las ondas de PIC, su monitorización y el registro de las mismas nos informan sobre la existencia de procesos que tienen como común denominador la HIC.
ConclusionesEl correcto registro de la PIC es fundamental para diagnosticar la HIC y, lo que resulta aún más importante, poder instaurar un tratamiento adecuado a tiempo.
In many patients affected by neurological disease, cerebral oedema and the intracranial hypertension (ICH) resulting from oedema are the common factors or direct pathways leading to brain death. The natural course of this condition inevitably leads to brain death. Studying, monitoring, and recording waves of intracranial pressure (ICP) provided data about the presence of different processes that develop alongside ICH. These processes mainly include severe head trauma (HT), cerebrovascular disease (intraparenchymal haematoma, spontaneous subarachnoid haemorrhage, malignant infarct of the middle cerebral artery, vascular malformation), and intracranial tumours.
Guillaume and Janny described the first experiences with ICP monitoring just over 60 years ago.1 Our study presents a review of brain physiology, ICP pathophysiology, and those aspects related to ICP monitoring. We also provide some comments regarding treatment of ICH.
DevelopmentBrain physiologyGeneral considerationsThe skull, once the sutures and fontanelles have closed, becomes a structure that permits no further expansion, so its volume will therefore remain constant regardless of its content. Under normal conditions, this content can be divided into 3 compartments (the Monro-Kellie doctrine): cerebral parenchyma (80%), cerebrospinal fluid (CSF) (10%), and blood (10%). When the volume of any of these 3 compartments increases, so does the pressure it exerts on the other 2 compartments.
Under normal circumstances, these variations are immediately compensated by means of CSF displacement into the lumbar cistern. Following that step, cerebral blood flow (CBF) decreases. In chronic situations only, the parenchyma changes shape as it loses part of extracellular water, and even neurons and glial cells.
However, when these buffer mechanisms fail, an increase in ICP may lead to a decrease in blood supply and subsequently elicit a decrease in cerebral perfusion pressure (CPP). This drop in pressure increases the probability of ischaemic lesions since CPP depends on two factors: mean arterial pressure (MAP) and ICP.2 In this interaction, we can distinguish 3 situations. In the first stage, the increased intracranial volume (IV) does not affect ICP since the CSF and cerebral blood are displaced in order to compensate the change in volume. In the second stage, this regulatory system is overloaded and can no longer compensate for the increase in pressure secondary to the increase in volume. In the third stage, the self-regulatory system ceases functioning and even small changes in volume can lead to a very pronounced rise in ICP.2–6
Cerebral blood flowThe brain receives between 15% and 25% of the cardiac output, with a CBF of 40 to 50mL/100g of brain tissue/min. Values of CBF are determined by the cerebral metabolic rate of oxygen consumption (CMRO2), which is in turn determined by the cerebral autoregulation by means of cerebral vascular resistance (CVR). CBF is also determined by CPP, the difference between MAP and ICP.2,3
Forty per cent of the CMRO2 corresponds to baseline energy expenditure (mainly to maintain membrane potential, so expenditure is thermosensitive but not modifiable with drugs). The remaining 60% corresponds to functional energy expenditure (it is not thermosensitive but can be altered with drugs). Ninety per cent of the CMRO2 corresponds to neural tissue and only 10% to the supporting tissue or glial cells (accounting for more than 50% of the total brain volume). CMRO2 ranges between 4 and 6mL/100g of brain tissue/min. Therefore, pathological situations including anaemia and hypoxia decrease arterial oxygen content which could lead to an improper supply of oxygen to the brain.
