Interferon-gamma release assays are widely used for the diagnosis of tuberculosis infection in low-prevalence countries. However, there is no consensus on their application. The objective of this study was to develop guidelines for the use of interferon-gamma release assays in specific clinical scenarios in Spain.
MethodsA panel of experts comprising specialists in infectious diseases, respiratory diseases, microbiology, pediatrics and preventive medicine, together with a methodologist, formulated the clinical questions and outcomes of interest. They conducted a systematic literature search, summarized the evidence and rated its quality, and prepared the recommendations following the GRADE (Grading of Recommendations of Assessment Development and Evaluations) methodology.
ResultsThe panel prepared recommendations on the use of interferon-gamma release assays for the diagnosis of tuberculosis infection in the contact-tracing study (both adults and children), health care workers, immunosuppressed patients (patients infected with human immunodeficiency virus, patients with chronic immunomediated inflammatory diseases due to start biological therapy and patients requiring organ transplant) and for the diagnosis of active tuberculosis. Most recommendations were weak, mainly due to the lack of good quality evidence to balance the clinical benefits and disadvantages of the interferon-gamma release assays as compared with the tuberculin skin test.
ConclusionThis document provides evidence-based guidance on the use of interferon-gamma release assays for the diagnosis of tuberculosis infection in patients at risk of tuberculosis or with suspicion of active disease. The guidelines will be applicable in specialist and primary care and in public health settings.
Las técnicas de detección in vitro de interferón-gamma (IGRA, del inglés interferon-gamma release assays) están ampliamente implantadas para el diagnóstico de infección tuberculosa en países de baja prevalencia. Sin embargo, no hay consenso sobre su aplicación. El objetivo fue desarrollar una guía de práctica clínica para el uso de los IGRA en los diferentes escenarios clínicos en España.
MétodosUn grupo de expertos compuesto por especialistas en enfermedades infecciosas, enfermedades respiratorias, microbiología, pediatría y medicina preventiva, junto con un metodólogo formularon las preguntas clínicas y los desenlaces de interés, llevaron a cabo una búsqueda sistemática de la literatura, sintetizaron la evidencia y graduaron su calidad, y formularon las recomendaciones siguiendo la metodología Grading of Recommendations of Assessment Development and Evaluations (GRADE).
ResultadosEl grupo de trabajo formuló las recomendaciones sobre el uso de los IGRA para el diagnóstico de infección tuberculosa en el estudio de contactos (adultos y niños), trabajadores sanitarios, pacientes inmunosuprimidos (pacientes infectados por el virus de la inmunodeficiencia humana, pacientes afectos de enfermedades inflamatorias inmunomediadas candidatos a terapias biológicas y pacientes que requieren trasplante de órganos), y en el diagnóstico de enfermedad tuberculosa activa. La mayor parte de las recomendaciones fueron débiles, principalmente debido a la falta de evidencia de calidad para establecer un balance entre beneficios y daños de los IGRA en comparación con la prueba de la tuberculina.
ConclusiónEste documento proporciona una guía basada en la evidencia para el uso de los IGRA en el diagnóstico de infección tuberculosa en pacientes en riesgo de tuberculosis o con sospecha de enfermedad activa. Esta guía es aplicable en la atención especializada y primaria, y salud pública.
The diagnosis of tuberculosis (TB) infection relied solely on the tuberculin skin test (TST) until a decade or so ago when in vitro immunodiagnostic tests, known as interferon-gamma release assays (IGRAs), became available. At present, the question of whether IGRAs should be used to complement or substitute the TST is under debate.
The aim of detecting TB infection is to identify and treat people at risk of developing active disease. Close-contacts of patients with TB, people infected with the human immunodeficiency virus (HIV), patients receiving immunosuppressive therapies, and health care workers are all at risk of active TB. Therefore, screening and preventive chemotherapy in cases of infection should be considered in these populations.1 The TST consists of the intradermal injection of PPD-S (Seibert's purified protein derivative), or the PPD-RT23 equivalent in Spain. However, this contains a mixture of more than 200 antigens that are shared by mycobacteria other than Mycobacterium tuberculosis, and includes the vaccine strain of Mycobacterium bovis bacillus Calmette-Guérin (BCG). Therefore, people sensitized by previous exposure to non-tuberculous mycobacteria (NTM) or the BCG vaccine may respond to a TST. Another major limitation is the loss of sensitivity of the test in certain groups, such as immunosuppressed patients and young children.2 It should also be remembered that a negative TST obtained early after the onset of infection with M. tuberculosis does not exclude infection because the test takes up to eight weeks to emerge as positive. This interval is usually referred to as the “window period.”
In the last decade, in vitro immunodiagnostic tests have been developed to overcome the deficiencies of the TST. Based on the in vitro quantification of the cellular immune response, IGRAs detect interferon-gamma (IFN-γ) release by sensitized T cells after stimulation with specific M. tuberculosis antigens.3 The two main antigens used are the 6-kD M. tuberculosis early-secreted antigenic target protein (ESAT-6) and the 10-kD culture filtrate protein (CFP-10), encoded in the region of difference 1 (RD1), which is present in M. tuberculosis but not in BCG nor in most NTM.4 Currently there are two commercially available assays: the QuantiFERON®-TB Gold assay (QFT-G) (Qiagen, Hilden, Germany) and the T-SPOT®.TB assay (Oxford Immunotec, Oxford, UK). The QFT-G tests are whole-blood assays that use enzyme-linked immunosorbent assays to detect IFN-γ produced in supernatants by stimulated T cells. The QFT-G in-tube version (QFT-GIT) includes a third antigen, TB7.7, which is encoded in RD11 and is missing from BCG strains and most of the common environmental mycobacteria.5 The T-SPOT.TB detects the number of IFN-γ-producing T cells after stimulation of a definite number of isolated peripheral blood mononuclear cells with ESAT-6 and CFP-10, separately, using an enzyme-linked immunospot assay. Both tests include a positive control that detects the capacity of T cells to produce IFN-γ upon stimulation with a mitogen. Like the TST, IGRAs have a window period of conversion after exposure to M. tuberculosis, but the duration of this window has not been clearly determined.
Scope and objectivesCurrently there is no consensus on the use of IGRAs in different clinical scenarios and settings. Despite the abundance of literature about IGRAs, there is a scarcity of good quality evidence evaluating important patient outcomes in given populations. This, in turn, is reflected in the inconsistency of the recommendations of official organizations and scientific societies, which largely rely on expert opinion.6,7 Moreover, recommendations that are suitable for one area or country may not be appropriate for another. To this end, two consensus documents were produced in Spain in 2008 and 2010 on the diagnosis and treatment of TB in adults8 and children,9 respectively, where some brief references are included about the use of IGRAs. Therefore, there was a need for updated recommendations, based on the best available evidence and formulated according to the modern methodology for the elaboration of guidelines.
The current document provides evidence-based guidance to the specialist and primary health-care providers and public health authorities on the use of IGRAs for diagnosing TB infection in both immunocompetent and immunosuppressed adults and children of any age at risk for or suspected of having active TB. The final objective of the document is to minimize the uncertainty and variability in the diagnosis of TB infection by the IGRAs. It is beyond the scope of these guidelines to give advice on when the screening of TB infection should be performed or when and how they should be treated. The authors refer the readers to other guidelines that provided specific guidance on that.10 Nonetheless, the authors stress the need to focus screening on people at risk of developing active TB based on the “Intention to test, intention to treat” principle.
MethodologyThe panelThese guidelines were developed by a panel of experts comprising in infectious diseases, respiratory diseases, microbiology, pediatrics and preventive medicine specialists, together with a methodology expert with experience in the development of clinical guidelines. All panel members were assigned to a specific subgroup (contact tracing, health care workers, children, HIV-infected people, patients with chronic inflammatory diseases, transplant candidates, and patients suspected of having active TB) according to their clinical profile. Each subgroup was coordinated by one of the three coordinators. The methodologist instructed the members of the panel in the methodology, performed the literature search, guided the deliberations and summarized the recommendations formulated by the panel. All panel members with the exception of the methodologist, participated in the discussions for the formulation of the recommendations. One of the coordinators (M.S.) drafted the manuscript, and finally, all the panel members reviewed and approved the final version. Former TB patients or the public in general were not invited to the panel meetings, since their participation would have required intensive training regarding the implications of test results on health outcomes. Instead, the panel members made inferences about patients’ critical and important outcomes considered in the guideline.
