Epidural analgesia for controlling pain in labour has been the gold standard over the past 2 decades as it is considered the least harmful technique for the newborn. In reality, however, it is not without risk. That said, there are few options for pain management in labour when epidural analgesia is contraindicated. A recent survey to investigate the use of alternatives showed remifentanil to be the first choice when using systemic analgesia intravenously, as short-acting opioids administered systemically relieve pain adequately without the need for epidural analgesia.
Another safe option for providing obstetric analgesia is dexmedetomidine, a selective alpha-2 agonist that improves the quality of analgesia and reduces opioid requirements. Dexmedetomidine promotes stability and maintains uterine/placental homeostasis.
La analgesia epidural ha sido el estándar de oro en las últimas 2 décadas para controlar el dolor en labor ya que es considerada la técnica menos nociva para el recién nacido, pero en realidad no está exenta de riesgos. Sin embargo hay pocas opciones para manejar el dolor en labor cuando la analgesia epidural está contraindicada. Una reciente encuesta para investigar el uso de alternativas demostró que se sugiere al remifentanil como la primera opción cuando se utiliza analgesia sistémica por vía intravenosa, ya que los opioides de corta acción administrados sistémicamente alivian el dolor adecuadamente sin usar analgesia epidural.
Otra opción segura para brindar analgesia obstétrica es la dexmedetomidina; un alfa 2 agonista selectivo que mejora la calidad de la analgesia y disminuye el requerimiento de opioides. La dexmedetomidina promueve la estabilidad y mantiene la homeostasis útero placentaria.
It is well known that the gold standard for obstetric analgesia is neuraxial block (epidural or subarachnoid block). However, when this is contraindicated, labour should not be left to progress without adequate pain relief.