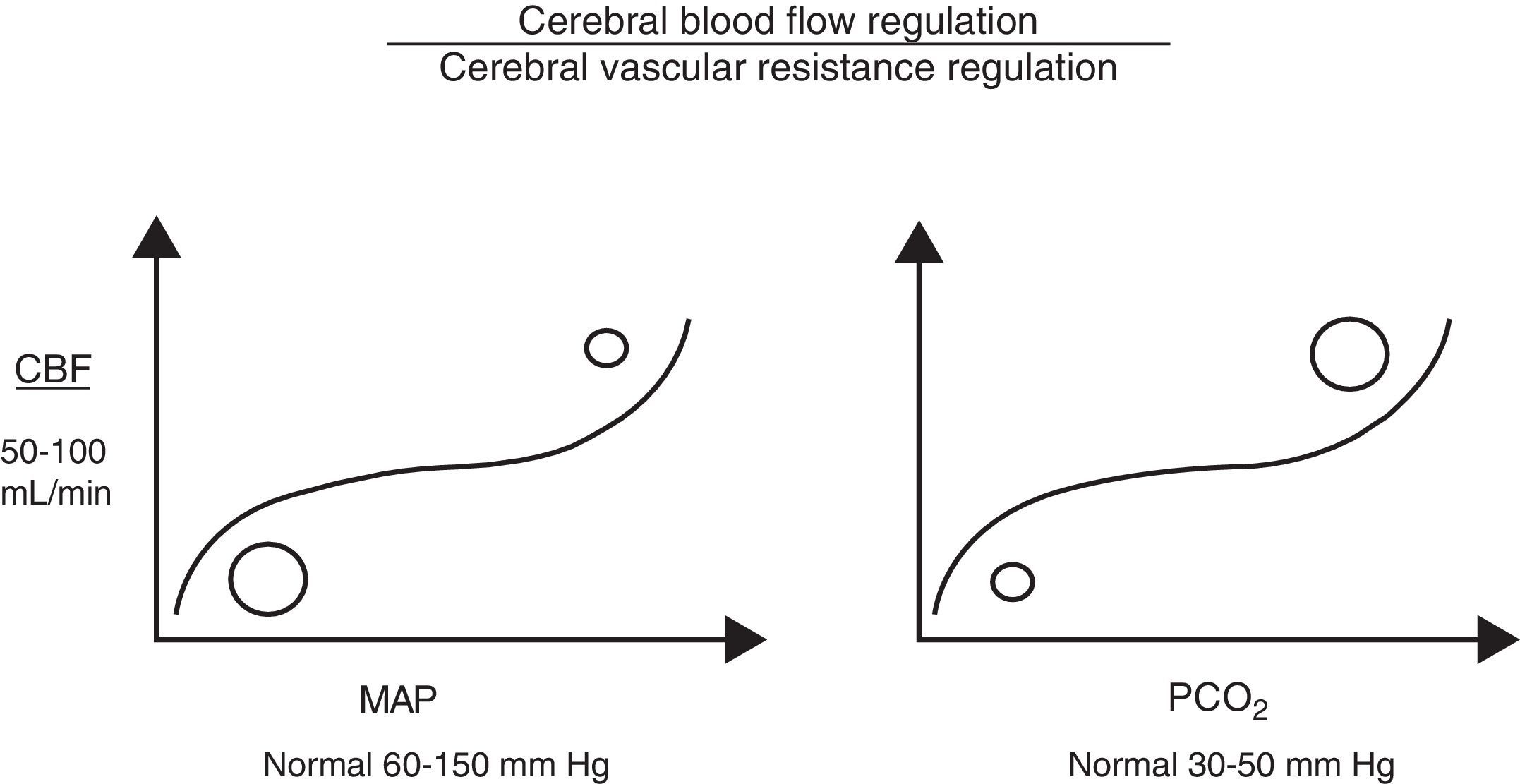
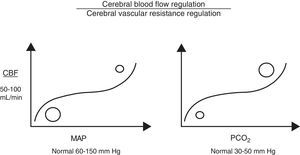
Cerebral autoregulation functions by modifying VCR (vasodilation or vasoconstriction) so as to maintain CBF at an appropriate level to meet the brain's metabolic oxygen requirements. Cerebral autoregulation is greatly determined by partial pressure of arterial carbon dioxide (PaCO2), MAP, and to a lesser extent, by partial pressure of arterial oxygen, adenosine, pH, etc. Therefore, when brain PaCO2 is high (increased metabolic work), VCR drops (vasodilation), and CBF and oxygen supply to the brain increases (CDO2). The opposite is true when PaCO2 decreases (reduced metabolic work; vasoconstriction). Under normotensive conditions, CBF is estimated to fluctuate by 4% for each mmHg of CO2. Something similar also occurs with MAP, since CBF is regulated to protect brain tissue from abrupt pressure spikes and drops that could compromise CDO2. However, when the upper or lower limits of these autoregulatory mechanisms are exceeded, CBF becomes absolutely dependent on MAP (Fig. 1).7
Cerebral perfusion pressureCPP is defined as the pressure necessary for perfusing nervous tissue to achieve adequate metabolic function. A CPP below 50mmHg results in a severe decrease in CBF and a subsequent risk of cerebral ischaemia. On the contrary, CPP values above 60 to 70mmHg are considered to be safe for adults (they have yet to be clearly determined for children, although we already know that immature brains, such those of newborns, tolerate lower CPP values better).2,3,8,9
Physiopathology of ICPICP is defined as the pressure inside the cranial vault. It is understood that the brain functions correctly when ICP values are between 10 and 20mmHg in adults, 3 and 7mmHg in children, and 1.5 and 6mmHg in newborns.2
ICP is the result of the interaction between the brain, CSF, and cerebral blood. As mentioned previously, cerebral parenchyma account for 80% of the intracranial content. Cerebral parenchyma has a water content of 75% to 80%; water is found in both the intracellular space (white matter and grey matter) and extracellular space (interstitial space).