Clinical questionsClinical questions were formulated according to the PICO structure (population, intervention [index test], comparison [reference test] and outcomes), with the outcomes of interest prioritized. No clinical trials had previously evaluated the efficacy of chemoprophylaxis for TB using baseline IGRA results; therefore, the diagnostic yield indexes were derived from observational studies, which constituted indirect evidence of the benefits and consequences of management based on IGRA results. This deficiency limited the quality of the evidence (confidence in the results) in all the questions evaluated. The same limitation was also applicable to the evaluation of IGRAs in the diagnosis of active disease.
Outcomes of interestGiven that the accuracy of the IGRAs for latent TB infection (LTBI) cannot be directly assessed because of the lack of a reference standard, the panel established the following outcomes, hierarchically classified according to their clinical relevance (from highest to lowest)11:
- 1.
The efficacy of chemoprophylaxis based on the IGRA results.
- 2.
The predictive values of the IGRAs for the development of active TB based on the IGRA results.
- 3.
The correlation between rate of exposure and risk factors for TB infection.
- 4.
The sensitivity and specificity of IGRAs for the diagnosis of active TB.
- 5.
The concordance between TST and IGRA results.
Whenever available, the panel formulated their recommendations based on the first two outcomes because they best reflected the target populations.
Literature searchSearch strategy was designed to identify systematic reviews of diagnostic studies and relevant individual studies that updated existing systematic reviews. Whenever they were available, the panel prioritized clinical trials and prospective observational studies from TB low-prevalence (<25 cases × 100,000 population) countries which used the commercial tests QTF-GIT and T-SPOT.TB performed in blood with 16–24h of incubation. When no data from these settings were available, studies from intermediate- or high-prevalence countries were also used. The panel excluded studies of non-commercial IGRAs or studies based on the old version of the QTF-G, as well as studies presenting non-original data, conference abstracts, editorials, reviews, guidelines, and studies conducted in animals. The search was conducted in the following electronic databases, without language or temporal limitations, up to March 2013: MEDLINE (accessed through PubMed) and EMBASE (accessed through Ovid). The panel was kept updated of new literature until June 2015, to ensure the inclusion of relevant studies published while the guidelines were being developed. Searches comprised a combination of terms that are shown in Appendix B. Publications on resource use and costs were identified by searching in the NHS EED database up to October 2014. The panel did not perform a specific literature search about patients’ values and preferences because they were not considered clinical outcomes, but it did assess the relative relevance of different outcomes of diagnostic yield.
Data extraction and synthesis of the evidenceTwo panel members from each subgroup independently compiled the data using a standardized data extraction sheet. Discrepancies were resolved by discussion and consensus between the two members and the coordinator. The following data were extracted: year of publication, period and country, number of participants, gender, test evaluated, TST results, development of active tuberculosis, and fractions of individuals with true positive, false negative, true negative and false positive results for the calculation of the test's sensitivity and specificity when necessary. The following definitions were used: Sensitivity refers to the proportion of culture-proven tuberculosis patients who had a positive IGRA test; specificity refers to the proportion of symptomatic non-tuberculosis patients who had a negative IGRA test; positive predictive value (PPV) refers to the proportion of patients with a positive IGRA test who had culture-proven tuberculosis, and negative predictive value (NPV) refers to the proportion of symptomatic patients with a negative IGRA test who did not have tuberculosis.
Quality of the evidenceThe quality of the evidence was assessed according to the GRADE (Grading of Recommendations of Assessment Development and Evaluations) international working group.12 The panel assessed the quality of the evidence for all outcomes of interest, using the following parameters: study limitations, consistency between the results of the different studies, availability of direct evidence, precision of the estimators of effect, and publication bias. After the evaluation process, the quality of evidence was classified into four categories for each outcome: high, moderate, low, and very low.
RecommendationsThe panel formulated the recommendations with the evidence available for each clinical question according to the GRADE methodology. For the direction (against/for an intervention) and the strength (strong/weak) of a recommendation, the panel weighed the overall quality of the evidence, the balance between benefits and harms, the relative importance of the outcomes, and the resource use and costs. The recommendations were established by consensus between the members of the panel. Methodological material such as data extraction forms, summaries of findings, tables and evidence to recommendation frameworks are available upon request.
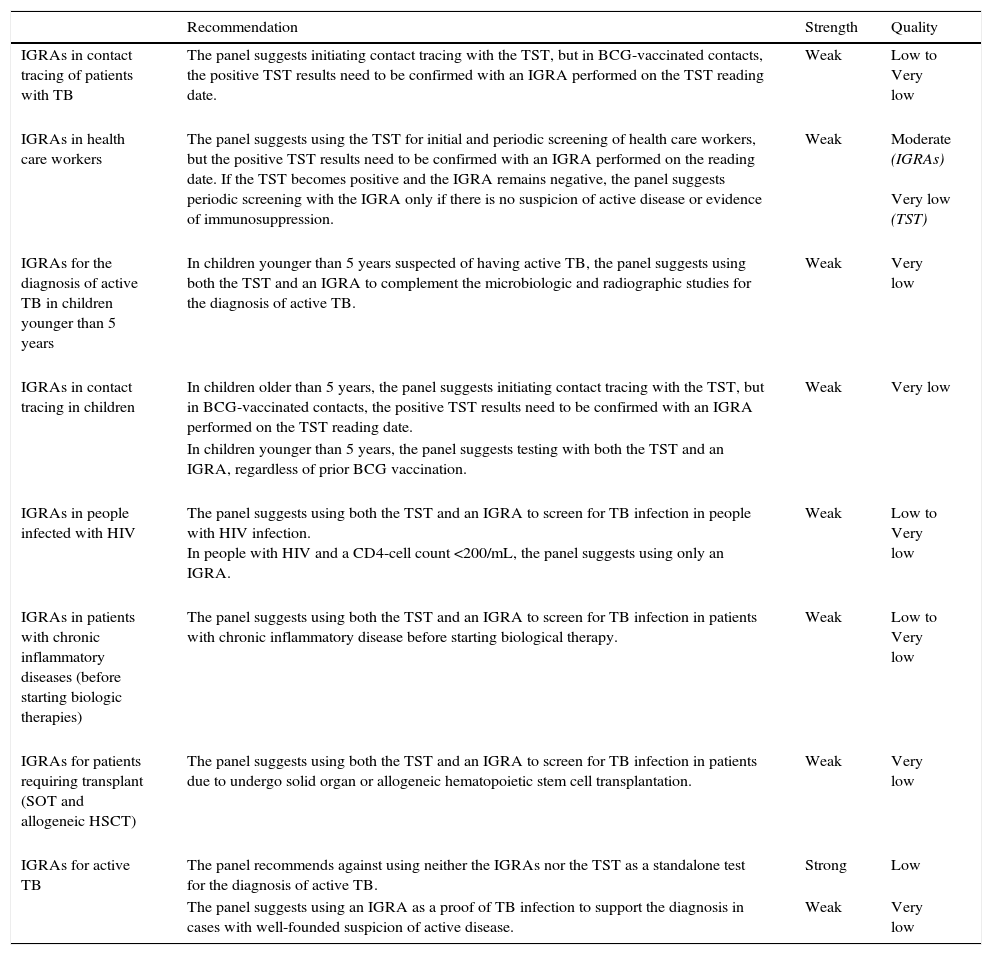
RecommendationsA summary of the recommendations is provided in Table 1.
