The aims of this study were to select microbial isolates from phyllosphere of maize and to examine their antagonistic activity against Exserohilum turcicum. Selection was performed through the ability of isolates to compete with the pathogen using an index of dominance and to affect growth parameters of E. turcicum. Most of the epiphytic populations obtained for the screening were bacteria. These isolates were found in the order of 6log CFU/g of leaf fresh weight. According to similar morphological characteristics and staining, 44 out of 111 isolates obtained were selected for testing antagonistic effects. At water potential, ψ, −1.38MPa and −4.19MPa, three Bacillus isolates showed dominance at a distance (5/0) and a significant reduction of growth rate of the pathogen. Three Bacillus isolates only decreased the growth rate of E. turcicum at −1.38MPa. At −4.19MPa the growth rate decreased with three isolates of Pantoea and three Bacillus. In this study a negative and significant correlation was observed between the growth rate of E. turcicum and the dominance index in the interaction of the pathogen with some bacteria. These results show that with decreasing growth rate of the pathogen the dominance index of the interaction increases. Eleven potential biocontrol agents against E. turcicum were selected.
El objetivo de este estudio fue seleccionar aislamientos microbianos de la filósfera de maíz y examinar su actividad antagonista contra Exserohilum turcicum. La selección se realizó a través de la capacidad de los aislamientos de competir con el patógeno usando un índice de dominancia y también la capacidad de afectar los parámetros de crecimiento de E. turcicum. La mayoría de las poblaciones epifíticas aisladas para la selección fueron bacterias. Estos aislamientos se encontraron en el orden de 6 log de UFC por gramo de peso fresco de hoja de maíz. En base a características morfológicas y tintóreas similares, se seleccionaron 44 de 111 aislamientos obtenidos para evaluar su capacidad antagónica. A los potenciales agua, ψ, −1,38MPa y −4,19MPa, tres aislados del género Bacillus mostraron dominancia a distancia (5/0) y una reducción significativa de la velocidad de crecimiento del patógeno. Tres aislamientos de Bacillus disminuyeron la velocidad de crecimiento de E. turcicum a −1,38MPa. A −4,19MPa la velocidad de crecimiento disminuyó con tres aislamientos de Pantoea y tres de Bacillus. En este estudio se observó una correlación negativa y significante entre la velocidad de crecimiento de E. turcicum y el índice de dominancia cuando el patógeno interactuó con algunas bacterias. Esto estaría indicando que cuando disminuye la velocidad de crecimiento del patógeno se incrementa el índice de dominancia de la interacción. Se seleccionaron once posibles agentes de biocontrol contra E. turcicum.
Maize (Zea mays L.) is one of the most important cereal crops in Argentina. The national average yield was 7270kg/ha during growing season 2012–20137. The increase in yield is conditioned by the improvement of several cultural practices. However, a negative factor is the emergence and re-emergence of some foliar diseases25,59. The common rust caused by Puccinia sorghi (Schwein) and the northern leaf blight caused by Exserohilum turcicum (Pass.) Leonard and Suggs (Syn. Helminthosporium turcicum Pass.) are two of the diseases that most affect the crop, causing a loss of yield8,22. Severe attacks of foliar diseases cause a reduction in the index of green leaf area, number of days with healthy leaf area and radiation interception. Therefore, because the photoassimilates are insufficient to grain filling, the plant begins remobilization of existing reserves in the stem immediately. Mobilization of nutrients leads to weakening of stems. This causes stalk breakage or lodging, favoring the increased occurrence of fungal diseases that cause stalk and root rot25. In Argentina, foliar diseases can cause loss up to 40% when these are endemic in the maize core area and occur each year with different levels of severity9. De Rossi et al.15 determined that severity values of 60% caused losses close to 40% in yield of susceptible hybrids in Córdoba province, Argentina. The leaf blight becomes important in maize sown in late December and January, after harvest of wheat. The residues on the soil surface, frequent artificial irrigation, and intense rainfall during the summer months and moderate temperatures favor the development of the disease16,21,24.
The expansion of emerging and reemerging diseases requires the prevention, control and eradication as technological tools necessary for the development of maize crop potential and the achievement of high yields62. The most widely used technique to control northern foliar blight is the selection of hybrids that show a better performance. Another alternative is based on cultural practices, avoiding monoculture. It is essential not to sow maize after maize or maize after sorghum, and to perform rotations with other species for one or two years26. Chemical control is the most used technique. The chemical fungicides used are mixtures of strobilurins and triazoles e.g. (NATIVO, Bayer), pyraclostrobin+epoxiconazole (OPERA, Basf), azoxystrobin+cyproconazole (AMISTAR XTRA, Syngenta) and picoxystrobin+cyproconazole (STINGER, DuPont). In general, these fungicides can reduce the severity and the epidemic rate of disease, showing good yields11. The application of these fungicides is performed at critical moments of the disease, depending on the hybrid of maize, climatic conditions and incidence of inoculum in the crop9,25. These chemicals are moderately hazardous Class II and to be effective must constantly protect new leaves, which is extremely expensive5.