Furthermore, CSF accounts for approximately 10% of the IV. CSF is mainly produced by the choroid plexus at a rate of 0.3 to 0.35mL/min. CSF production can be altered in several different situations, including inflammation of arachnoid villi and increases in ICP itself (cases of diffuse cerebral oedema or presence of intracranial masses, such as haemorrhages or tumours).
The blood in the brain refers to both cerebral blood volume (CBV) and CBF. CBV, the constant volume of blood in the brain, corresponds to approximately 10% of the total IV. CBV directly contributes to ICP, whereas the effect of CBF is indirect, through cerebral autoregulation.
ICP changes with position (standing vs decubitus) and also fluctuates with systemic arterial pressure and breathing.2 Valsalva manoeuvres, which increase intrathoracic or intra-abdominal pressure (coughing or defecating, for example), consequently increase pressure in the jugular veins and/or epidural venous plexus. Considering that cerebral veins have no valves, this increased venous pressure is transmitted to the intracranial space, resulting in a corresponding rise in ICP.
Under normal, physiological conditions, the graphic recording of ICP is stable and regular and ICP depends on the following factors:
- 1.
CSF production volume.
- 2.
System resistance to reabsorption of CSF.
- 3.
Venous pressure in the intracranial space, or equivalently, pressure in the superior longitudinal sinus.
The 3 compartments of the intracranial vault are essentially incompressible, and therefore total IV is constant. Under pathological conditions, if one of these compartments increases or a fourth one is created (by a mass-effect lesion such as tumour or haematoma), one or more of the other compartments must necessarily shrink to avoid an increase in ICP.
In case of a slow-growing cerebral lesion, the parenchymal compartment undergoes deformation or remodelling to compensate for increased ICP (loss of extracellular water, glial cells, and even neurons). It is clear that elderly patients tolerate the mass effect of a lesion better than younger patients, given that brain atrophy is natural in advanced age.
However, the parenchymal compartment cannot compensate for an abrupt increase in ICP, and therefore CBV and CSF will be responsible for absorbing that increase. CSF is the main compensatory system; in response to increased IV, it moves into the perimedullary subarachnoid space until the displaced brain structures block CSF flow.2
The vascular compartment's ability to compensate for ICP activates at a later stage and consists of reducing that compartment by displacing blood to the skull exterior by means of jugular drainage.
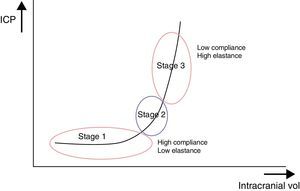
Interaction between cerebral volume and ICPThe quotient of volume differential (dV) and intracranial pressure differential (dP), that is, the volume necessary to obtain a known change in pressure, is known as cerebral compliance. We can therefore understand cerebral compliance as the cranial vault's adaptive capacity that lets it tolerate an increase in volume depending on compensation mechanisms. The inverse operation, that is, dP/dV, is known as cerebral elastance (pressure resulting from a known change in volume). It is understood as the resistance that opposes IV expansion.
