In South American countries it is necessary to have molecular methods as epidemiological tools as well as low costs and good level of discrimination capacity for polymorphisms that may enable molecular laboratories to perform the tasks for other regional laboratories having minimum standards. The methodology proposed in this article addresses this need.
We used the Proportion Method2 for susceptibility testing of Mycobacterium tuberculosis isolates against isoniazid (INH), rifampicin (RMP), ethambutol (EMB), para-aminosalicylic acid (PAS), thioacetazone (T), kanamycin (KM) and streptomycin (SM). Cultures were grown on Lowenstein Jensen medium at 37°C for 21 days, and slides were processed with the Ziehl–Neelsen stain. DNA from samples was extracted by using the phenol chloroform method6. In a simple PCR for M. tuberculosis strain differentiation, primer Mtb2 (5′-CGGCGGCAACGGCGGCA) was used with primers IS1 (5′-CGGACTCACCGGGGCGGTTCA) and IS2 (5′-CGGACATGCCGGGGCGGTTCA) that anneal at the inverted repeats flanking IS61107. PCR was done in a mixture containing 25pmol of each primer, 1U of Platinum Taq DNA polymerase (Invitrogen), 0.2mM of each deoxyribonucleotide triphosphate, 10mM Tris–HCl (pH 8.4), 1.65mM MgCl2, 50mM KCl, and 0.1% Triton X-100 and overlaid with mineral oil. Cycling conditions were as follows: denaturation at 94°C for 5min, followed by amplification for 35 cycles of 94°C for 1min, 62°C for 1min, and 72°C for 1min, followed by a final extension at 72°C for 10min. A total of 20μl of amplified DNA was subjected to electrophoresis in a 2% agarose gel, detected by ethidium bromide staining, and visualized under UV light. For the genetic polymorphism study, we used the Bionumerics program version 5.0 (Applied-Maths).
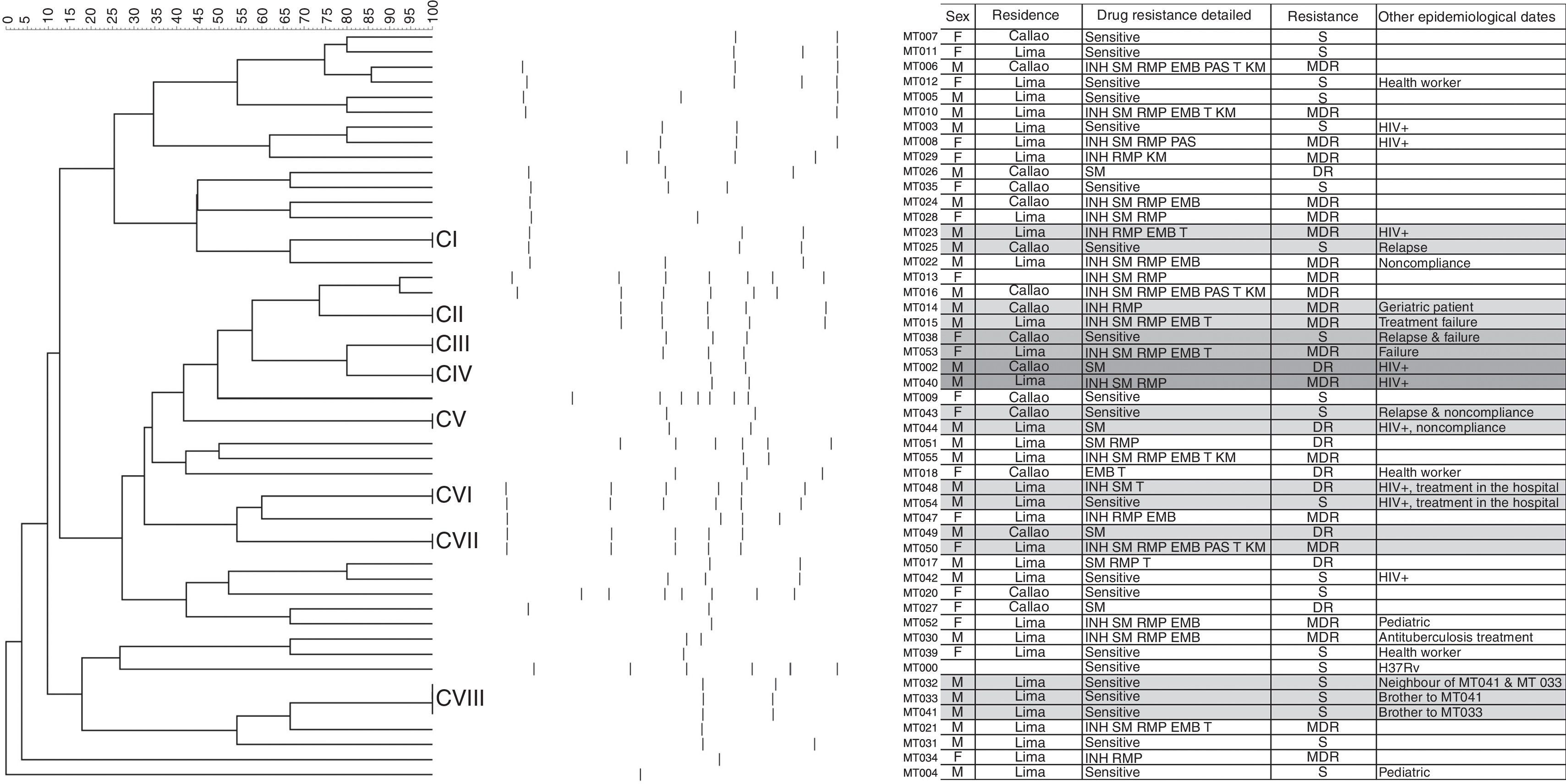
The drug resistance study showed that out of 49 strains belonging to TB patients in the Guillermo Almenara Irigoyen National Hospital, 18 strains (36.74%) were drug-sensitive, 10 (20.4%) were drug-resistant TB (DR) and 21 (42.8%) were multidrug-resistant TB (MDR), 3 of which were TB/HIV cases. Forty-two (42) different banding patterns were observed, which were classified into 10 clusters (Fig. 1). We suspect that cases MT009 and MT029 belong to heteroresistant strains, i.e. mixed wild-type and mutant strains because the banding pattern seems to overlap5.