Summary of the recommendations for different clinical settings.
| Recommendation | Strength | Quality | |
|---|---|---|---|
| IGRAs in contact tracing of patients with TB | The panel suggests initiating contact tracing with the TST, but in BCG-vaccinated contacts, the positive TST results need to be confirmed with an IGRA performed on the TST reading date. | Weak | Low to Very low |
| IGRAs in health care workers | The panel suggests using the TST for initial and periodic screening of health care workers, but the positive TST results need to be confirmed with an IGRA performed on the reading date. If the TST becomes positive and the IGRA remains negative, the panel suggests periodic screening with the IGRA only if there is no suspicion of active disease or evidence of immunosuppression. | Weak | Moderate (IGRAs) Very low (TST) |
| IGRAs for the diagnosis of active TB in children younger than 5 years | In children younger than 5 years suspected of having active TB, the panel suggests using both the TST and an IGRA to complement the microbiologic and radiographic studies for the diagnosis of active TB. | Weak | Very low |
| IGRAs in contact tracing in children | In children older than 5 years, the panel suggests initiating contact tracing with the TST, but in BCG-vaccinated contacts, the positive TST results need to be confirmed with an IGRA performed on the TST reading date. | Weak | Very low |
| In children younger than 5 years, the panel suggests testing with both the TST and an IGRA, regardless of prior BCG vaccination. | |||
| IGRAs in people infected with HIV | The panel suggests using both the TST and an IGRA to screen for TB infection in people with HIV infection. In people with HIV and a CD4-cell count <200/mL, the panel suggests using only an IGRA. | Weak | Low to Very low |
| IGRAs in patients with chronic inflammatory diseases (before starting biologic therapies) | The panel suggests using both the TST and an IGRA to screen for TB infection in patients with chronic inflammatory disease before starting biological therapy. | Weak | Low to Very low |
| IGRAs for patients requiring transplant (SOT and allogeneic HSCT) | The panel suggests using both the TST and an IGRA to screen for TB infection in patients due to undergo solid organ or allogeneic hematopoietic stem cell transplantation. | Weak | Very low |
| IGRAs for active TB | The panel recommends against using neither the IGRAs nor the TST as a standalone test for the diagnosis of active TB. | Strong | Low |
| The panel suggests using an IGRA as a proof of TB infection to support the diagnosis in cases with well-founded suspicion of active disease. | Weak | Very low |
TB, Tuberculosis; TST, Tuberculin skin test; BCG, Bacille Calmette-Guerin; IGRAs, Interferon-gamma release assays; HIV, Human immunodeficiency virus; SOT, Solid organ transplantation; HSCT, Hematopoietic stem cell transplantation.
Recommendation: The panel suggests initiating contact tracing with the TST, but in BCG-vaccinated contacts, the positive TST results need to be confirmed with an IGRA performed on the TST reading date. (Weak recommendation; low to very low quality of evidence.)
- 1.
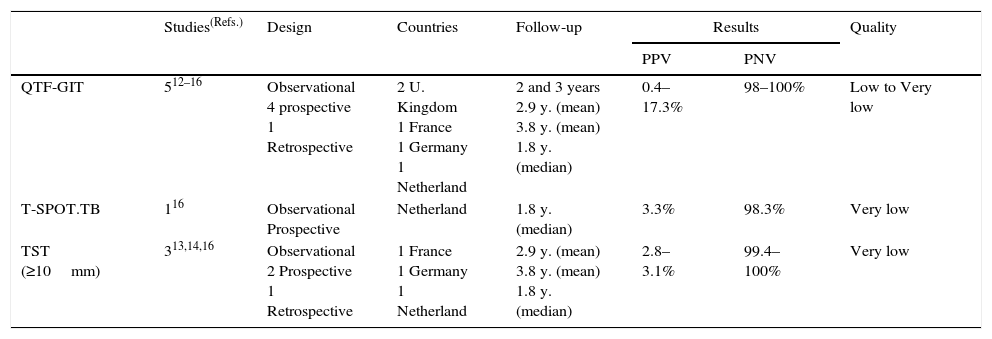
Summary of the evidence. Five observational studies assessed the predictive values of IGRAs for the development of TB in contacts in low-prevalence countries: four with QTF-GIT13–16 and one with both the QTF-GIT and T-SPOT.TB.17 Four studies were prospective13,15–17 and one was retrospective,14 and three also assessed the predictive value of the TST for the development of TB.14,15,17 The observational period was variable, but was at least 12 months in each study. The positive predictive value (PPV) for the QTF-GIT (622 subjects) ranged from 0.4% to 17.3%, for the T-SPOT.TB (181 subjects) was 3.3%, and for a TST ≥10mm (795 subjects) ranged from 2.8% to 3.1%. The corresponding negative predictive values (NPV) ranged from 98% to 100% for the QTF-GIT (2426 subjects), was 98.3% for the T-SPOT.TB (118 subjects), and from 99.4% to 100% for a TST ≥10mm (828 subjects) (Table 2).
Table 2.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in contact tracing of tuberculosis.
Studies(Refs.) Design Countries Follow-up Results Quality PPV PNV QTF-GIT 512–16 Observational
4 prospective
1 Retrospective2 U. Kingdom
1 France
1 Germany
1 Netherland2 and 3 years
2.9 y. (mean)
3.8 y. (mean)
1.8 y. (median)0.4–17.3% 98–100% Low to Very low T-SPOT.TB 116 Observational
ProspectiveNetherland 1.8 y. (median) 3.3% 98.3% Very low TST (≥10mm) 313,14,16 Observational
2 Prospective
1 Retrospective1 France
1 Germany
1 Netherland2.9 y. (mean)
3.8 y. (mean)
1.8 y. (median)2.8–3.1% 99.4–100% Very low TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
- 2.
Quality of the evidence. The overall quality of the evidence was low to very low. The main limiting factors were the lack of direct evidence of the outcomes evaluated and the risk of bias derived from the design of the studies.
- 3.
Justification. The percentage of patients with a negative result who did not develop TB was very high with either type of test (between 98% and 100%). However, because TST is generally more available than IGRAs, it can be used as the basis of screening. In case of a positive result in BCG-vaccinated patients, validation with an IGRA can better select those who require therapy, which should reduce unnecessary treatment. Such reduction, though, seems not to increase the risk of subsequent active disease.18 In BCG-vaccinated populations, the benefits of using IGRAs were considered to surpass the inconveniences, whereas in non-BCG-vaccinated people, the risks and benefits were thought to be more evenly balanced. Therefore, the impact of implementing this strategy will vary with the prevalence of BCG vaccination. Finally, although the lower direct cost would favor the use of TST, a cost-effectiveness study has suggested that the overall costs of including an IGRA in the strategy would be lower after including indirect cost savings from reduced treatment.19
Recommendation: The panel suggests using the TST for initial and periodic screening of health care workers, but the positive TST results need to be confirmed with an IGRA performed on the reading date. (Weak recommendation; moderate and very low quality of evidence for IGRAs and TST respectively.)
If the TST becomes positive and the IGRA remains negative, the panel suggests periodic screening with IGRAs only if there is no suspicion of active disease or evidence of immunosuppression. (Weak recommendation; very low quality of evidence.)
- 1.