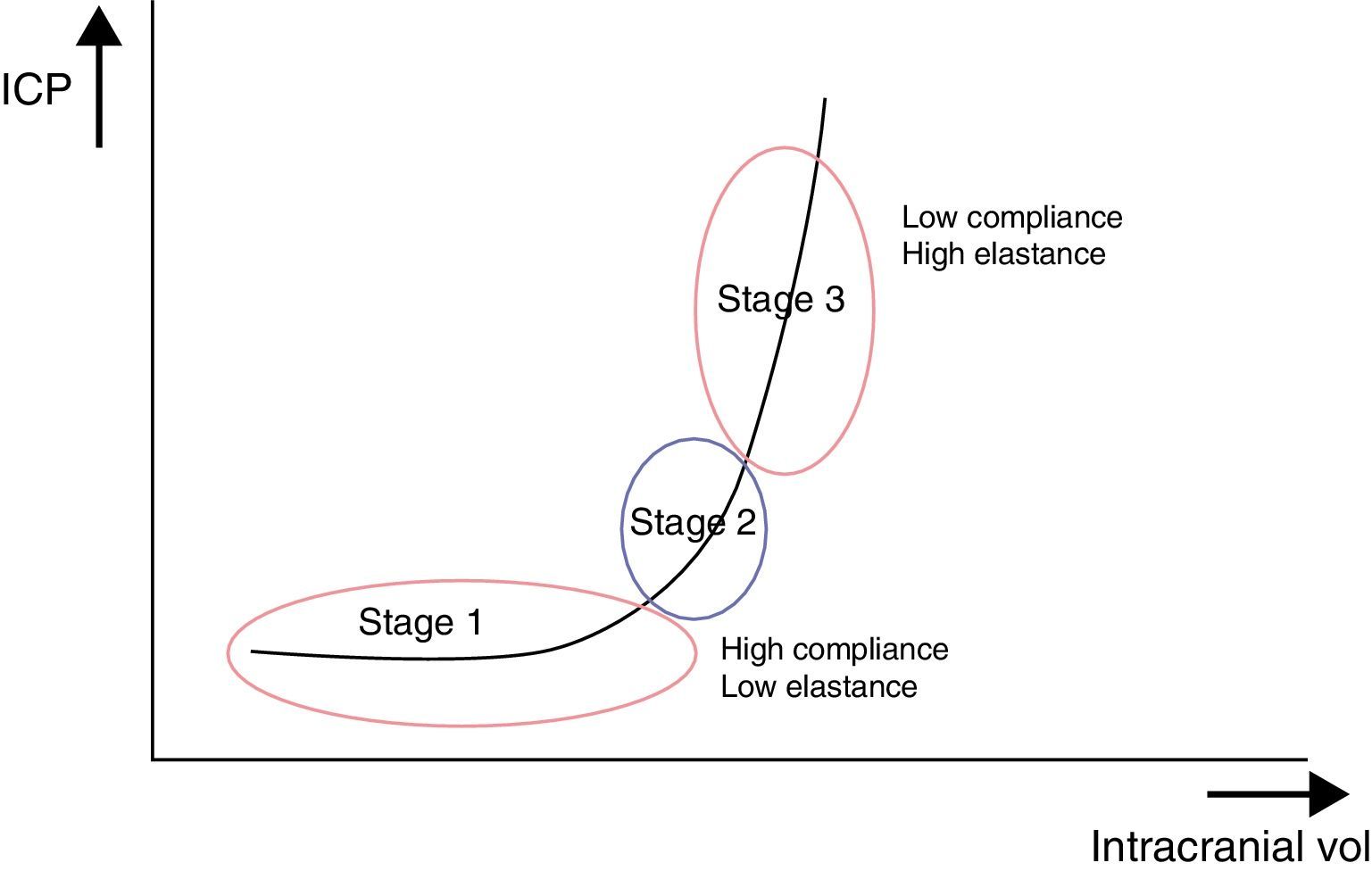
These ICP buffer systems are limited as can be deduced from the intracranial pressure-volume curve. This curve displays the relationship between changes in ICP and IV. It is made up of 3 stages (Fig. 2):
- –
Initial stage (stage 1): this is the stage dominated by high compliance and low ICP. Despite the increase in volume, there is scarcely any increase in ICP (CSF and CBV absorb the increase in volume).
- –
Transition stage (stage 2): this stage is characterised by low compliance and low ICP, but the latter starts to increase slowly.