The dendogram of fragment to Multiplex PCR of 50 strains of M. tuberculosis was constructed using the similarity coefficient of Dice and UPGMA, using a tolerance of 1.2% and optimization of 0.17%. The clusters CI, CII, CIII, CIV, CV, CVI, CVII y CVIII are indicate in the dendogram. The banding patterns obtained by PCR-based methodology are in the right of the dendogram. The attached table contains demographic and drug resistant date of the cases.
The transmission study showed Cluster II. Two male patients with MDR-TB, one of whom (MT014) had undergone previous MDR-TB treatment, were both hospitalized; Cluster IV. Two male patients with TB/HIV co-infection, both residing in the same district; Cluster VI. Two male patients with TB/HIV co-infection, both living in the same district and receiving their treatment in the same hospital, one of them (MT048) with DR TB and the other (MT054) with a sensitive case; Cluster VIII. Three male patients aged 44, 34 and 28 years, respectively, two of whom were brothers (MT033 and MT041) and the other a neighbor (MT032), all of them sensitive cases. The other clusters did not have an epidemiological link. A statistical risk study was performed4,13 and the result was that the patients with HIV infections had the highest contagion risk in our population (p=0.174; OR=3.150; CI=0.568–17.477). The repetition rate was good (Cronbach's alpha=0.82). This genotyping method could be an alternative for other PCR-based typing procedures, such as spoligotyping and MIRU-VNTR typing as cited in other studies7 and could help in the study of transmission relationship with heteroresistance, HIV-TB patients and outbreaks. Our TB survey system has many complications3,9,11 and the lack of surveillance in DOTS3 results in patients having a great diversity of genotypes and drug-resistant profiles1,5, as well as heteroresistance of wild type to resistant, resistant to resistant, and wild type to MDR strains1,4,8,10,12,13. Our country needs a strategy based on epidemiology with molecular tools that will assist us in the analysis of the genetic diversity existing in Peru.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingUniversidad Nacional Mayor de San Marcos: CON-CON 2006 – Con asignación a la Investigación y con incentivo al investigador.
The authors acknowledge to Dr. Pablo Ramirez Roca and Dra. Debora Alvarado Iparraguirre for the use of the Molecular Microbiology Laboratory (Universidad Nacional Mayor de San Marcos, Peru), Dr. Ernesto Montoro and Dr. Raul Diaz of Instituto Pedro Kouri (Cuba) for the advice and review of this investigation.