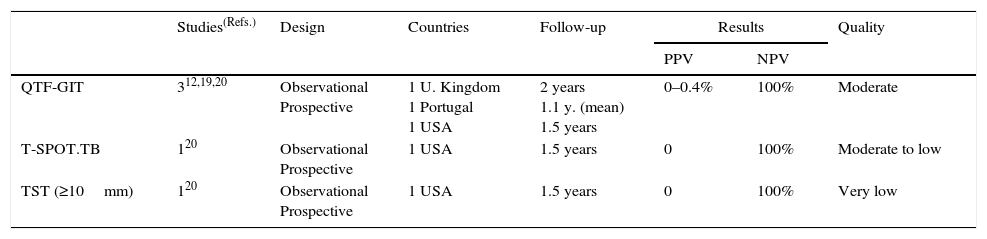
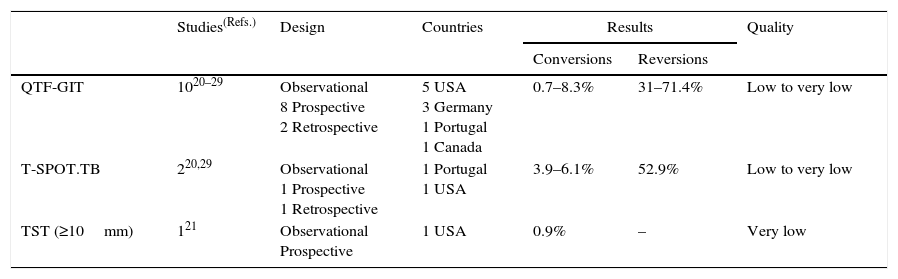
Summary of the evidence. Three prospective observational studies assessed the predictive values of IGRAs for the development of TB in health care workers in low-prevalence countries over one to three years; two with QTF-GIT13,20 and one with both the QTF-GIT and T-SPOT.TB.21 One of these studies also assessed the predictive value of the TST.21 The three studies were prospective. The PPVs for the development of TB ranged from 0% to 0.4% for QTF-GIT (891 subjects), 0% for T-SPOT.TB (144 subjects from only one study), and 0% for a TST ≥10mm (or 5mm in immunosuppressed patients) (125 subjects from only one study). The corresponding NPVs were 100% for each of the QTF-GIT (4171 subjects), T-SPOT.TB (2274 subjects), and TST (2293 subjects) (Table 3a). Ten studies assessed the conversion and reversion rates of IGRA20–29; eight did so with the QTF-GIT20,22–28 and two with both the QTF-GIT and T-SPOT.T.21,29 One study also assessed the conversions and reversions of the TST.21 All ten studies were observational, but eight were prospective20–26,29 and two were retrospective.27,28 Conversions ranged from 0.7% to 8.3% for QTF-GIT, from 3.9% to 6.1% for T-SPOT.TB, and were 0.9% for the TST. In the only study evaluating both IGRAs and the TST in the same population,21 the conversion rates were 8.3%, 6.1%, and 0.9% for the QTF-GIT, T-SPOT.TB, and TST ≥10mm (or 5mm in immunosuppressed patients), respectively. Reversions ranged from 31% to 71% for the QTF-GIT and were 52.9% for the T-SPOT.TB in one study. There were no studies assessing the reversion rates of the TST (Table 3b).
Table 3a.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in health care workers (development of tuberculosis).
Studies(Refs.) Design Countries Follow-up Results Quality PPV NPV QTF-GIT 312,19,20 Observational
Prospective1 U. Kingdom
1 Portugal
1 USA2 years
1.1 y. (mean)
1.5 years0–0.4% 100% Moderate T-SPOT.TB 120 Observational
Prospective1 USA 1.5 years 0 100% Moderate to low TST (≥10mm) 120 Observational
Prospective1 USA 1.5 years 0 100% Very low TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
Table 3b.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in health care workers (conversions and reversions).
Studies(Refs.) Design Countries Results Quality Conversions Reversions QTF-GIT 1020–29 Observational
8 Prospective
2 Retrospective5 USA
3 Germany
1 Portugal
1 Canada0.7–8.3% 31–71.4% Low to very low T-SPOT.TB 220,29 Observational
1 Prospective
1 Retrospective1 Portugal
1 USA3.9–6.1% 52.9% Low to very low TST (≥10mm) 121 Observational
Prospective1 USA 0.9% – Very low TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
- 2.
Quality of the evidence. The overall quality of the evidence was moderate for the evaluation of the predictive values of IGRAs, and was very low for the TST. The main limiting factors were the lack of direct evidence for the evaluated outcomes and the imprecision caused by the small number of cases.
- 3.
Justification. In cases with a negative result (NPV 100%), both the TST and the IGRAs offer high security against the development of active TB. The extended availability of the TST, together with the high rates of conversions and reversions of IGRAs, justify the use of the TST as the initial test for the screening of health care workers. However, confirming positive TST results with an IGRA will improve the selection of subjects for therapy and should reduce the number of treatments prescribed.18,30
Although the lower direct cost would favor the use of TST, the overall costs of including an IGRA in the strategy would be lower after including indirect cost savings derived from reduced treatment, as a cost-effectiveness study has suggested.19 However, the costs of the IGRAs would be excessive against their use in the periodic screening of health care workers.
Use of IGRAs for the diagnosis of active TB in children younger than 5 yearsRecommendation: In children younger than 5 years suspected of having active TB, the panel suggests using both the TST and an IGRA to complement the microbiologic and radiographic studies for the diagnosis of active TB. (Weak recommendation; very low quality of evidence.)
- 1.
Summary of the evidence. Only one study has assessed the yield of IGRAs and the TST for the diagnosis of active TB in children under five years of age in low-prevalence countries.31 It was prospective in design and compared IGRAs and the TST head-to-head. For the QTF-GIT (68 subjects), the sensitivity and specificity for diagnosing active TB were 93% (95% CI 77–99%) and 100% (95% CI 91–100%), respectively; for the T-SPOT.TB (68 subjects), were 93% (95% CI 77–99%) and 98% (95% CI 87–100%), respectively; and for the TST (73 subjects), were 100% (95% CI 88–100%) and 58% (95% CI 42–73%), respectively.
- 2.
Quality of the evidence. The overall quality of the evidence was very low. The main limiting factors were the lack of direct evidence of the outcomes evaluated and the imprecision due to the small number of cases.
- 3.
Justification. Detection of TB infection (by TST or IGRA) as a complementary test for the diagnosis of active disease is important to ensure early treatment in children, where the yields are otherwise poor from smear stains and cultures for mycobacteria. The panel prioritized the need to detect all cases of active TB in children with suspected disease, giving less weight to the potential increase in unnecessary treatment. Since children are particularly vulnerable, the panel deemed that the advantages of the combination of both tests would surpass any associated inconvenience or additional cost. In fact, some members argued for the use of both IGRAs simultaneously, if available.
Recommendation: In children older than 5 years, the panel suggests initiating contact tracing with the TST, but in BCG-vaccinated contacts, the positive TST results need to be confirmed with an IGRA performed on the TST reading date. (Weak recommendation; very low quality of evidence.)
In children younger than 5 years, the panel suggests testing with both the TST and an IGRA, regardless of prior BCG vaccination. (Weak recommendation; low quality of evidence.)
- 1.
Summary of the evidence. Two observational studies assessed the predictive values of the QTF-GIT and the TST for the development of TB in children of high-risk contacts in low-prevalence countries.14,32 The PPVs for the development of TB were 5.3% and 75% for the QTF-GIT (32 subjects), and were 4% and 14% for the TST (82 subjects). The corresponding NPVs were 100% for both the QTF-GIT (135 subjects) and the TST (84 subjects) (Table 4).
Table 4.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in contact tracing of tuberculosis in children.
Studies(Refs.) Design Countries Follow-up Results Quality PPV NPV QTF-GIT 214,32 Observational
2 prospective1 Germany
1 Spain3.8 years (mean) 5.3–75% 100% Very low TST 4–14% 100% TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
- 2.
Quality of the evidence. The overall quality of the evidence was very low. The main limiting factor was the lack of direct evidence of the outcomes evaluated; moreover, one of the studies14 did not provide the number of children younger than 5 years. The results were also imprecise because of the small number of subjects.
- 3.
Justification. The rationale for using two different strategies in children depending on the age threshold of five years was that the sensitivity of IGRAs was considered worse in children under five. In children older than five years, the extended availability of the TST justifies its use as the first-choice test for screening. In BCG-vaccinated subjects with positive TST results, validation with an IGRA will help to better select those who require therapy, which should reduce unnecessary treatments. In children younger than five years, the panel suggests performing the TST and an IGRA because of the importance of maximizing detection of those infected, and that are at high risk of progression to active disease. The panel deemed that the potential advantages would surpass the inconveniences and additional costs.
Recommendation. The panel suggests using both the TST and an IGRA to screen for TB infection in people with HIV infection. (Weak recommendation; low quality of evidence.)
In people with HIV and a CD4-cell count <200/mL, the panel suggests using only an IGRA. (Weak recommendation; very low quality of evidence.)
- 1.