- –
Ascending stage (stage 3): stage of low or null compliance and high ICP (beginning decompensation). Compensatory mechanisms stop working and small changes in volume elicit high increases in pressure.
The sigmoidal behaviour of the ICP-IV curve shows that major pressure changes are elicited by small increases in IV. Because of cerebral distensibility and buffering capacity, a change in volume will give rise to a tenfold increase in the numeric value of ICP. This gives us the intracranial pressure-volume index (PVI). Therefore, a PVI>18mL indicates a low risk of ICH, whereas a PVI<13mL suggests a practically untreatable ICP value. In clinical practice, increases in volume that elicit increases in ICP in excess of 25mmHg are considered to start the decompensation stage. The problem here is how to clinically determine if a patient with normal ICP values is in the initial phase (high compliance) or in the ascending phase (low compliance). Several methods for studying cerebral compliance have been developed, including an analysis of the morphology of the ICP curve.2,10
Another issue to be considered is that the pressure-volume curve corresponds to the craniospinal axis when the 2 spaces communicate freely. If CSF flow is blocked between the 2 compartments, as in a case of transtentorial or transforaminal herniation, the curve shows left displacement (cranial curve) with accompanying lower compliance.
Monitoring methodsThe standard method for monitoring ICP consists of placing a catheter inside the ventricular system. This system also enables treatment of high ICP by draining CSF. Multiple devices are currently available, with different intracranial locations and using different transducer tips (fluid-coupled or non-fluid coupled catheters, including fibre-optic catheters in the latter case).4,5,10–14
Monitoring device location- 1.
Intraventricular: this is the gold standard since it enables treatment of ICH in addition to being the simplest and most cost-efficient method. Following precoronal trepanation, the intraventricular device is placed ideally on the hemisphere with the most lesions visible in imaging studies, since an interhemispheric pressure gradient may exist. The main complication is infection, especially for catheters that remain inserted for more than 5 days (2%-22%). Tunnelling the catheter as far as possible from the incision site is recommended to minimise this risk.15 Antibiotic prophylaxis is not currently recommended while the catheter is in place, and therefore strict aseptic conditions are required during the procedure. Other problems deriving from this technique are haemorrhages and obstruction in the tract (1%-6%), especially if an associated intraventricular haemorrhage is present. In some occasions, due to ventricular collapse or displacement, the catheter cannot be placed in this location and another site will have to be chosen.
- 2.
Intraparenchymal: the catheter can be introduced bedside in the critical care unit, unlike all other catheter systems. Some of these devices are also able to offer doctors data other than ICP, such as brain temperature. The insertion process is very similar to that of an intraventricular catheter and it has fewer complications, but it cannot be used to drain CSF. Once it has been placed, experts recommend recalibrating it as little as possible to avoid damaging the fibre optic transducer.16
- 3.
Subarachnoid: this approach is no longer in use. While it reduces the risk of bleeding since the catheter does not penetrate the parenchyma, the readings it delivers include many artefacts.
- 4.
Subdural: these catheters are generally placed after surgical evacuation of mass-effect lesions. They may even be placed inside the same surgical site for postsurgical monitoring. This system tends to underestimate real ICP.
- 5.
Epidural: this is a far less invasive technique but it tends to overestimate absolute values of ICP, which can lead to an iatrogenic effect as doctors attempt to treat false ICH situations.17
- 6.
Lumbar: catheter can be placed easily by means of lumbar puncture. Devices that prevent associated loss of CSF must also be used. In situations in which CSF flow is obstructed between the lateral ventricles and the lumbar cistern, these readings are not reliable.
The clearest indication for monitoring ICP is severe HT (Glasgow coma scale≤8). According to the guidelines for managing HT, patients benefiting from ICP monitoring are those at risk for ICH,3–5,18 listed below.
- 1.
Patients with pathological cranial computed tomography (CT), except for individuals with diffuse axonal lesion since their risk of ICH is very low.
- 2.
When 2 of the following conditions are met:
- –
Age>40 years.
- –
Unilateral or bilateral decerebrate posturing.
- –
Pupillary abnormalities.
- –
Only 13% of the patients with severe HT and initial cranial CT showing no lesions will present raised ICP needing treatment. This percentage will almost always include those patients for whom CT was performed early (during the first 2 or 3 h after trauma). In fact, for these patients, the imaging study should be repeated within the following 8 hours.
