(p = NS). Conclusiones. La intervención mediante mensajes de alerta al teléfono móvil de hipertensos no ha mejorado el cumplimiento terapéutico.
Introduction
The high prevalence of hypertension (HT) in Spain and the low percentage of patients in whom the disorder is well controlled1 are indications of the magnitude of this primary care problem. To improve the degree of control of HT, interventions aimed at different health care professionals are a key approach to providing them with the conviction and capacity to put into clinical practice current expert group recommendations and consensus guidelines for HT.1
Compliance can be defined as the extent to which the patient accepts the physician's rules or advice in relation to recommended lifestyle, habits or prescribed pharmacological treatment. Compliance should result from a well-reasoned decision-making process, and it should be free of any connotations of submission that the term implies for the patient.1
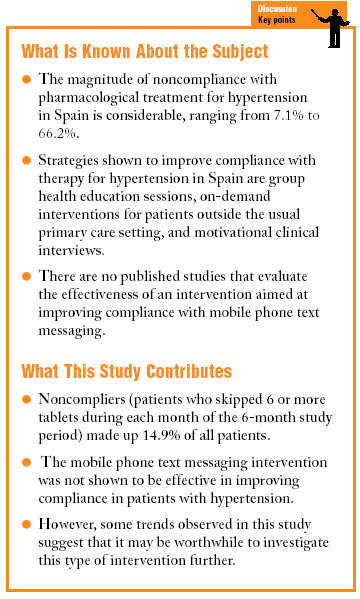
The magnitude of noncompliance in Spain ranges from 7.1% to 66.2%, figures similar to those reported for other countries.2 The medical literature contains few studies in which the main objective was to investigate compliance with HT therapy. Even more relevant is the lack of studies that investigate interventional strategies to improve compliance in Spain1-3 and other countries.4-7 This makes clear the need for studies of different compliance-enhancing strategies that are easy to apply in practice.
The results of a survey of 2363 patients treated at 37 HT units in Spain8 showed that 43.5% were willing to receive health advice via their mobile phone. Moreover, text messaging with Short Message System (SMS) technology has been used as a support measure in young patients with asthma.9
The main objective of the present study was to determine whether an intervention based on mobile telephone text messaging to send alerts and reminders improved compliance with antihypertensive therapy.
Methods
Design
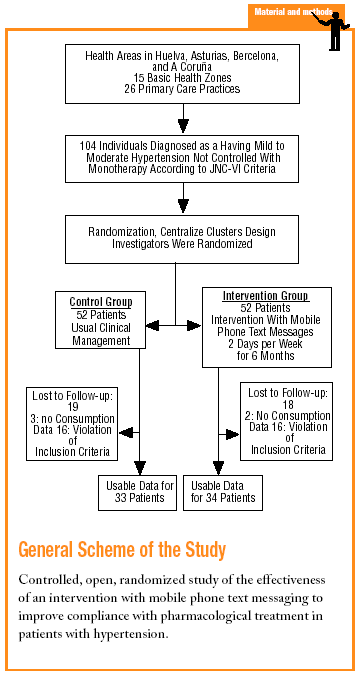
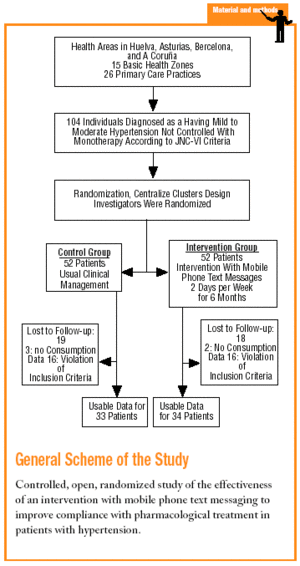
This was a prospective, multicenter, controlled, randomized, open study. The participants were individuals diagnosed as having mild to moderate HT according to JNC-VI diagnostic criteria. The patients were chosen by 26 researchers at 15 urban and rural primary care centers in the provinces of Huelva, Asturias, Barcelona, and A Coruña in Spain. Each researcher was responsible for recruiting 4 individuals.