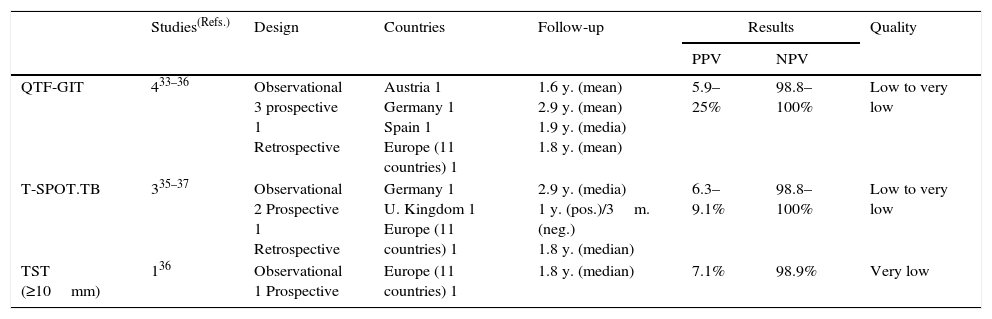
Summary of the evidence. Five observational studies have assessed the predictive values of IGRAs for the development of TB in HIV-infected people in low-prevalence countries: two with the QTF-GIT only,33,34 two with both the QTF-GIT and T-SPOT.TB,35,36 and one with the T-SPOT.TB only.37 Four studies were prospective33,34,36,37 and one was retrospective.35 One study also assessed the predictive values of the TST.36 The PPVs for the development of TB ranged from 5.9% to 25% for the QTF-GIT (888 subjects), from 6.3% to 9.1% for the T-SPOT.TB (133 subjects), and was 7.1% for the TST (42 subjects). The corresponding NPVs were 98.8–100% for the QTF-GIT (1524 subjects), 98.8–100% for the T-SPOT.TB (684 subjects), and 98.9% for the TST (584 subjects) (Table 5).
Table 5.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in people infected with HIV.
Studies(Refs.) Design Countries Follow-up Results Quality PPV NPV QTF-GIT 433–36 Observational
3 prospective
1 RetrospectiveAustria 1
Germany 1
Spain 1
Europe (11 countries) 11.6 y. (mean)
2.9 y. (mean)
1.9 y. (media)
1.8 y. (mean)5.9–25% 98.8–100% Low to very low T-SPOT.TB 335–37 Observational
2 Prospective
1 RetrospectiveGermany 1
U. Kingdom 1
Europe (11 countries) 12.9 y. (media)
1 y. (pos.)/3m. (neg.)
1.8 y. (median)6.3–9.1% 98.8–100% Low to very low TST (≥10mm) 136 Observational
1 ProspectiveEurope (11 countries) 1 1.8 y. (median) 7.1% 98.9% Very low TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
The review identified three systematic reviews that included studies evaluating the use of IGRAs in low-prevalence countries.38–40 The panel selected the newest and most comprehensive review from which to extract sensitivity and specificity data for the detection of active TB and to assess the impact of immunosuppression on the yield of IGRAs in HIV-infected people.39 The sensitivities and specificities were 61% and 72% for the QTF-GIT, and 65% and 70% for the T-SPOT.TB, respectively. In both IGRAs, an increase in the number of indeterminate results was observed with a CD4 count <200cells/mL (8.1% for the QTF-GIT and 4% for the T-SPOT.TB).
- 2.
Quality of the evidence. The overall quality of the evidence was low to very low. The main limiting factors were the lack of direct evidence of the outcomes evaluated, the imprecision due to the small number of subjects, and the risk of bias due to the design of the studies.
- 3.
Justification. The panel prioritized the need to detect all cases of infection, while relativizing the potential increase in unnecessary treatment. Since people infected with HIV are at high risk of progression, the panel deemed that the advantages of using both tests would surpass the inconveniences and additional costs. As justification for screening people with a CD4 count <200 cells/mL with an IGRA exclusively, the panel considers that the TST rarely identifies infection that has not been detected by IGRAs in patients with advanced immunosuppression.
The implementation of this recommendation is unlikely to be problematic because most HIV-infected patients are treated in specialist hospital units with appropriate laboratory facilities.
Use of IGRAs to screen for TB infection in patients with chronic inflammatory disease before treatment with biologic therapiesRecommendation: The panel suggests using both the TST and an IGRA to screen for TB infection in patients with chronic inflammatory disease before starting biological therapy. (Weak recommendation; low to very low quality of evidence.)
- 1.
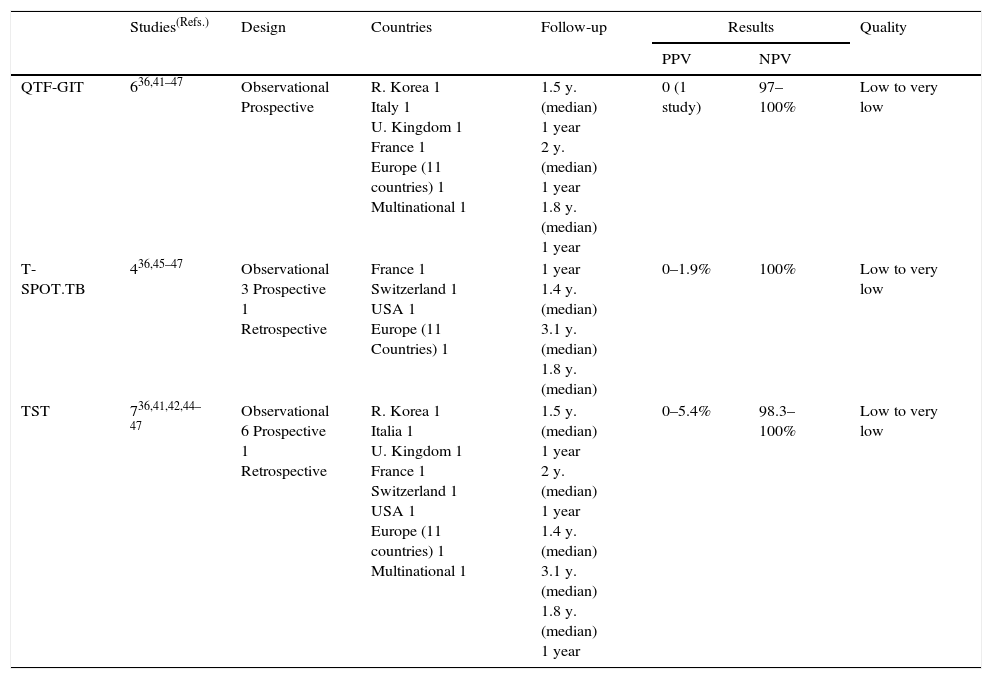
Summary of the evidence. Eight observational studies assessed the predictive values of IGRAs for the development of TB in patients treated with biological therapies in TB low-prevalence countries: four with the QTF-GIT only,41–44 two with both the QTF-GIT and T-SPOT.TB,36,45 and two with the T-SPOT.TB only.46,47 Only two of them assessed the PPV in a small number of patients.36,47 Seven studies were prospective36,41–45,47 and one was retrospective.46 Seven studies also assessed the predictive values of the TST for the development of TB.36,41,42,44–47 The PPVs for the development of TB were 0% for the QTF-GIT (20 subjects), between 0% and 1.9% for the T-SPOT.TB (131 subjects), and between 0% and 5.4% for the TST (237 subjects). The corresponding NPVs were 97–100% for the QTF-GIT (2535 subjects), 100% for the T-SPOT.TB (over 563 subjects), and 98.3–100% for the TST (2468 subjects) (Table 6).
Table 6.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in patients with chronic inflammatory diseases, prior to treatment with biologic therapies.
Studies(Refs.) Design Countries Follow-up Results Quality PPV NPV QTF-GIT 636,41–47 Observational
ProspectiveR. Korea 1
Italy 1
U. Kingdom 1
France 1
Europe (11 countries) 1
Multinational 11.5 y. (median)
1 year
2 y. (median)
1 year
1.8 y. (median)
1 year0 (1 study) 97–100% Low to very low T-SPOT.TB 436,45–47 Observational
3 Prospective
1 RetrospectiveFrance 1
Switzerland 1
USA 1
Europe (11 Countries) 11 year
1.4 y. (median)
3.1 y. (median)
1.8 y. (median)0–1.9% 100% Low to very low TST 736,41,42,44–47 Observational
6 Prospective
1 RetrospectiveR. Korea 1
Italia 1
U. Kingdom 1
France 1
Switzerland 1
USA 1
Europe (11 countries) 1
Multinational 11.5 y. (median)
1 year
2 y. (median)
1 year
1.4 y. (median)
3.1 y. (median)
1.8 y. (median)
1 year0–5.4% 98.3–100% Low to very low TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
Five studies assessed the impact of immunosuppression on the test results (both IGRAs and the TST).48–52 Four studies, with a total of 747 cases, looked at the prevalence of indeterminate results with the QTF-GIT.48,49,51,52 In all four studies, there was an association between immunosuppression and risk of indeterminate results, while one showed a tight dose–effect correlation with prednisone.48
- 2.