Therefore new strategies must be developed to give up the chemical paradigm. Biological control is presented as an alternative aimed to minimize yield losses caused by foliar diseases. This control strategy has the advantage of avoiding the accumulation of xenobiotics in the biosphere, avoiding the application of harmful products for those who manipulate them and reducing the costs of product applications. The use of microorganisms that antagonize foliar pathogens is risk-free when these organisms come from the same ecosystem. The inhabitants of the phyllosphere are termed epiphytes and may consist of a variety of bacteria, yeasts or filamentous fungi40. Microorganisms within the phyllosphere can include those that are pathogenic to the plant, but can also include non-pathogenic organisms that prevent the colonization of leaf by pathogens38,40. Diverse bacteria and yeast were tested as potential antagonists of different foliar diseases in crops27,38,58,64,67. Moreover, the success of biological control of foliar diseases is difficult because microbes of phyllosphere are located in a fluctuating environment. In addition, with global climate change phyllospheric microbes are also exposed to additional changes in the physical environment54. To achieve the selection of a potential biocontrol agent it is important to consider the relationship between biological interactions and environmental stress factors49. It is also important to use criteria to determine the result of several interactions. The index of dominance compares the competitiveness of microbial species to dominate under a particular set of environmental conditions. Mostly, water availability, temperature and substrate have been reported influencing several interactions39. Numerous changes in environmental factors cause an impact that can be decisive in determining the co-existence level or dominance of species in a particular ecological niche43,45. Mainly, it is important to show that any potential biocontrol agent has the ability to decrease the growth of the pathogen.
Our study was carried out to obtain information on the potential of possible antagonists of E. turcicum and was aimed to pursue the following objectives: (a) evaluate bacteria from phyllosphere of maize; (b) determine the sensitivity of E. turcicum to osmotic stress; (c) evaluate the ability of bacterial antagonistic isolates to compete with E. turcicum using an index of dominance and antibiosis, under different water availabilities, and (d) determine the effect of bacteria on growth parameters of E. turcicum.
Materials and methodsCollection of samples and microbial isolation from phyllosphereThere have been a large number of studies that report the existence of microbial competition on leaves1,23,68 between pathogens and possible antagonists37. Therefore, the isolation of microorganisms that live in the same ecosystem with the pathogen, allows the selection of potential antagonists. On this basis, the selection of bacteria was performed on leaves of maize with blight lesions from fields of three cultivars in Chucul, Río Cuarto and Vicuña Mackenna, all in Córdoba province, Argentina. Each sample contained fifteen plants and two leaves per plant were chosen for the assays. Leaves fully developed, but not senescent, were picked from the field and transferred to the laboratory. Samples were stored at 4°C before processing.
To isolate epiphytic microorganisms the samples were subjected to three different techniques. For the first and second techniques, suspensions were prepared as follows. From each plant, ten discs of 1cm from each leaf were cut with a sterile cork borer. The discs were transferred into tubes containing 10ml of phosphate buffered saline (PBS: 0.1M phosphate buffer containing 0.1% Bacto Peptone, pH 7). In the first technique the suspension was vortexed for 2min. In the second technique another PBS suspension was sonicated at a frequency of 40KHz for 4min in an ultrasonic cleaning bath (TESTLAB – TB10TA, Argentina) to displace microorganisms4,10,47,68. The third technique consisted of a surface disinfection of the leaf discs in order to reduce inoculum of opportunistic and epiphytic pathogens, which could interfere with the isolation of potential antagonists. Ogliari et al.51 methodology was followed with some modifications. Briefly, disks of infected tissues were placed in a solution of sodium hypochlorite at 2% for 3min and then rinsed several times with sterile distilled water.
Serial dilutions to 10−4 were performed in PBS from all the obtained suspensions. Aliquots of 100μl suspensions were plated on malt extract agar (MEA: malt extract 20g, peptone 1g, glucose 20g, agar 15g, distilled water 1000ml, pH 5) and trypticase soy agar (TSA: tryptone 17g, soytone 3g, dextrose 2.5g, NaCl 5.0g, K2HPO4 2.5g, agar 15g, distilled water 1000ml, pH 7.3±0.2) (TSA, Britania, Argentina). Plates were incubated at 25°C. Populations observed after 24–48h were expressed as log CFU per gram of leaf fresh weight.
Colonies were grouped and listed according to their morphology, appearance and bacterial Gram stain. Some of the bacteria that showed consistent antifungal activity were selected for further identification according to Bergey's Manual of Systematic Bacteriology31. API Test kit was used to identify Gram-negative bacteria of the Enterobacteriaceae family and other Gram-negative bacilli (API®20 E, bioMèrieux, Argentina).