Participants
The sample needed was calculated10 for studies that use proportions as the main outcome measure and that require two-sided contrast analysis. The differences considered clinically significant10,11 were 25% for compliance between groups, 65% for estimated prevalence of compliance in the control group, and 90% for the proportion of compliance in the intervention group. This yielded a sample size of 52 individuals per group. The study period lasted for 12 months starting in January 2002. The total number of subjects included was 104. A cluster method of randomization was used. Researchers were randomized to one of the 2 groups with a random number table, so that all patients recruited by the same investigator were assigned to the same group. The 2 groups compared in this study were: a) control group (CG): 52 patients with HT who received their usual care from their general practitioner, and b) intervention group (IG): 52 patients with HT whose disease was managed with the intervention described below in addition to their usual care. The inclusion criteria were: a) ambulatory patients of both sexes older than 18 years; b) patients whose HT was not well uncontrolled with monotherapy (SBP greater than or equal to 140 mm Hg or SBP greater than or equal to 90 mm Hg in patients without diabetes; SBP>=130 mm Hg or SBP>=85 mm Hg in patients with diabetes); c) patients with HT eligible for starting treatment with a combination of a single-dose angiotensin II antagonist and a diuretic; d) patients with a mobile telephone for their personal use or whose life partner had a mobile phone; e) patients who knew how to retrieve and read text messages on their mobile phone; and f) patients who gave their informed consent to participate in the study. The exclusion criteria were: a) patient on treatment with 2 or more antihypertensive drugs to control their blood pressure; b) patients with secondary HT; c) patients with known contraindications for any of the antihypertensive drugs to be used; d) patients whose clinical condition might have interfered with the study; e) patients unable to give their informed consent; f) patients participating in other research studies; and g) patients who lived with a person who was being treated with the same antihypertensive drug. Finally some withdrawal criteria were established: a) insufficient therapeutic effect necessitating an increase of more than 20% in the number of scheduled follow-up visits; b) patient´s decision to withdraw from the study; c) patient´s health compromised, in the physician's opinion, because of adverse effects or concomitant disease; and d) lack of cooperation or noncompliance with follow-up by the patient after inclusion.
Intervention
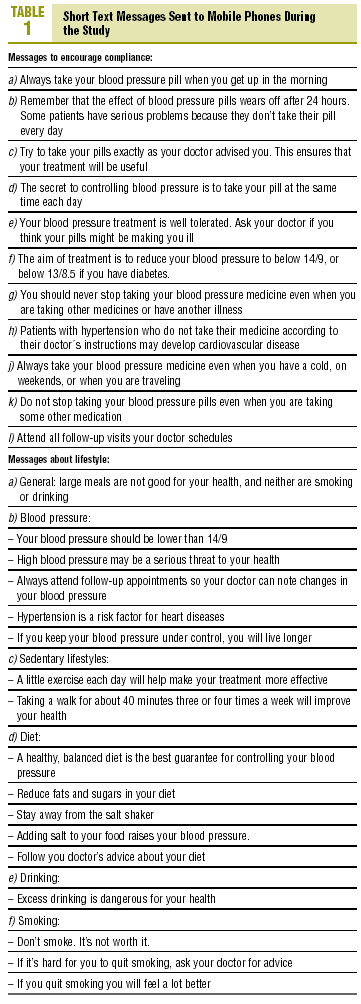
Each patient was given printed information about HT, and each was subscribed to a Short Message System (SMS) mobile telephone alerting system. Two messages were sent to the patient's mobile phone per week between 11:00 AM and 14:00 PM on randomly chosen weekdays from Monday to Friday during the 6-month study period. The aim of the messages was to provide information on HT, foment compliance, and good health and
dietary habits, and remind patients to take their medication (table 1). The messages were sent randomly from an SMS platform programmed to generate random messages by Infociencia, S.L., and MyAlert, Inc. Receipt of the messages was free to participants in the study and independent of their telephone service operator.
Follow-up and Work Plan
Four visits to the health center were scheduled: the first appointment (inclusion) and 3 subsequent visits at 4, 12, and 24 weeks.
1. First appointment: fulfillment of the inclusion and exclusion criteria was checked, the patient was given verbal and written information about the study, and informed consent was requested. The patient was questioned about his or her illness and given a physical examination. Weight and height were recorded, and 2 blood pressure measurements were taken. Verbal and printed health education information was provided. Single-dose antihypertensive treatment was prescribed, with medication to be taken each day on arising. The date of the prescription, number of tablets in the package, and day the first tablet was to be taken were recorded. A follow-up appointment was scheduled for 4 weeks later. The patient was instructed to bring to this appointment all the packages of the prescribed medication (used, partly used, and unused packages), and not to take the medication on the day of the appointment. Patients with HT in the IG were subscribed to the short text messaging program via a telephone call made by the physician or the patient to a toll-free (900) number.