Quality of the evidence. The overall quality of the evidence was low to very low. The main limiting factors were the lack of direct evidence of the outcomes evaluated and the risk of bias derived from the design of the studies.
- 3.
Justification. The panel prioritized the need to detect all cases of infection, while relativizing the potential increase in unnecessary treatment. Patients treated with biological therapies are at an increased risk of developing active TB, and when they do, it is frequently as a disseminated or severe; therefore, the panel deemed that the advantages of the combination of both tests would surpass the inconveniences and additional costs. The scarcity of evidence precluded specific recommendations for different patient groups, such as those with rheumatic diseases, psoriasis, and inflammatory bowel disease.
The implementation of this recommendation is unlikely to be problematic because most patients requiring biological therapies are treated in specialist hospital units with appropriate laboratory facilities, and the resources needed to implement IGRA testing are minimal.
Use of IGRAs to screen for TB infection before transplantationRecommendation: The panel suggests using both the TST and an IGRA to screen for TB infection in patients due to undergo solid organ or allogeneic hematopoietic stem cell transplantation. (Weak recommendation; very low quality of evidence.)
- 1.
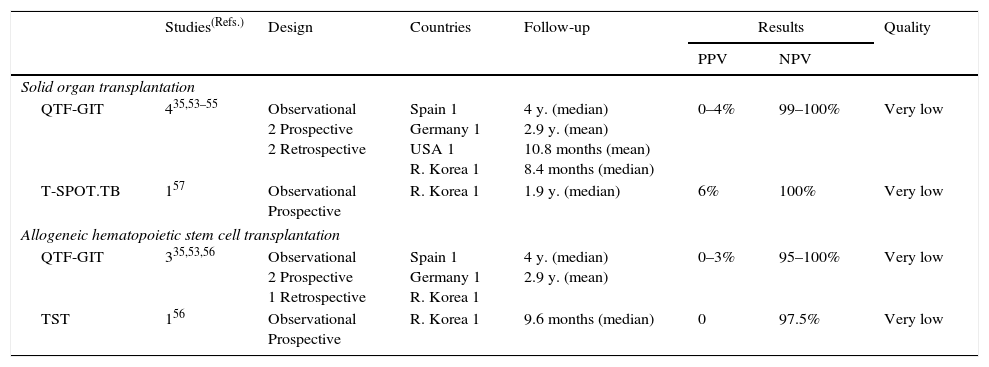
Summary of the evidence. Three observational studies assessed the predictive values of IGRAs for the development of TB in patients undergoing solid organ transplantation (SOT) or allogeneic hematopoietic stem cell transplantation (HSCT) in low-prevalence countries (all three studies used the QTF-GIT).35,53,54 In addition, three studies have assessed these predictive values in an intermediate- to high-prevalence country (two with QTF-GIT,55,56 and only one study was performed using the T-SPOT.TB).57 One study also assessed the predictive values of the TST for the development of TB.56 Four studies were prospective53,55–57 and two were retrospective.35,54 The PPVs for the development of TB in SOT ranged from 0% to 4% for the QTF-GIT (47 subjects) and were 6% for T-SPOT.TB (71 subjects). The corresponding NPVs were 99–100% (954 subjects) and 100% (201 subjects). The PPVs in allogeneic HSCT ranged from 0% to 3% for the QTF-GIT (48 subjects) and 0% for the TST (39 subjects). The corresponding NPVs were 95–100% for the QTF-GIT (265 subjects) and 97.5% for the TST (205 subjects) (Table 7).
Table 7.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in patients prior undergoing sold organ or allogeneic hematopoietic stem cell transplant.
Studies(Refs.) Design Countries Follow-up Results Quality PPV NPV Solid organ transplantation QTF-GIT 435,53–55 Observational
2 Prospective
2 RetrospectiveSpain 1
Germany 1
USA 1
R. Korea 14 y. (median)
2.9 y. (mean)
10.8 months (mean)
8.4 months (median)0–4% 99–100% Very low T-SPOT.TB 157 Observational
ProspectiveR. Korea 1 1.9 y. (median) 6% 100% Very low Allogeneic hematopoietic stem cell transplantation QTF-GIT 335,53,56 Observational
2 Prospective
1 RetrospectiveSpain 1
Germany 1
R. Korea 14 y. (median)
2.9 y. (mean)0–3% 95–100% Very low TST 156 Observational
ProspectiveR. Korea 1 9.6 months (median) 0 97.5% Very low TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube version assay.
- 2.
Quality of the evidence. The overall quality of the evidence was very low. The main limiting factors were the lack of direct evidence of the outcomes evaluated and the use of data from intermediate- to high-incidence settings. Confidence in the results was also limited by short follow-up periods, imprecision, and risk of bias in the studies designs.
- 3.
Justification. The panel prioritized the need to detect all cases of infection, while relativizing the potential increase in unnecessary treatment. Patients with SOT and HSCT who are latently infected are at high risk of developing active TB; therefore, the panel deemed that the advantages of using both tests would surpassed the inconveniences and additional costs. However, although the available evidence allowed only for an overall recommendation for transplants as a whole, the panel decided to focus their recommendation on allogeneic HSCT because the risk of TB in autologous transplant is not clearly higher than that of the general population.58
The implementation of this recommendation is unlikely to be problematic because most transplant candidates are treated in specialist hospital units with appropriate laboratory facilities, and the resources needed to implement IGRA testing are minimal.
Use of IGRAs for the diagnosis of active TBRecommendation: The panel recommends against using the IGRAs or TST as a standalone tests for the diagnosis of active TB. (Strong recommendation; low quality of evidence.)
The panel suggests using an IGRA as a proof of TB infection to support the diagnosis in cases with well-founded suspicion of active disease. (Weak recommendation; low quality of evidence.)
- 1.
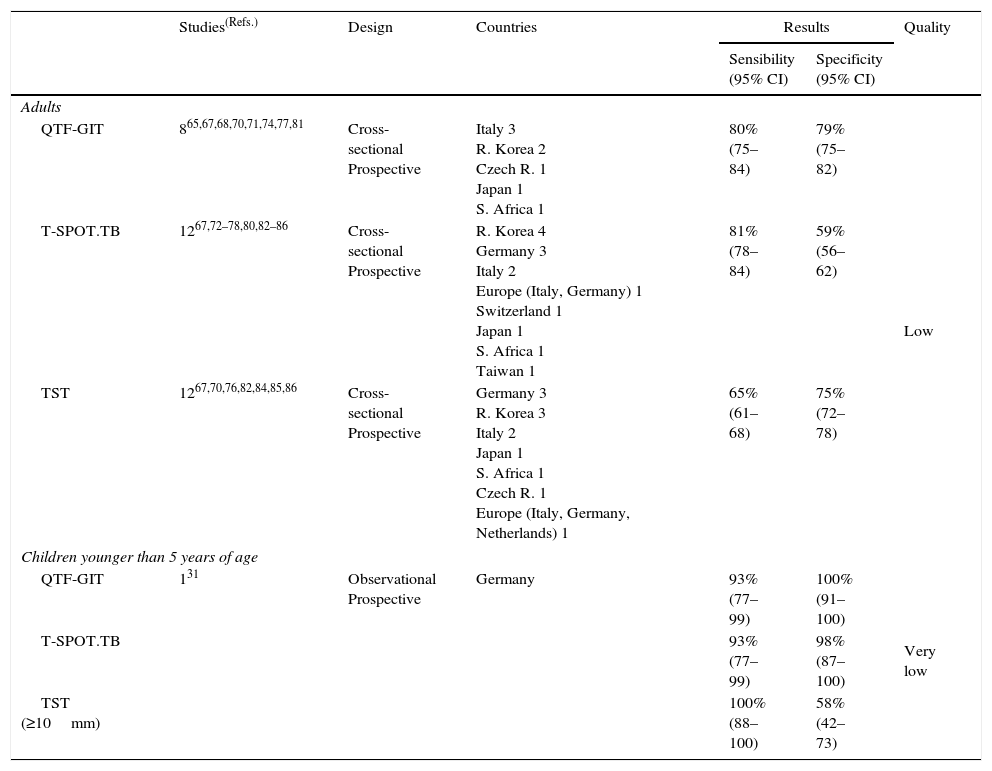
Summary of the evidence. The review identified five systematic reviews assessing the sensitivity and specificity of IGRAs for detecting active TB.59–63 The panel chose a review that included 20 studies,62 of which eleven assessed the QTF-GIT64–71,74,77,81 (four provided data in non-blood fluids64–66,69), and 16 assessed the T-SPOT.TB64,66,72–80,82–86 (eight provided data for non-blood fluids64,66,72,73,76,80,83,84). Twelve studies also assessed the sensitivity and specificity of the TST.67,70–76,82,84–86
The sensitivities and specificities in the blood were 80% and 79% for the QTF-GIT and 81% and 59% for the T-SPOT.TB, respectively. The corresponding values in non-blood fluids were 48% and 82%, and 88% and 82% for the QTF-GIT, the T-SPOT.TB and the TST respectively. The sensitivity and specificity for the TST were 65% and 75%, respectively (Tables 8a and 8b).