Fungal isolateThe E. turcicum fungal strain used was previously isolated from maize (DK 190) growing on Campus Santa Julia of Universidad Nacional de Córdoba (UNC), in Córdoba province, Argentina. The isolate was maintained at 4°C on slants of potato dextrose agar medium (PDA: dextrose 20g, potato extract 4g, agar 15g, distilled water 1000ml, pH 5.6±0.2) and in 15% glycerol at −80°C.
E. turcicum sensitivity to osmotic stress: media, water potential modification and inoculationTwo media were used: potato dextrose agar medium (PDA) and maize leaves agar medium (MLA). MLA medium was made by boiling 30g fresh maize leaves in 1 l water for 60min and filtering the suspension through a double layer of muslin. The volume was made up to 1 l with distilled water. This medium was specifically chosen because E. turcicum was isolated from fresh maize leaves. All experiments were carried out over a water potential range ψ of −1.38 to −12.9MPa. The ψ of the unmodified medium was −1.38MPa, and this was selected as the control treatment. The water potential of PDA medium was adjusted to −2.78, −4.19, −5.62, −7.06, −8.52, −9.99, −11.5 and −12.9MPa [=0.98, 0.97, 0.96, 0.95, 0.94, 0.93, 0.92 and 0.91 water activity (aw), respectively] by the addition of known amounts of the non-ionic solute glycerol12. According to growth obtained in PDA medium, ψ of −4.19MPa and −8.52MPa were chosen to modify MLA medium. The aw of the media was determined using an equipment AquaLab (Series 4, TE, USA). The media were autoclaved at 121°C for 20min before cooling to 50°C and pouring into 9cm sterile plastic Petri plates.
Petri plates containing the different media were inoculated aseptically with E. turcicum by transferring 4mm diameter agar plugs of 10-day old culture of the pathogen to the center of PDA and MLA media. Petri plates of the same ψ values were sealed in polyethylene bags. The inoculated plates were incubated at 25°C for 20 days or until the colony covered the plate. The colony radius was measured daily. For each colony, two radii, measured at right angles to one another, were averaged to find the mean radius for that colony. All colony radii were determined by using three replicates for each treatment. The radial rate (mm/d) was then calculated by linear regression of the linear phase for growth, and the time at which the line intercepted the x-axis was used to calculate the lag phase.
Index of dominance (ID)Petri plates containing MLA modified with glycerol to −1.38MPa, −4.19MPa and −8.52MPa12 were used. A streak of each epiphytic microorganism suspension grown for 24h in trypticase soy broth (TSB) was inoculated in the middle of each Petri plate. The Petri plates were inoculated with two agar plugs of the pathogen E. turcicum at two points equidistant from the center and edge of the plate. Treatments were incubated in polyethylene bags for 15 days at 25°C49. The ID was developed to measure the ability of a species to dominate under a particular set of environmental conditions43. The type of interaction was determined macroscopically. Controls of fungal pathogen and antagonistic bacteria were inoculated in separate plates. The diameter of the fungal colony and the width of the streak of the bacterial colony were measured in controls and compared with the interactions. The methodology used by Magan and Lacey43 to assign scores to obtain ID was adapted for interactions between fungus and bacteria49. The scores were based on mutual intermingling (1/1), mutual inhibition on contact (2/2), mutual inhibition at a distance (3/3), dominance of one species on contact (4/0) and dominance at a distance (5/0)43. This assessment was carried out with at least three separate replicates per treatment.
Antifungal effect of epiphytic microorganisms on E. turcicum growth parametersThe MLA medium at −1.38MPa, −4.19MPa and −8.52MPa was prepared following the procedure mentioned above. Before cooling, MLA medium was inoculated with 100μl of 109CFU/ml suspension of each epiphytic microorganism and poured into Petri plates. An agar plug of E. turcicum was inoculated in the center of the plate. Cultures were incubated at 25°C for 20 days in polyethylene bags28,49. The experiments were carried out three times for single and paired cultures. The inhibitory activity on lag phase and growth rate of screened epiphytic microorganisms against E. turcicum were evaluated as described previously.
Statistical analysisThe analysis of variance (ANOVA)19 was used to compare counts of epiphytic microorganisms in different sampling sites, differences between sample processing techniques and differences in growth rate. Means were compared with DGC test (p<0.05) 20. The Pearson correlation coefficient was used to evaluate correlations between growth rate of E. turcicum at different water potentials and dominance index. A significant level of p<0.0001 was used.