Labour is considered to be one of the most painful experiences in the life of a woman. Different studies have assessed the perception of pain during this experience, with about 20% of women reporting “unbearable pain” and another 60% describing it as “severe pain”.1
This makes pain management and comfort for the mother and neonate one of the most challenging tasks for the anaesthetist. Various techniques have been used, from “natural” techniques, for example water immersion and acupuncture, to pharmacological, such as regional analgesia, considered the gold standard (either spinal or epidural), and systemic analgesia with opioids.2–4 The aim of this study was to review the available literature on the use and the effects on the mother and child of two drugs that reduce pain in labour, remifentanil and dexmedetomidine.
RemifentanilAlthough opioid anaesthetics are usually avoided in the induction of general anaesthesia in obstetrics as they cross the placental barrier and can cause respiratory depression in the neonate, in view of their properties, they have often been resorted to when the mother has a coexisting condition that can increase blood pressure and heart rate, as in the case of cardiac, neurological or liver disease, and disorders related to the pregnancy itself, such as preeclampsia.5 In fact, these drugs, mainly fentanyl, have been commonly used in regional epidural analgesia during the first stage of labour.2,6
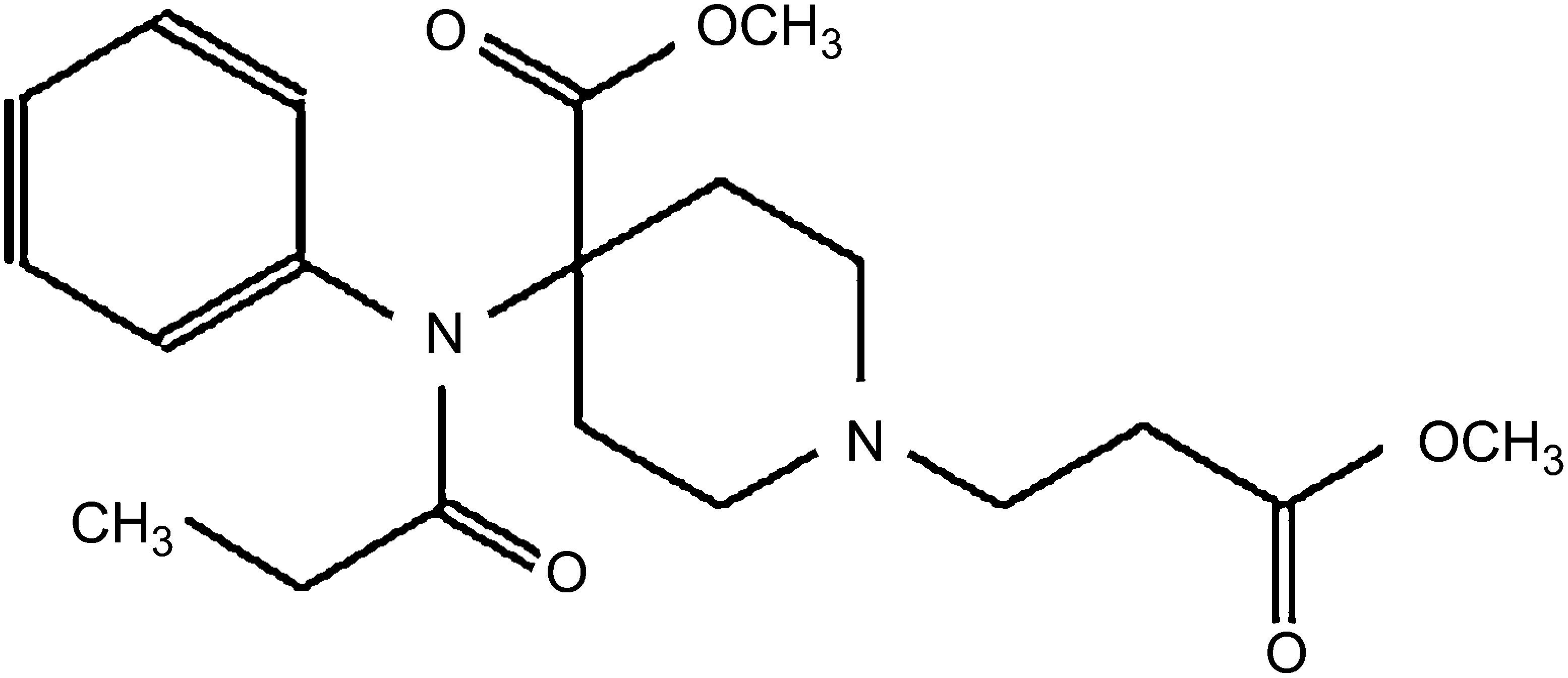
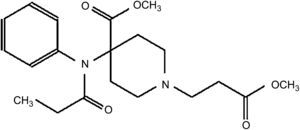
Remifentanil, a 4-anilidopiperidine derivative of fentanyl, is a potent, ultra-short-acting, synthetic μ-opioid receptor agonist. It contains a propionic acid ester linkage, so it is easily inactivated by non-specific plasma and tissue esterases.4,7 The chemical structure is shown in Fig. 1.