2. First follow-up visit: 4 weeks after the start of treatment, blood pressure and weight were recorded, and the physician discreetly counted the number of tablets consumed to avoid patient-related bias. The patient was asked about the possible appearance of side effects. If the target blood pressure value was not reached, the physician-researcher considered adding another antihypertensive in the light of his or her clinical experience. A second medication was prescribed if necessary, and the second and third follow-up visits at the health center were scheduled for 12 and 24 weeks after the start of treatment.
3. Second follow-up visit: the procedure at this visit, 12 weeks after the start of treatment, was similar to that used for the 4-week follow-up visit.
4. Final visit: the third and last follow-up visit took place 24 weeks after the start of treatment. The procedure at this visit was similar to that used for the 4-week and 12-week follow-up visits.
Main Outcome Measures
To evaluate compliance with treatment, we counted the number of tablets consumed,3 and then calculated percentage compliance (PC) with the formula
Individuals were considered compliers if PC was between 80% and 110%.3 The data analyzed for this study were PC at the end of the study, cumulative PC at the end of follow-up (last follow-up visit or withdrawal), monthly PC, and change in PC from one follow-up visit to the next.
Statistical Analysis
The following statistical analyses were done:
Similarity of the 2 groups was verified for age, sex, time since diagnosis, number of diseases, and number of tablets consumed, as variables that might influence compliance.
The percentage of compliers and mean PC at the end of the study were compared between groups.
The degree of control of HT was calculated. Control of HT was defined as values <140/90 mm Hg in patients without diabetes, and <130/85 mm Hg in patients with diabetes.
The reduction in absolute risk (RAR) and in relative risk (RRR) was calculated, along with the number needed to treat (NNT) to avoid noncompliance.
The chi-squared test was used for qualitative variables, and Students t test for quantitative variables. Statistical significance was said to exist when P<.05.
Ethical Considerations
The study was approved by the Primary Care Ethical Committee of the Jordi Gol i Gurina Foundation in Barcelona. Written informed consent was obtained and good clinical practice guidelines were followed.
Results
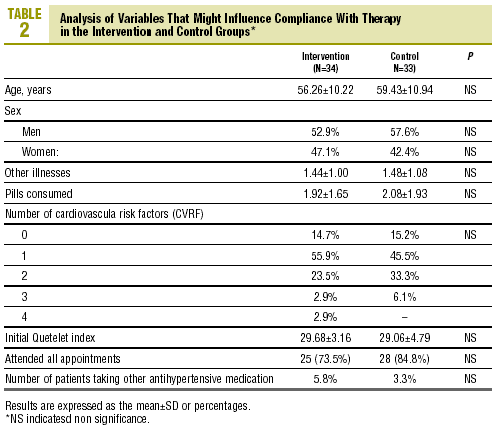
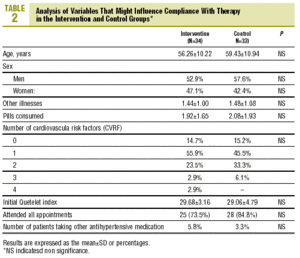
A total of 72 individuals (69.23% of the calculated sample) were recruited; 5 patients were excluded because they had no record of the number of tablets consumed. Usable results were available for 67 individuals (37 [55.2%] men and 30 [44.8%] women) with a mean age of 57.8±10.6 years. The IG consisted of 34 individuals (18 men, 16 women) with a mean age of 56.3±10.2 years, and the CG consisted of 33 persons (19 men, 14 women) with a mean age of 59.4±10.9 years. At the start of the study there were no differences between groups in variables that might have affected compliance (table 2).
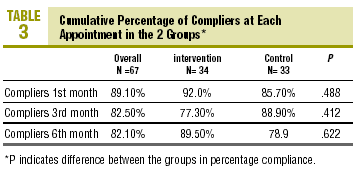
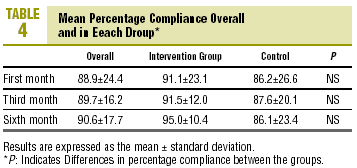
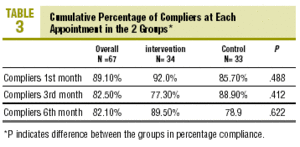
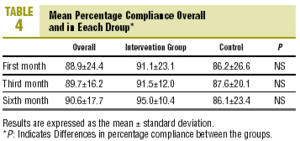
At the end of follow-up, compliers comprised 85.1% of the sample (CI, 74.9%-95.3%), i.e., 85.7% (CI, 70.5%-100.0%) in the CG and 84.4% (CI, 70.7%-95.3%) in the IG (P=NS, table 3). There were no differences between groups in PC at any of the visits. Mean PC at the end of follow-up was 90.2%±16.3% overall, 88.1%±20.8% in the CG and 91.9%±11.5% in the IG (table 4; P=NS). There were no differences in mean PC between groups.