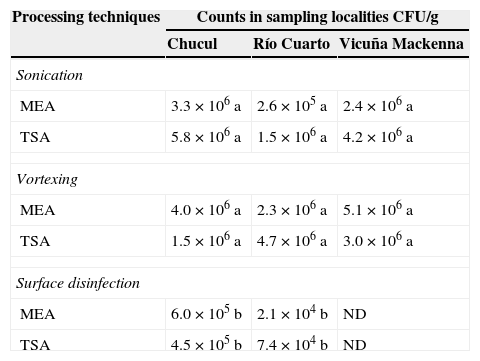
ResultsIsolates of epiphytic microorganismsMost of the counts were in the order of 6 log of CFU/g (Table 1), and no significant differences were observed between sampling localities, although there were differences between processing methods. The counts obtained by performing surface disinfection of the samples were significantly lower (p<0.001). A total of 111 epiphytic isolates were obtained. Grouping of total isolates showed the following composition: 46.8% Gram positive, 52.3% Gram-negative and 0.9% yeast (data not shown). According to Gram stain and morphology 9.9% were Gram-positive rods, 11.7% Gram-positive irregular rods, 15.3% Gram-positive spore-forming rods, 9.9% Gram-positive cocci, 20.7% Gram-negative rods and 31.5% Gram-negative irregular rods. According to similar morphological characteristics and staining 44 isolates were selected for antagonistic testing.
Total count on maize leaves of epiphytic microorganisms according to sampling localities and processing techniques.
| Processing techniques | Counts in sampling localities CFU/g | ||
|---|---|---|---|
| Chucul | Río Cuarto | Vicuña Mackenna | |
| Sonication | |||
| MEA | 3.3×106 a | 2.6×105 a | 2.4×106 a |
| TSA | 5.8×106 a | 1.5×106 a | 4.2×106 a |
| Vortexing | |||
| MEA | 4.0×106 a | 2.3×106 a | 5.1×106 a |
| TSA | 1.5×106 a | 4.7×106 a | 3.0×106 a |
| Surface disinfection | |||
| MEA | 6.0×105 b | 2.1×104 b | ND |
| TSA | 4.5×105 b | 7.4×104 b | ND |
ND: not determined; MEA: malt extract agar medium; TSA: trypticase soy agar medium.
Different letters indicate significant differences between processing techniques for each sampling locality, according to the DGC test (p<0.001).
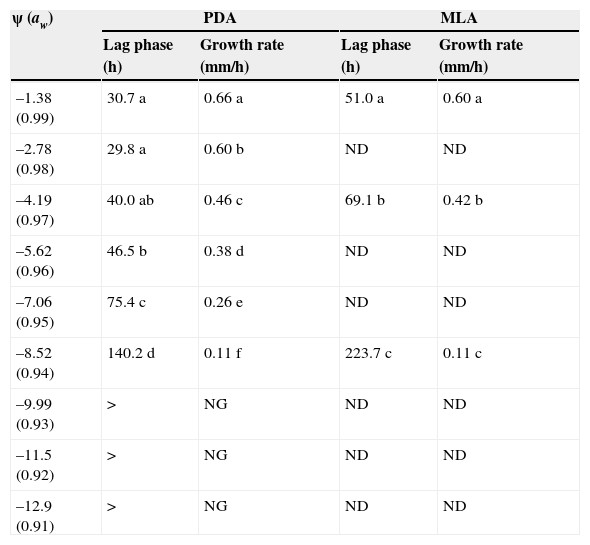
The effect of osmotic stress in PDA and MLA media on lag phase and growth rate of E. turcicum is shown in Table 2. Water potential showed a significant effect (p<0.001) on the lag phase and growth rate of E. turcicum in both culture media. When water potential decreased, lag phase increased and growth rate decreased in both media. Lag phase showed significant differences between the culture media PDA and MLA (p<0.001). The lag phases were higher in MLA medium than in PDA medium at −1.38MPa, −4.19MPa and −8.52MPa. However, in PDA medium lag phase was not significantly different at −1.38MPa, −2.78MPa and −4.19MPa (p<0.05). No growth was observed in PDA medium at −9.99MPa, −11.5MPa and −12.9MPa. Growth rate of E. turcicum was higher at −1.38MPa. The values observed were 0.66 and 0.60mm/h in PDA and MLA media, respectively. The growth rate decreased in both media at −4.19MPa and −8.52MPa. According to these results, ψ of −1.38MPa, −4.19MPa and −8.52MPa were selected for the following assays.
Osmotic stress sensitivity of E. turcicum in PDA and MLA media.
| ψ (aw) | PDA | MLA | ||
|---|---|---|---|---|
| Lag phase (h) | Growth rate (mm/h) | Lag phase (h) | Growth rate (mm/h) | |
| –1.38 (0.99) | 30.7 a | 0.66 a | 51.0 a | 0.60 a |
| –2.78 (0.98) | 29.8 a | 0.60 b | ND | ND |
| –4.19 (0.97) | 40.0 ab | 0.46 c | 69.1 b | 0.42 b |
| –5.62 (0.96) | 46.5 b | 0.38 d | ND | ND |
| –7.06 (0.95) | 75.4 c | 0.26 e | ND | ND |
| –8.52 (0.94) | 140.2 d | 0.11 f | 223.7 c | 0.11 c |
| –9.99 (0.93) | > | NG | ND | ND |
| –11.5 (0.92) | > | NG | ND | ND |
| –12.9 (0.91) | > | NG | ND | ND |
NG: no growth; ND: not determined; PDA: potato dextrose agar medium; MLA: maize leaf agar medium.