Patients requiring sedation for reasons that are not brain-related, or those presenting conditions that can potentially cause ICH (closed abdominal trauma resulting in abdominal hypertension, severe respiratory distress needing extrinsic positive end-expiratory pressure [PEEP], etc.) could also benefit from ICP monitoring.
Study of ICP wavesIn addition to obtaining the absolute value of ICP, we can analyse its wave morphology and any changes that might be indicative of an autoregulatory defect. On this basis, we can draw up a treatment plan for ICP before irreversible lesions emerge.
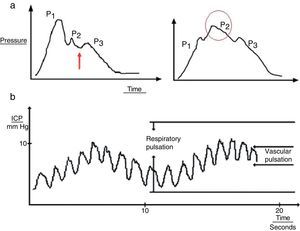
Several upstrokes can be distinguished in an ICP waveform19:
- 1.
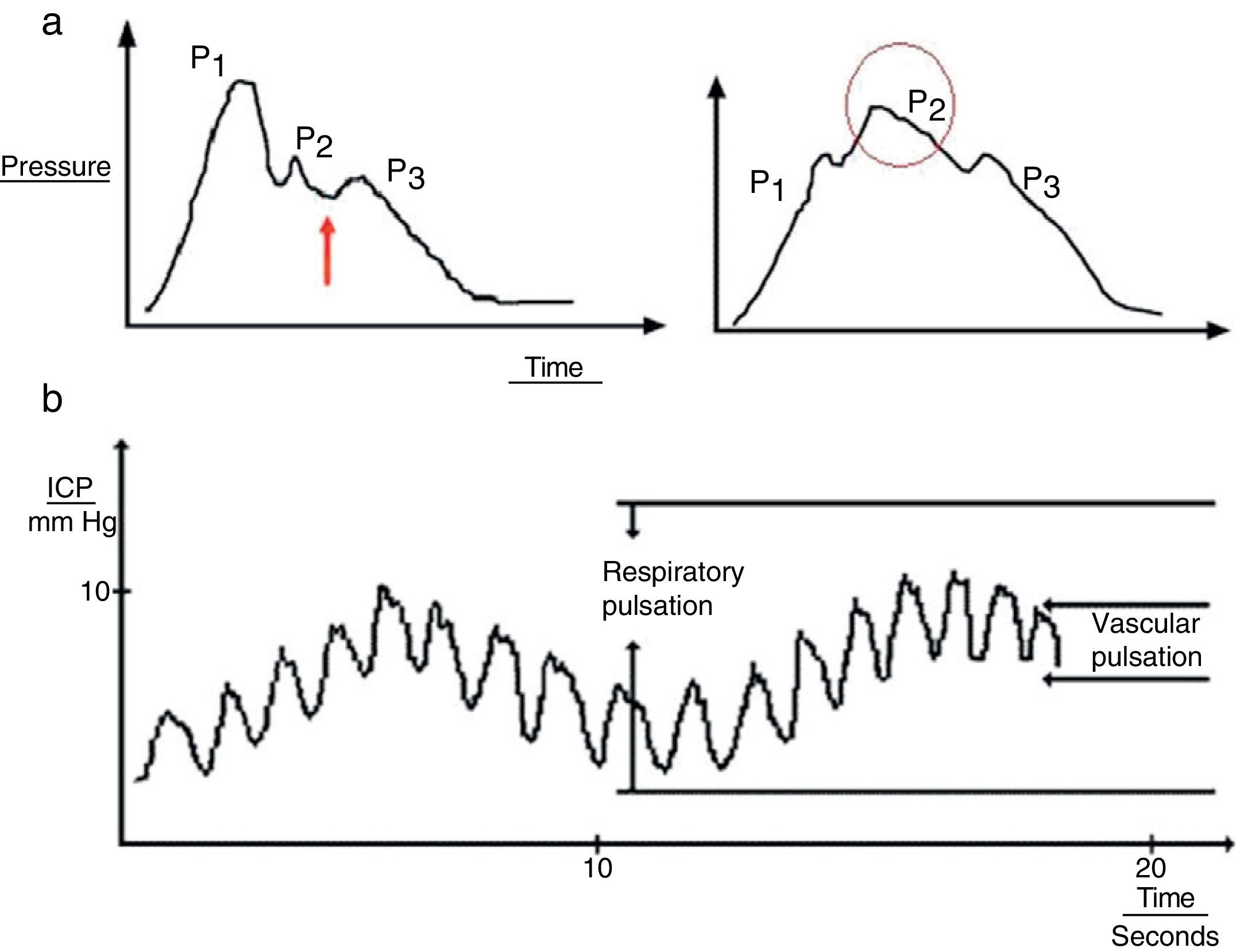
Cardiac waves: these waves result from transmission of the pulse in cerebral blood vessels; their morphology resembles that of the arterial pulsation waveform and 3 upstrokes: P1 (percussion wave), P2 (tidal wave), and P3 (dicrotic wave). Although the precise origin of these peaks remains unclear, P1 is assumed to be due to the arterial pulsation on the choroid plexi and reflects cerebral flow, while P2 and P3 are due to the retrograde venous pulse of the jugular against the cortical veins. The dicrotic notch indicating the pulse is found between these peaks. The change in the morphology of the second wave (P2) can predict the failure of the cerebral autoregulation systems, and therefore serves as an early indicator of ICP (Fig. 3a).