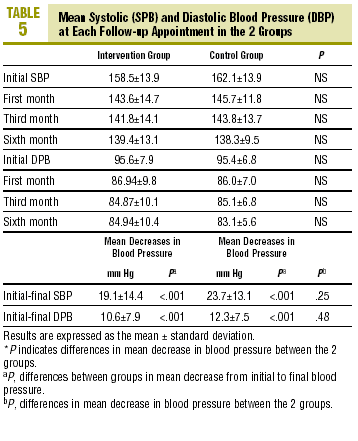
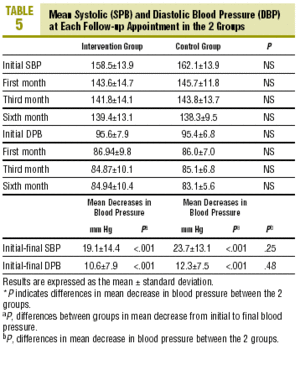
Table 5 shows mean differences in blood pressure at each visit in the 2 groups; none of the differences between groups was significant. The decrease in SPB and DPB was significant in each group, with no difference in the mean decreases.The percentage of patients in whom blood pressure was controlled was 33.3% (CI, 17.2%-49.4%) in the CG and 44.1% (CI, 27.4%-60.8%) in the IG (P=NS) after 4 weeks. These figures were 36.4% (CI, 20.4%-52.8%) in the CG and 61.8% (CI, 45.5%-78.1%) in the IG (P<.05) after 12 weeks, and 51.5% (CI, 34.4%-68.6%) and 64.7% (CI, 48.6%-80.8%) respectively (P=NS) after 24 weeks. The percentage of patients whose blood pressure was controlled at the final follow-up visit was significantly higher in the CG.
Mean initial body weight was 79.8±13.6 kg in the CG and 82.0±10.9 kg in the IG (P=NS), and mean final body weight was 79.6±13.2 kg and 76.84±8.92 kg respectively. The decrease in body weight was significant in the IG (P<.001).
Discussion
The rate of noncompliance in this study was 14.9%. Although compliance was slightly higher in the IG, there were no significant differences between the two groups in PC or mean PC. If the sample had been larger and if the trend had remained stable, the differences would probably have reached significance. However, the effectiveness of the intervention was not supported by our data. In comparison to other studies, the percentage of compliers and percentage compliance were high in both of our groups, and only 15.6% of the patients in the IG had skipped 6 or more tablets each month of the study. Compliance was highest in both groups after 4 weeks of treatment, and decreased thereafter. Similar results have been reported in other studies of patients with HT2 or dyslipidemia.13In Spain the prevalence of noncompliance ranges from 7.1% to 66.2% depending on the study.14 In a review of all studies of compliance based on tablet counts published in Spain until 2001,14 the weighted mean for noncompliance among all 2313 patients was 45%. In other clinical studies run in Spain, Márquez et al2 found a noncompliance rate of 16.7%, Pertussa et al reported a figure of 54%,15 Raigal et al set the figure at 52.6%,16 and Tortajada et al17 noted a rate of 45%. A number of reviews and metaanalyses have looked into strategies to improve compliance with HT treatment, including the review by Márquez et al1 on compliance in general, a Cochrane review by Haynes et al,4 and reviews published by Kravitz et al,5 Roter et al,6 and Haynes et al.7 The strategies found to be effective in improving compliance and the degree of control of HT are: regular sessions of individual verbal and written health education; group health education sessions about HT; documents prepared according to specific methods; single-dose antihypertensive treatment regimens; diary cards for patients with HT; associating antihypertensive medication with a habitual daily activity; using medication dispensers; use of an alarm clock or other device to remind patients to take their medication; rewards for compliance with treatment better than 90%; on-demand primary care interventions outside the physician's office or health center, and motivational clinical interview. The best strategy consists of a combination of several of these individual approaches.1 In Spain, Márquez et al2 investigated the effectiveness of health education in group sessions with reminders sent by mail. After 2 years of intervention to improve compliance with HT therapy, the authors found that compliance was indeed better in the intervention group. Raigal et al16 tested an intervention to improve knowledge about the disorder with oral and written health education materials. The CG received on-demand primary care (5 minutes per visit) and the intervention group attended scheduled visits (10 minutes per visit). After 14 weeks the difference between groups was significant (P=.01) for percentage compliance and reduction in blood pressure. A randomized clinical trial reported by Tortajada et al10 compared the usual methods for HT therapy combined with telephone reminders to attend follow-up visits, versus these measures in combination with motivational interviews and an on-demand visit outside the usual setting of the follow-up visits. Significant differences were found for compliance and for the reduction in blood pressure. However, Zaragoza et al,11 in a study of the effects of an initial home interview to foment compliance along with brief written instructions similar to those habitually provided by physicians, found that this intervention did not improve compliance. These authors concluded that brief counseling was not useful, and that instead, longer-term strategies and extensive written information about HT and antihypertensive treatment should be used.