>:480h.
Different letters indicate significant differences between different ψ for growth rate and lag phase in each media, according to the DGC test (p<0.05).
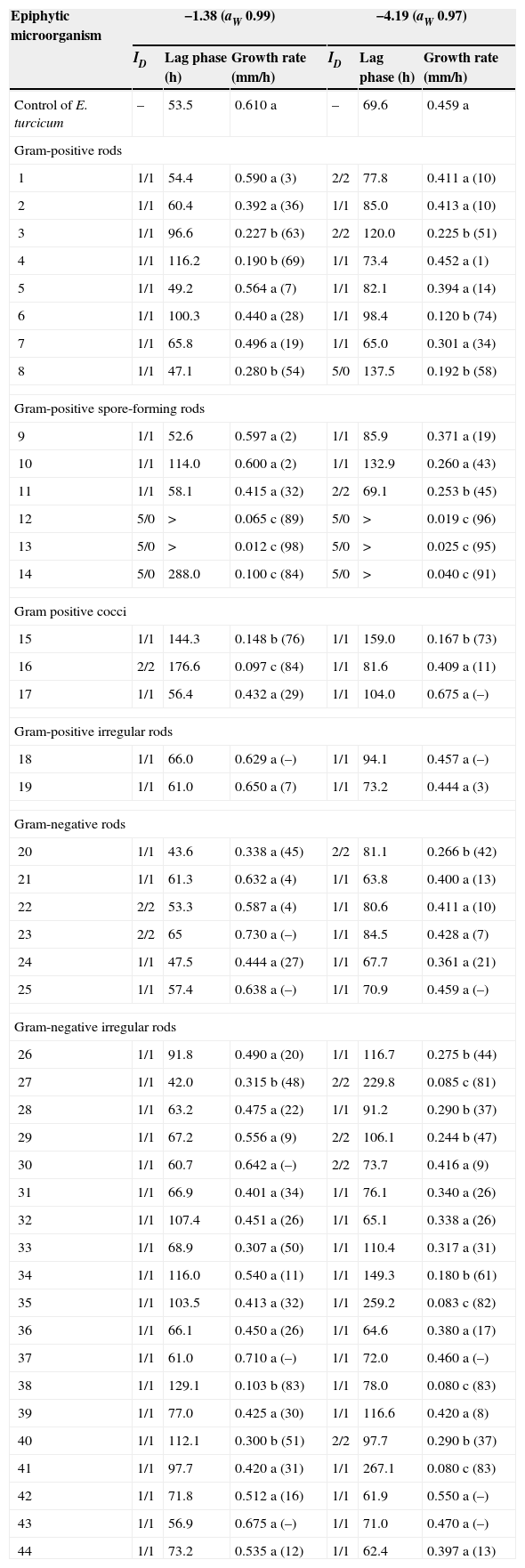
Table 3 shows the effect of biological interaction among the pathogen E. turcicum and 44 epiphytic microorganisms selected. The predominant interaction between bacterial antagonists and fungus in dual culture was mutual intermingling (ID=1/1). At −1.38MPa three isolates (16, 22 and 23) showed a mutual inhibition on contact (2/2). Other three isolates (12, 13 and 14) showed dominance at a distance (5/0). The isolates 12, 13 and 14 showed a significant reduction of the pathogen growth rate compared with the control. These isolates caused the same effect on the pathogen at −4.19MPa. At this ψ also the isolate 8 showed an interaction 5/0 and other eight isolates showed an interaction 2/2. Most of the isolates that showed spatial dominance also showed significant increase in the lag phase and reduction of the growth rate. The isolates 15, 34, 35 and 38 showed mutual intermingling at −4.19MPa and −1.38MPa; however these isolates reduced growth rate of E. turcicum. At −8.52MPa none of the epiphytic microorganisms were able to grow, so there was no interaction or effect on growth of the pathogen (data not shown).