Onset of action is 90s and it has a half-life of 3min. In terms of metabolism, 16–18% of remifentanil is metabolised in the muscle tissue, brain, lungs and intestine, and less than 3% in the liver and kidneys, so it is not contraindicated by impairment of these organs.7,8
Reported side effects are similar to those of the other substances in its class, such as nausea, vomiting, muscle stiffness, itching, bradycardia and respiratory depression. It is contraindicated in epidural or spinal analgesia due to the glycine used as preservative being neurotoxic.7,8
The use of remifentanil and its safety in obstetrics patients has already been established. The initial studies were conducted on patients undergoing caesarean section under epidural anaesthesia combined with continuous infusion of remifentanil (0.1μg/kg/min), in which the concentrations of remifentanil were measured in maternal arterial blood, the umbilical artery and the umbilical vein.
High placental transfer of remifentanil has been demonstrated, with umbilical cord/maternal ratios of greater than 0.88ng/ml, but with rapid metabolism and elimination (umbilical artery/umbilical vein ratio of 0.29ng/ml), and without causing effects in the neonate according to APGAR scores (greater than 7 at 5min) and neonatal neurological adaptive capacity scores.9
As mentioned above, remifentanil crosses the placental barrier (up to 80%) and it has been found that the foetus is able to metabolise about 50% of this, with reports of some cases of neonatal respiratory depression, although reversed after administration of naloxone.7,8
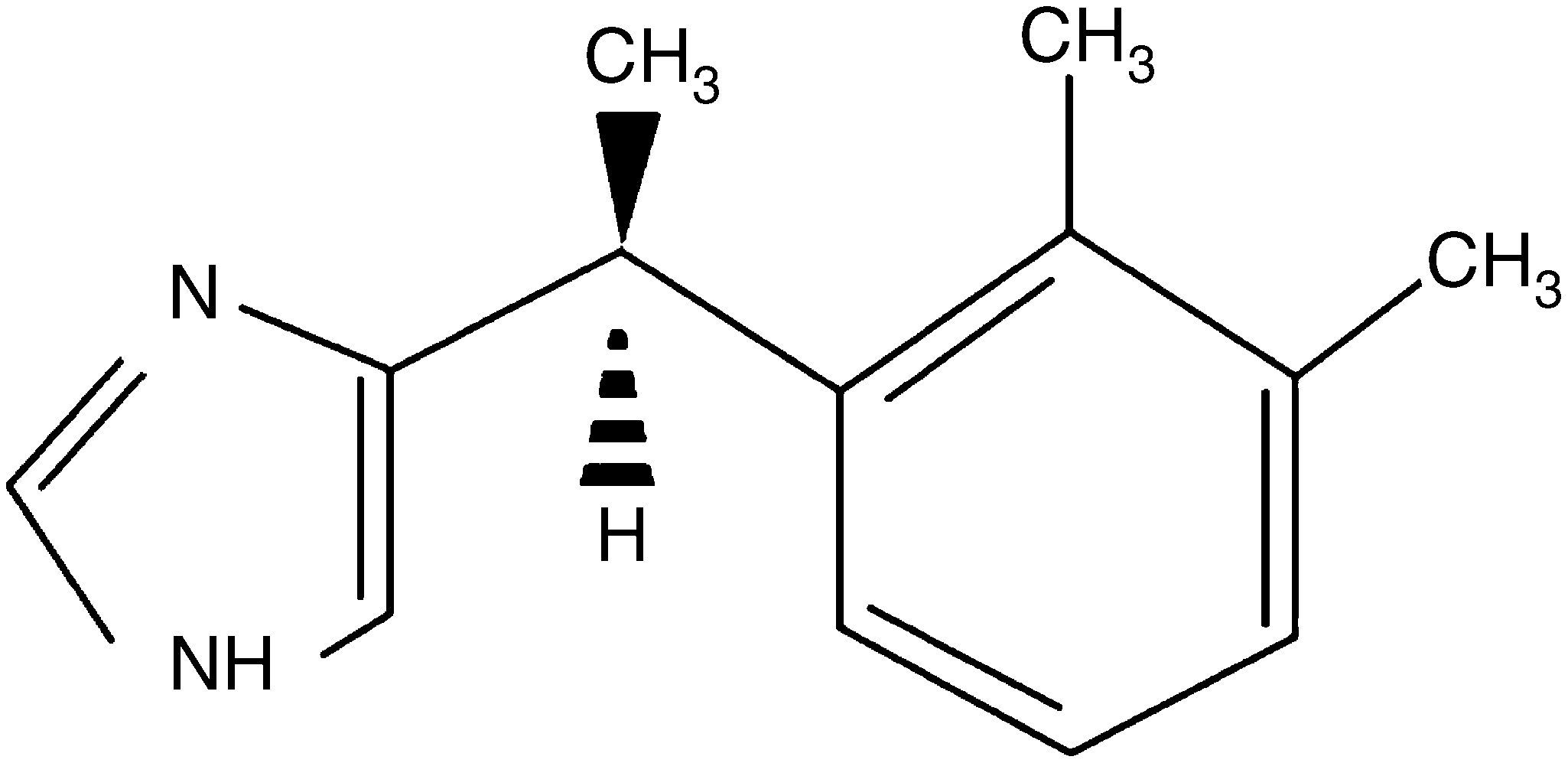
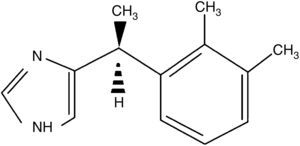
DexmedetomidineAnother of the drugs used in obstetrics for its sedative and analgesic properties is dexmedetomidine. This substance is a d-enantiomer of medetomidine, α2-adrenergic receptor agonist imidazole derivative. The chemical structure is shown in Fig. 2.