Figure 3.(a) Graph presenting the cardiac wave of intracranial pressure (ICP). The image on the left shows a non-pathological recording; the arrow points to the dicrotic notch. The image on the right shows a pronounced P2 wave in situations of low distensibility. (b) Pulse waveform of ICP.
(0.15MB). - 2.
Respiratory waves: these waves give the reading its sinusoid form (Fig. 3b).
Lundberg, the first to describe the change in morphology in the global ICP waveform, observed 3 different patterns20,21:
- 1.
‘A’ or plateau waves: increases in ICP that persist over time (5-20min) and display a large amplitude (50-100mmHg). Although ‘A’ waves can be observed in healthy asymptomatic individuals, long-term presence in pressure readings indicates CPP compromise. These ICP spikes will provoke global ischaemia followed by brain death. Examination often reveals clinical signs of pain where ‘A’ waves are present.
- 2.
‘B’ waves: waves with an amplitude of 20 to 50mmHg and duration of 1 to 2min. These waves can progress to ‘A’ waves and are related to changes in the physiological or pathological CBF.
- 3.
‘C’ waves: these waves have no pathological significance. They present an amplitude of less than 20mmHg and a duration shorter than 5min. They result from the transmission of arterial pressure waves.
ICP absolute values, in an adult patient, oscillate between 5 and 15mmHg, and the average pressure is 12mmHg. When values exceed 20mmHg, the patient is considered to have ICH. We should not forget that ICH does occur during different physiological situations, including the REM sleep phase or any Valsalva manoeuvre (coughing, sneezing, defecating, etc.). Intubated patients also present causes of increased ICP that are not brain-related: fever, CO2 retention, venous compression (in the jugular region because of head posture, in the chest due to high PEEP or pneumothorax, in the abdomen due to compartment syndrome), and others.
Management of patients with ICH-cerebral oedema aims to ensure an ICP lower than 20mmHg and a CPP higher than 60mmHg. CPP values of less than 60mmHg point to increased risk of ischaemic events; high ICP favours cerebral oedema as hydrostatic pressure increases.3–5 From the therapeutic perspective, we distinguish first-line and second-line measures to combat ICH22:
First level measures- 1.
Promote jugular venous outflow:
- –
Patient's head in neutral position, with the head of the bed elevated to 30°. The correct position for pregnant women is left lateral decubitus.
- –
Decrease abdominal pressure using muscle relaxants, laxatives, or decompression, in cases of suspected abdominal hypertension.
- –
Decrease intrathoracic pressure, especially in patients requiring high PEEP.
- –
- 2.
Decrease cerebral metabolic rate of oxygen consumption:
- –
Sedation.
- –
Analgesia.
- –
Maintain normal temperature.
- –
- 3.
Improve cerebral oxygenation:
- –
Achieve moderate hyperventilation with carbon dioxide pressure (pCO2) between 25 and 30mmHg. Be mindful that intense hyperventilation (pCO2≤25mmHg) is contraindicated in the first 24 hours.
- –
Maintain oxygen saturation above 90% and oxygen pressure over 80mmHg.