Table 8a.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in blood in the diagnosis of active tuberculosis in adults and children under 5 years of age.
Studies(Refs.) Design Countries Results Quality Sensibility
(95% CI)Specificity
(95% CI)Adults QTF-GIT 865,67,68,70,71,74,77,81 Cross-sectional
ProspectiveItaly 3
R. Korea 2
Czech R. 1
Japan 1
S. Africa 180%
(75–84)79%
(75–82)Low T-SPOT.TB 1267,72–78,80,82–86 Cross-sectional
ProspectiveR. Korea 4
Germany 3
Italy 2
Europe (Italy, Germany) 1
Switzerland 1
Japan 1
S. Africa 1
Taiwan 181%
(78–84)59%
(56–62)TST 1267,70,76,82,84,85,86 Cross-sectional
ProspectiveGermany 3
R. Korea 3
Italy 2
Japan 1
S. Africa 1
Czech R. 1
Europe (Italy, Germany, Netherlands) 165%
(61–68)75%
(72–78)Children younger than 5 years of age QTF-GIT 131 Observational
ProspectiveGermany 93%
(77–99)100%
(91–100)Very low T-SPOT.TB 93%
(77–99)98%
(87–100)TST (≥10mm) 100%
(88–100)58%
(42–73)TST, Tuberculin skin test; IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold in-tube assay.
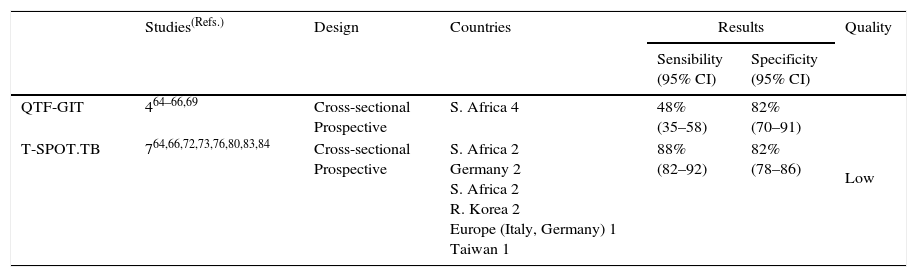
Table 8b.Synthesis and quality of the evidence used for the recommendation on the use of IGRAs in non-blood fluids in the diagnosis of active tuberculosis in adults.
Studies(Refs.) Design Countries Results Quality Sensibility
(95% CI)Specificity
(95% CI)QTF-GIT 464–66,69 Cross-sectional
ProspectiveS. Africa 4 48%
(35–58)82%
(70–91)Low T-SPOT.TB 764,66,72,73,76,80,83,84 Cross-sectional
ProspectiveS. Africa 2
Germany 2
S. Africa 2
R. Korea 2
Europe (Italy, Germany) 1
Taiwan 188%
(82–92)82%
(78–86)IGRAs, Interferon-gamma release assays; QFT-GIT, QuantiFERON®-TB Gold In-tube assay.
- 2.
Quality of the evidence. The overall quality of the evidence was low. The main limiting factors were the lack of direct evidence of the outcomes evaluated, the use of data from high-incidence settings, and the wide range of patients (adult, child, immunocompetent, and immunosuppressed patients).
- 3.
Justification. Although the quality of evidence was low, it was consistent with regard the sub-optimal performance of all tests when used as standalone tests for the diagnosis of active TB. The dangers of making a diagnosis of TB based exclusively on either TSTs or IGRAs exceed the potential benefits. Nonetheless, the panel considered that a positive test result support the diagnosis of active TB in a patient in whom there was a well-founded suspicion of active disease. A positive test result has been recognized as an independent risk factor for active TB in immunosuppressed patients, and particularly those infected with HIV.60 The panel deemed that the T-SPOT.TB could be considered in particular cases when using non-blood fluids (bronchoalveolar lavage, cerebrospinal, and pleural fluids).
The panel noted the following additional considerations:
- 1.
The resources needed for IGRAs to be implemented are low. However, implementation in non-urban settings, where a close laboratory for rapid handling of samples is required, may be difficult. This was acknowledged to be particularly important when using the T-SPOT.TB, which should be performed within the first 24h, or in the case of the T-SPOT.TB® Extend, within 48h after incubation.87 Furthermore, the need for venous punctures in large collectives and requirement of a reference laboratory to handle sample batches, could make the use of IGRAs difficult for contact tracing at a community level.
- 2.
Although there was no attempt to distinguish between the two IGRAs in the final guidelines, some panel members favored the use of the T-SPOT.TB in HIV-infected (CD4<200 cells/mL) and children, based on the perception of higher sensitivity over the QTF-GIT. However, it was acknowledged that the available evidence showed no clear difference between the two tests.39,88
- 3.
An emphasis was placed on the importance of screening before any underlying disease becomes too advanced, because this can compromise the yield of IGRAs. This was particularly relevant for patients with HIV and for candidates for transplantation or biologic therapies. Likewise, performing the QTF-GIT when leucopenia is present (particularly in HSCT) should be avoided because it increases the likelihood of indeterminate results.
- 4.
There was no specific recommendation on whether the TST and an IGRA should be performed simultaneously or sequentially (start with TST and follow with an IGRA in case of negativity) when both tests are recommended. The panel considers that the choice of strategy should be guided by the circumstances of a given patient and the singularities of each center.
- 5.
Regarding the use of IGRAs in the diagnostic work-up of TB, the panel emphasizes:
- •
Ensure rational use of IGRAs by using them only when there is a well-founded clinical suspicion.
- •
A positive IGRA in patients with compatible symptoms, particularly in immunosuppressed patients and children, must prompt active investigation for active TB.
- •
It remains important to establish a diagnosis of TB by conventional microbiologic and/or molecular methods.
- •
- 6.
If a baseline test is negative (IGRAs or the TST) in HIV-infected patients with very low CD4 cell counts, retesting once immunity has improved with antiretroviral therapy. This approach may be useful to detect additional positive cases.
- 7.
In general, an IGRA is preferred when testing those who are unlikely to return for TST reading, such as patients with alcoholism, drug abuse disorders, and the homeless. In these groups, the use of IGRAs may increase test completion rates.
- 8.
The panel emphasizes the need for health care workers to adhere to TB prevention protocols in place.
The panel does not expect major limitations to the applicability of these guidelines in Spain because IGRAs are widely available, particularly the QTF-GIT, and because the cost burden would be easily assumed by the public health system. However, the panel anticipates more difficulty when implementing community contact studies (e.g., in schools, workplaces, and other settings). In such settings, the need for multiple venous punctures and sample collections may be regarded as added inconveniences by public health authorities.