Its peak concentration is reached at 6min and it has a half-life of 2h.3,10 It undergoes cytochrome P450-dependent metabolism in the liver and 95% of the drug is excreted by the kidneys. Reported adverse effects include hypotension, hypertension, bradycardia, nausea, dry mouth and arrhythmias.11–13
The main benefits observed in obstetrics are as an anxiolytic, an adjunct in the haemodynamic management of the mother and a stimulant of uterine contractions, and even for prevention of postoperative delirium. Similarly, being lipophilic, it is retained in the placental tissue. It therefore does not reach the foetus and does not cause neonatal respiratory depression.12,13
Due to its high lipophilicity and maternal foetal ratio of 0.77, dexmedetomidine is retained in the placental tissue and does not cross into the foetal circulation. The risk of causing foetal bradycardia is therefore significantly lower because of these properties.13
Evidence in obstetric analgesiaBoth drugs have been the subject of different studies examining the side effects in both the mother and the newborn. One example is the analysis by the anaesthetist Jesus Fresa, in which, after reviewing the available information on the use and effects of remifentanil and dexmedetomidine in obstetric analgesia from 2000 to 2013, he concluded that in pregnant women with co-existing conditions requiring strict haemodynamic monitoring during labour, dexmedetomidine is the drug of choice and, due to its “opioid-sparing” properties, it can be used in conjunction with opioids in order to properly manage pain, provide comfort to the patient and reduce effects on the neonate.14
In 2014, through a controlled clinical trial with 100 pregnant women with pre-eclampsia, Helmy et al. analysed different haemodynamic parameters, both in the mother and the newborn, after administration to 50 of the women of one dose of dexmedetomidine 1μg/kg prior to induction of general anaesthesia, followed by a continuous infusion at 0.4μg/kg/h until the end of the procedure, and administration to the remaining 50 of one dose of fentanyl 1μg/kg prior to induction of general anaesthesia, followed by a continuous infusion at 1μg/kg/h until the end of the procedure. The researchers concluded that, between these two drugs, dexmedetomidine was the drug of choice given the apparent lack of effects on the mother and newborn and its benefits in terms of haemodynamic control.15
In 2015, various groups of researchers studied the effects of remifentanil and/or dexmedetomidine on the mother and foetus. One such study was that by Li et al.,16 in which the haemodynamic changes in the mother and foetus were monitored in 44 patients without pre-existing heart or cerebrovascular disease, who were scheduled for caesarean section under general anaesthetic. The patients were divided into two groups: the 22 in the first group were administered one dose of remifentanil 2μg/kg, followed by a continuous remifentanil infusion at 2μg/kg/h; the 22 in the second group received one dose of dexmedetomidine 0.4μg/kg, followed by a continuous dexmedetomidine infusion at 0.4μg/kg/h. The team showed that, at the doses administered, both drugs were effective for maternal haemodynamic control during general anaesthesia, with similar findings in terms of safety for the newborn. However, remifentanil can cause transient neonatal respiratory depression, making dexmedetomidine the better option.16
In another study, following a review of available information on the use of dexmedetomidine in obstetrics, Nair and Sriprakash determined that in patients for whom tachycardia and hypertension are totally undesirable, taking their liver and kidney function into account, and also considering that its effects on the neonate are practically nil, the drug is a useful adjunct in general anaesthesia.17
Similarly, Yu et al. studied the effects in the neonate after administering an infusion of dexmedetomidine 0.6μg/kg for 10min prior to propofol 1.5–2mg/kg and remifentanil 1.5–2μg/kg, followed by a continuous infusion of dexmedetomidine 0.4μg/kg/h, to 19 pregnant women with contraindication for regional anaesthesia; a similar group of pregnant women were administered saline solution only. The team concluded that, although a minimal amount of dexmedetomidine does cross the placenta, it does not cause adverse effects in the newborn and, on the contrary, it is helpful for haemodynamic control in the mothers.18
Noskovaet et al. published the results of a controlled clinical trial in which 76 patients undergoing caesarean section under general anaesthetic were given one dose of remifentanil 1μg/kg 30s before induction of anaesthesia and compared to a control group not given the substance. They found that, although the symptoms were short-lived, at that dose, remifentanil increased the risk of neonatal respiratory depression.19
Solek et al. reviewed the literature on the use of remifentanil in pain management during labour, concluding that, due to its pharmacokinetic and pharmacodynamic properties, remifentanil is the ideal opioid for labour pain control. There are, however, certain risks to the neonate such as respiratory depression which, although transient, should be studied in more detail in controlled clinical trials.1
Last of all, in 2016, Stourac et al. assessed the analgesic efficacy of remifentanil during labour through a meta-analysis, and determined that the drug is effective, but that the dose-response effects in the mother and the newborn should be evaluated in controlled clinical trials.20
ConclusionsAfter reviewing the available literature on the use of remifentanil and dexmedetomidine in obstetric analgesia, evidence supports the fact that these drugs are effective and safe for induction of general anaesthesia in pregnant women and for use in intravenous obstetric analgesia, particularly when optimal haemodynamic control is required. Furthermore, since it has been shown that remifentanil can cause neonatal respiratory depression, a thorough anaesthetic assessment should be carried out, ensuring that neonatology and perinatology have full understanding of the use of these drugs, in order to provide greater safety to mother and foetus and avoid adverse effects. Controlled clinical trials are needed to evaluate the dose-response and reactions when combining remifentanil with dexmedetomidine since, thanks to its opioid-sparing effect, dexmedetomidine may contribute to a reduction in the risk of respiratory depression in the newborn due to the lower dose of remifentanil required.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.