Index of dominance (ID) and interactions on growth parameters between epiphytic microorganisms and E. turcicum in maize leaf agar at different ψ.
| Epiphytic microorganism | −1.38 (aW 0.99) | −4.19 (aW 0.97) | ||||
|---|---|---|---|---|---|---|
| ID | Lag phase (h) | Growth rate (mm/h) | ID | Lag phase (h) | Growth rate (mm/h) | |
| Control of E. turcicum | – | 53.5 | 0.610 a | – | 69.6 | 0.459 a |
| Gram-positive rods | ||||||
| 1 | 1/1 | 54.4 | 0.590 a (3) | 2/2 | 77.8 | 0.411 a (10) |
| 2 | 1/1 | 60.4 | 0.392 a (36) | 1/1 | 85.0 | 0.413 a (10) |
| 3 | 1/1 | 96.6 | 0.227 b (63) | 2/2 | 120.0 | 0.225 b (51) |
| 4 | 1/1 | 116.2 | 0.190 b (69) | 1/1 | 73.4 | 0.452 a (1) |
| 5 | 1/1 | 49.2 | 0.564 a (7) | 1/1 | 82.1 | 0.394 a (14) |
| 6 | 1/1 | 100.3 | 0.440 a (28) | 1/1 | 98.4 | 0.120 b (74) |
| 7 | 1/1 | 65.8 | 0.496 a (19) | 1/1 | 65.0 | 0.301 a (34) |
| 8 | 1/1 | 47.1 | 0.280 b (54) | 5/0 | 137.5 | 0.192 b (58) |
| Gram-positive spore-forming rods | ||||||
| 9 | 1/1 | 52.6 | 0.597 a (2) | 1/1 | 85.9 | 0.371 a (19) |
| 10 | 1/1 | 114.0 | 0.600 a (2) | 1/1 | 132.9 | 0.260 a (43) |
| 11 | 1/1 | 58.1 | 0.415 a (32) | 2/2 | 69.1 | 0.253 b (45) |
| 12 | 5/0 | > | 0.065 c (89) | 5/0 | > | 0.019 c (96) |
| 13 | 5/0 | > | 0.012 c (98) | 5/0 | > | 0.025 c (95) |
| 14 | 5/0 | 288.0 | 0.100 c (84) | 5/0 | > | 0.040 c (91) |
| Gram positive cocci | ||||||
| 15 | 1/1 | 144.3 | 0.148 b (76) | 1/1 | 159.0 | 0.167 b (73) |
| 16 | 2/2 | 176.6 | 0.097 c (84) | 1/1 | 81.6 | 0.409 a (11) |
| 17 | 1/1 | 56.4 | 0.432 a (29) | 1/1 | 104.0 | 0.675 a (–) |
| Gram-positive irregular rods | ||||||
| 18 | 1/1 | 66.0 | 0.629 a (–) | 1/1 | 94.1 | 0.457 a (–) |
| 19 | 1/1 | 61.0 | 0.650 a (7) | 1/1 | 73.2 | 0.444 a (3) |
| Gram-negative rods | ||||||
| 20 | 1/1 | 43.6 | 0.338 a (45) | 2/2 | 81.1 | 0.266 b (42) |
| 21 | 1/1 | 61.3 | 0.632 a (4) | 1/1 | 63.8 | 0.400 a (13) |
| 22 | 2/2 | 53.3 | 0.587 a (4) | 1/1 | 80.6 | 0.411 a (10) |
| 23 | 2/2 | 65 | 0.730 a (–) | 1/1 | 84.5 | 0.428 a (7) |
| 24 | 1/1 | 47.5 | 0.444 a (27) | 1/1 | 67.7 | 0.361 a (21) |
| 25 | 1/1 | 57.4 | 0.638 a (–) | 1/1 | 70.9 | 0.459 a (–) |
| Gram-negative irregular rods | ||||||
| 26 | 1/1 | 91.8 | 0.490 a (20) | 1/1 | 116.7 | 0.275 b (44) |
| 27 | 1/1 | 42.0 | 0.315 b (48) | 2/2 | 229.8 | 0.085 c (81) |
| 28 | 1/1 | 63.2 | 0.475 a (22) | 1/1 | 91.2 | 0.290 b (37) |
| 29 | 1/1 | 67.2 | 0.556 a (9) | 2/2 | 106.1 | 0.244 b (47) |
| 30 | 1/1 | 60.7 | 0.642 a (–) | 2/2 | 73.7 | 0.416 a (9) |
| 31 | 1/1 | 66.9 | 0.401 a (34) | 1/1 | 76.1 | 0.340 a (26) |
| 32 | 1/1 | 107.4 | 0.451 a (26) | 1/1 | 65.1 | 0.338 a (26) |
| 33 | 1/1 | 68.9 | 0.307 a (50) | 1/1 | 110.4 | 0.317 a (31) |
| 34 | 1/1 | 116.0 | 0.540 a (11) | 1/1 | 149.3 | 0.180 b (61) |
| 35 | 1/1 | 103.5 | 0.413 a (32) | 1/1 | 259.2 | 0.083 c (82) |
| 36 | 1/1 | 66.1 | 0.450 a (26) | 1/1 | 64.6 | 0.380 a (17) |
| 37 | 1/1 | 61.0 | 0.710 a (–) | 1/1 | 72.0 | 0.460 a (–) |
| 38 | 1/1 | 129.1 | 0.103 b (83) | 1/1 | 78.0 | 0.080 c (83) |
| 39 | 1/1 | 77.0 | 0.425 a (30) | 1/1 | 116.6 | 0.420 a (8) |
| 40 | 1/1 | 112.1 | 0.300 b (51) | 2/2 | 97.7 | 0.290 b (37) |
| 41 | 1/1 | 97.7 | 0.420 a (31) | 1/1 | 267.1 | 0.080 c (83) |
| 42 | 1/1 | 71.8 | 0.512 a (16) | 1/1 | 61.9 | 0.550 a (–) |
| 43 | 1/1 | 56.9 | 0.675 a (–) | 1/1 | 71.0 | 0.470 a (–) |
| 44 | 1/1 | 73.2 | 0.535 a (12) | 1/1 | 62.4 | 0.397 a (13) |
(): percentage of growth rate inhibition.