The present study did not investigate the clinical relevance of the intervention, since no benefits were observed. However, the NNT with the strategies tested here were 2.49 according to Márquez et al,2 5.5 in a study by Tortajada et al10, and 4 according to a report by Raigal et al.16 When we analyzed the effect of the intervention on blood pressure values, we found no significant differences between the 2 groups at the end of the study. However, the percentage of patients who achieved good control at the third (and last) follow-up visit was significantly higher in the IG than in the CG. In the former, we noted a significant mean decrease in body weight as compared to the CG at the end of the study. This decrease may have been related with the SMS alerts received, as some messages--programmed to be sent between 11:00 AM and 13:00 specifically recommended healthy eating habits and exercise. However, this finding should be interpreted with caution, as in the long term (after 2 years, for example), the effect of most educational measures regarding weight control are found to be transient. When we examined the validity of the study and its biases, we noted a number of problems that need to be considered in order to interpret the results appropriately. Although the criteria for studies on compliance recommended by Haynes et al7 were taken into account, some of them were not satisfied. For example, the diagnosis of HT was appropriate and in accordance with the JNC VI recommendations. The methods used to measure outcomes and to count the tablets consumed were those recommended by authorities at McMaster University in Canada,7 although it would have been preferable to determine use of medication with unscheduled home visits. Our methods were nonetheless suitable given that the recommended method is a double-blind randomized clinical trial, with appropriate assurances that the groups are initially comparable, and with an analysis of compliance based on measures of the actual disease or disorder involved (blood pressure values, in this case). Follow-up data should have been available for more than 80% of the participants during a period of 6 months, and more than 50 patients per group should have been included in the final calculation of the magnitude of clinical relevance (RRR, RAR and NNT). Statistical significance should have been calculated with confidence intervals. These criteria were not satisfied in the present study, as the final sample comprised less than 80% of the initial number of patients, and there were fewer than 50 individuals in each group. A larger sample would probably have improved the favorable results in the IG. Despite these biases, we feel it is worthwhile to report the findings and explain why it was not possible to obtain a larger sample.
The sample size makes it impossible to draw conclusions about the possible clinical relevance of the intervention, and the good results for compliance in the CG suggest that the participating researchers might not have been representative of the "average" Spanish primary care physician, since members of this collective, in general, have little interest in or motivation to participate in this type of study. The use of mobile phone messaging in motivated researchers who were already able to obtain high rates of compliance from their patients might not have revealed any additional benefits. Our impression was that the technology is difficult to implement in everyday practice, as it requires patients with HT who own a mobile phone, who know how to retrieve and read SMS messages, and who are preferably noncompliers with poorly controlled HT.
Regardless of the lack of evidence of effectiveness of this intervention, further research on the usefulness of this technology for health care is advisable given that there are currently 30 million mobile phone users in Spain, and it is likely that more than 40% of all persons with HT will own a mobile phone. These numbers make SMS alerts a simple technique to implement, at reasonable cost.
One of the challenges of future research in this area is to recruit a random sample of primary care physicians and train or otherwise motivate health professionals, to the extent necessary, to participate in studies of this type and to show an acceptable degree of adherence to procedural requirements. Once enough health professionals have been trained and motivated, patients should be recruited anew and randomized to one group or the other, and the study should be repeated with a sample large enough to provide useful data and to compensate for withdrawals and drop-outs. Training in research procedures should not significantly influence the physicians' habits and practices in the long term regarding how they manage their patients with HT. This approach, arduous as it is, would improve the external validity of the results, and would ensure that if they were again found to be negative, this could be considered definitive evidence of a lack of effect of mobile phone text messaging to improve compliance with treatment in patients with hypertension.
Acknowledgments
We thank the researchers of the HTA-Alert Study and the Grupo de Cumplimiento (Compliance Group) of the Asociación de la Sociedad Española de Hipertensión (Spanish Hypertension Society) and the Liga Española para la Lucha contra la HTA (Spanish League for the Fight against Hypertension) (SEH-LELHA).