- –
Normal perfusion pressure: systolic blood pressure higher than 90mmHg, haematocrit levels of 30% to 33%, and haemoglobin levels of 8 to 10g/dL.
- –
- 4.
Decrease cerebral oedema:
- –
Monitor ICP through external ventricular drain if possible, since CSF drainage can be used to decrease ICP.
- –
Osmotherapy: either 20% mannitol or hypertonic saline (3%, 7.2%, 20%, or 23.4%). Intermittent boli of 20min duration should be administered every 4 hours, with a maximum of one litre per day. Serum osmolality should be maintained below 320mOsm/kg and serum Na+ lower than 155mEq/L.
- –
- 5.
Use prophylactic antiepileptics:
- –
These drugs are recommended since seizures are accompanied by increases in both ICP and tissue oxygen demand. Furthermore, many seizures are subclinical.
- –
- 6.
Repeat cranial CT:
- –
This study is performed to rule out exacerbated and/or new intracranial lesions needing surgical evacuation, as well as appearance of massive cerebral oedema (brain swelling).
- –
When ICP is resistant to previous treatments and pressure values exceed 20mmHg, second-line measures should be applied: intense hyperventilation, moderate hypothermia, barbiturate-induced coma, or decompressive craniectomy.
Limitations of ICP readingsThe studies conducted by Sahuquillo et al.23 demonstrated the presence of ICP gradients between the two brain hemispheres, although CPP analyses did not find statistically significant differences. The best practice is therefore to monitor ICP in the hemisphere ipsilateral to the largest lesion or lesions.24
Temporal lesions, above all where there has been a rupture in the left temporal lobe (cerebral contusion with subarachnoid haemorrhage and/or associated subdural haematoma), should be monitored with daily imaging tests. This is because uncal herniation may develop with no prior increase in ICP.
On the other hand, lesions in the posterior fossa cannot be monitored using ICP recordings, and CSF overdrainage through an intraventricular catheter can lead to an inverted transtentorial herniation in these cases.
If cerebral haemorrhage is suspected due to aneurysm or arteriovenous malformation, keeping ICP extremely low is contraindicated since this would promote hypothetical rebleeding of the vascular lesion. This circumstance, however, is not a limitation of ICP recordings as such.
Finally, we should mention that information obtained from ICP recordings provides a global view of intracranial status that should be complemented with specific information on cerebral oxygenation and metabolic status, from both healthy and pathological territories.
Aids in ICP monitoringAnother parameter that is helpful in monitoring ICP and optimising treatment of these patients is tissue oxygen pressure (PtiO2). This is a local measurement that provides information on tissue hypoxia in a specific brain region. Normal values of PtiO2 in the brain white matter range from 20 to 30mmHg. Hyperaemia is diagnosed when values are higher than 30mmHg. In contrast, values between 15 and 10mmHg are indicative of moderate tissue hypoxia and values below 10mmHg point to severe tissue hypoxia requiring an intervention.25 These values are only significant if they remain constant over time, typically for more than 30min. PtiO2 is beginning to replace jugular oxygen saturation as the parameter used to assess cerebral hypoxia. This is because jugular oxygen saturation, unlike PtiO2, is a global measurement that can be interpreted erroneously.
The PtiO2 probe should be placed on the cerebral white matter, either in the area of the penumbra (peri-lesional region with secondary damage that is potentially reversible if ischaemia resolves), or on the apparently normal parenchyma. Since this is a purely local measurement, we should choose the region of the brain to be monitored and then check the position of the device using head CT.
The information provided by the PtiO2 probe is reliable 30 to 40min after the probe is placed, but tissue temperature should also be ascertained, since an increase of 1°C elicits an increase in oxygen extraction of 8% to 12%.26
ConclusionsTreatment of ICH requires an in-depth knowledge of brain physiopathology and of ICP monitoring. ICH medication errors can produce undesirable iatrogenic effects. An adequate ICP recording is essential for diagnosing ICH and, more importantly, for starting an appropriate treatment before it is too late.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodríguez-Boto G, Rivero-Garvía M, Gutiérrez-González R, Márquez-Rivas J. Conceptos básicos sobre la fisiopatología cerebral y la monitorización de la presión intracraneal. Neurología. 2015;30:16–22.