>:480h.
Different letters indicate significant differences for the same ψ on growth rate of E. turcicum interacting with each epiphytic microorganism isolate, according to the DGC test (p<0.05). Index of Dominance ID: 1/1 mutual intermingling, 2/2 mutual inhibition on contact, 3/3 mutual inhibition at a distance, 4/0 dominance of one species on contact and 5/0 dominance at a distance.
Epiphytic microorganisms had significant effects on increasing the lag phase. There was a significant increase in the lag phase in treatments where there were spatial interactions or dominance of epiphytic microorganisms on E. turcicum. Twenty-seven percent and 43% of the bacterial isolates had significant inhibitory effects on the mycelial growth of E. turcicum at ψ −1.38 and −4.19, respectively. However, none of the bacterial isolates inhibited the growth of the pathogen completely. Growth rate of E. turcicum was reduced in significant percentages (p<0.001) with the isolates 16 (84%), 12 (89%), 13 (98%) and 14 (84%) at −1.38MPa. And at −4.19MPa the growth rate decreased with the isolates 27 (81%), 35 (82%), 38 (83%), 12 (96%), 41 (83%), 13 (95%) and 14 (91%).
Identification of epiphytic microorganismsEleven isolates demonstrating significant reducing effect on growth rate or dominance on E. turcicum were identified at the genera level. Isolates 27, 34, 35, 38, and 40 showed characteristics of the genus Pantoea of the family Enterobacteriaceae. Three isolates, 12, 13 and 14 were identified as Bacillus, and isolates 3 and 8 were compatible with Corynebacterium features. Finally, isolate 15 was identified as Enterococcus.
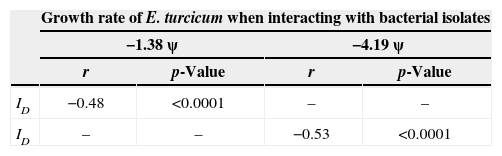
Correlation between biological interactions of E. turcicum and epiphytic microorganismsTable 4 shows the Pearson correlation coefficients’ values obtained. Negative and significant correlations were observed between the effect of epiphytic microorganisms on growth rate of E. turcicum and index of dominance in the biological interaction at −1.38MPa and −4.19MPa.
Pearson (r) correlation coefficients values between growth rate and index of dominance (ID) of E. turcicum and selected bacterial isolates interacting in maize leaf agar at two different ψ.
| Growth rate of E. turcicum when interacting with bacterial isolates | ||||
|---|---|---|---|---|
| −1.38ψ | −4.19ψ | |||
| r | p-Value | r | p-Value | |
| ID | −0.48 | <0.0001 | – | – |
| ID | – | – | −0.53 | <0.0001 |
p<0.0001 indicates a significant relationship between the two variables.