Most recommendations in these guidelines are weak mainly due to the lack of good quality evidence for anticipating and balancing the clinical benefits and disadvantages of TST or IGRA testing. In the case of weak recommendations, clinicians should recognize that different choices might be appropriate for different patients. Policymaking in this context requires substantial debate and the involvement of many stakeholders, and so health policies are likely to vary between regions.
It is unlikely that in the near future the existing knowledge gaps on the field could be overcome. Not only are there difficulties in conducting appropriate clinical trials but also improvements are expected in the marketed IGRAs, platforms, and latency antigens that will presumably reduce the interest in conducting studies with the current IGRAs. Recently, two prospective longitudinal studies with a large cohort of adults and children have confirmed the poor ability of IGRAs to predict the development of active TB, while confirming their high NPVs; nevertheless, the IGRAs were still shown to be superior to the TST.32,89 In the meantime, the present evidence-based guidelines try to support decision making for practitioners who use IGRAs in daily clinical practice as well as for stakeholders in public health.
Updating of the guidelinesThe panel considers that these guidelines should be updated within five years of their publication, or earlier if relevant information becomes available.
Funding and editorial independenceThe panel received no funding from any for-profit companies or organizations. The Spanish Society of Respiratory Diseases and Thoracic Surgery (SEPAR) hosted the in-person meetings, while SEPAR and the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) funded the travel and housing costs for members to attend the meetings. Neither the SEIMC nor SEPAR have direct or indirect influence on the elaboration of the guidelines.
Conflict of interest statementAll the authors provided a conflict of interest statement. M.S. declared the following conflicts of interest: he received a fee for speaking at two conferences sponsored by Inverness Medical Iberica, S.A.U., supplier of the QuantiFERON®-TB Gold In-tube in Spain, and he is the PI of a Clinical Trial assessing QuantiFERON®-TB Gold In-tube in contact-tracing, which was supplied with blood collection tubes by Cellestis, Inc. (Carnegie, Australia). Any declared conflicts of interest were not deemed relevant to preclude involvement in the deliberations of the panel.
External reviewers: Fernando Alcaide (Service of Microbiology, Bellvitge University Hospital, University of Barcelona, L’Hospitalet de Llobregat, Barcelona), Ángel Domínguez (Service of Infectious Diseases, Hospital Virgen Macarena, Sevilla), Jordi Dorca (Service of Pneumology, Bellvitge University Hospital, University of Barcelona, L’Hospitalet de Llobregat, Barcelona), María J. Mellado Peña (Service of General Pediatrics and Infectious and Tropical Diseases, Hospital Universitario Infantil La Paz, Madrid), Juan J. Palacios (Regional Laboratory for Mycobacteria, Hospital Universitario Central de Asturias, Oviedo, Asturias), Antonio Rivero (Service of Infectious Diseases, Hospital Reina Sofía, Córdoba), Anna Rodés (Program of Prevention and Control of Tuberculosis, Public Health Agency of Catalonia), Juan Ruíz Manzano (Service of Pneumology, Hospital Germans Trias i Pujol, Badalona, Barcelona).
The working group thanks SEPAR for providing the venue and infrastructure for the panel's meetings.
- 1.
Miguel Santin. Service of Infectious Diseases, Bellvitge University Hospital-IDIBELL, C/Feixa Llarga, s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain; Department of Clinical Sciences, University of Barcelona, Barcelona, Spain.
- 2.
José-María García-García. Clinical Unit of Pneumology, Hospital San Agustín, C/Camino de Heros, 4, 33400, Avilés, Asturias, Spain.
- 3.
David Rigau. Iberoamerican Cochrane Centre – Biomedical Research Institute Sant Pau (IIB Sant Pau), C/Sant Antoni Maria Claret, 167, 08025, Barcelona, Spain.
- 4.
Neus Altet. Unit of Tuberculosis Valle de Hebron, Institute of Research in Primary Assistance Jordi Gol, Unidad Clínica de Tratamiento Directamente Observado “Servicios Clínicos”, C/Garcia Mariño, 4, 08022, Barcelona, Spain.
- 5.
Luis Anibarro. Unit of Tuberculosis, Infectious Diseases, Service of Internal Medicine, Complexo Hospitalario de Pontevedra, 36001, Pontevedra, Spain.
- 6.
Irma Casas. Service of Preventive Medicine, Hospital Gremans Trias i Pujol, Carretera del Canyet, s/n, 08916 Badalona, Barcelona, Spain; Department of Pediatrics, Obstetrics-Gynecology and Preventive Medicine, Universidad Autónoma de Barcelona, Barcelona, Spain.
- 7.
Nuria Díez. Service of Pediatrics, Hospital Universitario Rio Hortega, Calle Dulzaina, 2, 47012, Valladolid, Spain.
- 8.
Mercedes García-Gasalla. Service of Internal Medicine/Infectious Diseases, Hospital Son Llàtzer, Carretera de Manacor, 4, 07198, Palma de Mallorca, Spain.
- 9.
Xavier Martínez-Lacasa. Unit of Tuberculosis Control, Hospital Universitario Mutua de Terrassa, Pza. Dr Robert, 5, 08224, Terrassa, Barcelona, Spain.
- 10.
Antón Penas. Unit of Tuberculosis, Service of Pneumology, Hospital Universitario Lucus Augusti, C/San Cibrao, 27003, Lugo, Spain.
- 11.
Elvira Pérez-Escolano. Service of Internal Medicine, Infectious Diseases, Hospital de Jerez, Ctra. Circunvalación, s/n, 11407, Jerez de la Frontera, Cádiz, Spain.
- 12.
Francisca Sánchez. Service of Infectious Diseases, Instituto Mar de Investigaciones Médicas (IMIM), Hospital del Mar; Dr. Aiguader, 88, 08003 Barcelona, Spain.
- 13.
José Domínguez. Service of Microbiology, Instituto de Investigación Trias i Pujol, Hospital Gremans Trias i Pujol, Carretera del Canyet, s/n, 08916 Badalona, Barcelona, Spain; Department of Genetics and Microbiology, Universidad Autónoma de Barcelona, Spain; CIBER Respiratory Diseases.
MEDLINE (PubMed; 13.03.2013)
| #2 | "Interferon-gamma Release Tests"[Mesh] | 199 |
| #3 | Interferon-Gamma Release Assay*[tiab] | 629 |
| #4 | Interferon-Gamma Assay*[tiab] | 159 |
| #5 | Interferon-Gamma test*[tiab] | 23 |
| #6 | IGRA[tiab] | 319 |
| #7 | IGRAS[tiab] | 242 |
| #8 | QuantiFERON[tiab] | 802 |
| #9 | QFT[ti] | 16 |
| #10 | T-SPOT.TB[tiab] | 239 |
| #11 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 1438 |
| #12 | systematic[sb] | 197472 |
| #13 | systematic review[tiab] OR Meta-Analysis[pt] OR Meta-Analysis as Topic[mh] OR Meta-Analys*[tiab] OR "Cochrane Database Syst Rev"[Journal:__jrid21711] | 95337 |
| #15 | #11 AND #13 | 38 |
| #16 | #11 AND #12 | 60 |
| #17 | #15 OR #16 | 63 |
| #18 | #11 NOT #17 | 1375 |
EMBASE (Embase Classic+Embase <1947 to 2013 Week 10>; 13.03.2013)
- 1.
exp interferon gamma release assay/ (657)
- 2.
Interferon-Gamma Release Assay*.ti,ab. (592)
- 3.
Interferon-Gamma Assay*.ti,ab. (107)
- 4.
Interferon-Gamma test*.ti,ab. (15)
- 5.
IGRA.ti,ab. (528)
- 6.
IGRAS.ti,ab. (366)
- 7.
QuantiFERON.ti,ab. (1234)
- 8.
QFT.ti. (25)
- 9.
T-SPOT?TB.ti,ab. (295)
- 10.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (2100)
- 11.
exp "systematic review"/ (58051)
- 12.
meta analysis/ (69369)
- 13.
systematic review.ti. (29199)
- 14.
meta?nalysis.ti. (807)
- 15.
meta analys*.ti. (31304)
- 16.
11 or 12 or 13 or 14 or 15 (117109)
- 17.
10 and 16 (49)
- 18.
10 not 17 (2051)