This study presents the results of the selection steps of possible biological control agents of E. turcicum, by taking into consideration ecological parameters relevant to the agroecosystem. In our study we isolated possible antagonistic epiphytic microorganisms from leaves of maize with blight lesions. Most epiphytic population consisted of bacteria which were found in the order of 6 log CFU per gram of maize leaf, similar to results obtained in other studies30,35,70. Therefore, the interaction of phyllosphere microorganisms can play an important role for plant health and protection3. Previous studies performed on peanut phyllosphere showed that most of the identified strains were Gram-positive63, with Bacillus that accounted 39% of the total33. In our study Gram-positive rods represent 36.9% of the isolates. On the other hand most of the isolates were grouped in Gram-negative bacteria. The microbial ecology of the phyllosphere has been viewed mainly through the biology of Gram-negative bacteria like plant-pathogenic microorganisms40. However, some Gram-negative rods were considered antagonistic bacteria of different phyllosphere diseases42,69. Antagonistic effect was observed with Bacillus subtilis and Pseudomonas fluorescens against E. turcicum in dual culture in vitro29. Bacteria, especially the pseudomonads and bacilli have been shown to play a key role in the suppression of plant pathogens in different cropping systems57. Other studies showed in vitro antagonism of filamentous fungi like Trichoderma harzianum and T. viride against E. turcicum29,56. Phyllosphere microbes live in a physical environment of continuous fluctuation17,30. Consequently, the leaf surface is considered to be a hostile location for microbial colonization41. Fluctuating water availability, incident irradiation and low nutrient availability on leaves are likely selective pressures that influence the composition of epiphytic bacteria6,41. Bacteria selected as potential biological control agents must be able to tolerate continuous microclimatic changes. Also these bacteria must demonstrate good growth under similar conditions, such as water potential and temperature. In this study E. turcicum was able to grow at a range of water potential of −1.38MPa to −8.52MPa in culture media, synthetic potato dextrose agar and maize leaves agar. However, the antagonistic bacteria selected were able to grow at −1.38MPa and −4.19MPa. From the selected bacterial isolates, Bacillus is known to be more tolerant to environmental changes due to the presence of stress resistant endospores60. Gram-positive bacteria are generally resistant to drought stress61. A study conducted by Jacobs and Sundin33 revealed that Bacillus coagulans showed a phenotype of tolerance for solar UV radiation in peanut leaves. In our study, three Bacillus isolates showed a dominance of the pathogen at a distance, and a reduction of E. turcicum growth. Possibly, these isolates have the ability to synthesize a diffusible substance with inhibitory capacity. Kishore et al.38 showed that Bacillus circulans GRS 243 was considered a chitinolytic bacterium and inhibited the germination of conidia of Cercospora arachidicola, Phaeoisariopsis personata and urediniospores of Puccinia arachidis. US EPA65 reported that Bacillus subtilis strain QST 713 controlled the growth of certain pathogenic fungi, presumably by competition for nutrients, growth sites in plants and direct colonization and adhesion to fungi.
Taking into account the antagonistic effects on plant pathogens of Gram-negative bacteria, Howell et al.32 reported that volatile compounds such as ammonia produced by Enterobacter cloacae were involved in the suppression of cotton seedling damping-off caused by Pythium ultimum. In our study, five isolates selected and identified as Pantoea showed high percentage of growth inhibition of the pathogen or mutual inhibition on contact. However, in most of the interactions mutual intermingling was observed. This interaction suggests that competition for space and nutrients did not occur between these isolates and E. turcicum under the conditions evaluated. For instance, the isolate 38 did not show dominance over the pathogen (ID=1/1) but produced 83% of growth inhibition. Therefore synthesis of diffusible inhibitory substances and competition for nutrients and space are not the mechanisms used by this bacterium to inhibit the growth of the pathogen and possibly competitive exclusion is the mechanism used by this antagonist. The most abundant non-pathogenic microorganisms associated with plants are thought to protect the plant by rapid colonization36. The pear and apple disease caused by the bacterium Erwinia amylovora was controlled using Pantoea agglomerans strain whose mechanism of action is not the synthesis of antibiotics34,55. In this study a negative and significant correlation was observed between the growth rate of E. turcicum and dominance index when the pathogen interacts with some bacteria (3, 8, 12, 13, 14, 16, 27 and 40). This means that with decreasing growth rate of the pathogen the dominance index of the interaction increases.
On the other hand, it is known that Pantoea stewartii subsp. stewartii is the causal agent of Stewart's wilt of sweet maize. This phytopathogen is a yellow-pigmented and Gram-negative bacterium50. Symptoms of bacterial leaf blight of maize caused by P. stewartii in maize fields of central Argentina were observed2. Many microorganisms are known to produce pigments44,48, like members of Pantoea genus. Since solar radiation influences the ecology of the phyllosphere, pigmented bacteria dominate the leaf surfaces33,63. In the present study, other than Bacillus, eight of the eleven isolates selected were pigmented.
Several investigations have demonstrated the antagonistic role of the Corynebacterium genus13,14. C. nebraskense was isolated from maize field and was described as pathogenic species66. On the other hand, others species of Corynebacterium were used to control different plant fungal diseases like Pythium damping-off and some fungi that cause root rot18,53.
Finally, one of the isolates that showed high inhibition percentage of E. turcicum was Enterococcus. Mata et al.46 described different Enterococcus strains with antagonist bacterial effects against phytopathogenic species Clavibacter michiganensis, Erwinia carotovora and Xanthomonas axonopodis in vitro. Although, Enterococcus species have shown a wide distribution in the phyllosphere of plants, many investigations are conducted to evaluate the possible pre-harvest contamination of plants with human pathogens52. Further studies on the detrimental effects of the potential antagonists of E. turcicum need to be conducted.
At present, studies are carried out to evaluate the eleven potential biocontrol agents obtained against E. turcicum in greenhouse conditions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This work was supported by a grant from the Agencia Nacional de Promoción Científica y Tecnológica de la República Argentina, PICT 2268/12. M. Sartori was provided with a post-doctoral fellowship, A. Nesci and M. Etcheverry are members of Carrera del Investigador Científico from CONICET.