Asthma and chronic rhinosinusitis with nasal polyps (CRSwNP) are two common chronic respiratory conditions that frequently coexist and significantly impact the quality of life. The relationship between asthma and CRSwNP is complex and multifactorial, involving shared inflammatory pathways, genetic predispositions, and environmental factors.1 Epidemiological studies have consistently demonstrated a higher prevalence of CRSwNP in asthmatics compared to the general population.2,3 Furthermore, the presence of CRSwNP in asthma is associated with more severe asthma symptoms, increased exacerbation rates, and poorer control.4–8 The underlying pathophysiological mechanisms linking asthma and CRSwNP involve a type 2 inflammatory response characterized by the release of cytokines such as interleukin (IL)-4, IL-5, and IL-13, which promote eosinophilic inflammation and tissue remodelling in both the respiratory and sinonasal mucosa.1 Recent advances in understanding the molecular pathways driving type 2 inflammation have led to the development of targeted biologic therapies that have shown efficacy in treating both asthma and CRSwNP. Biologic agents targeting IL-4, IL-5, and IL-13 have demonstrated promising results in reducing nasal polyp burden, improving sinonasal symptoms, and enhancing asthma control in patients with comorbid CRSwNP and asthma.4–8
In order to facilitate the diagnosis and treatment of both diseases, there are different clinical guidelines, but they address each pathology individually: Spanish Guideline on the Management of Asthma (GEMA) for asthma and Multidisciplinary Consensus Guideline on CRSwNP (POLINA) for CRSwNP.7,8 However, in routine clinical practice, different scenarios can be found that result from the combination of both diseases (severe asthma previously controlled or not controlled with biological treatment; CRSwNP previously operated or not operated). Given the lack of evidence in these scenarios, the selection of the most appropriate treatment for each case can be a challenge for less experienced healthcare professionals. In decision-making, patient input is essential. Their perspective provides valuable insights into individual needs and preferences, fostering shared decision-making. By incorporating patient viewpoints, healthcare professionals ensure treatment aligns with patient goals, promoting trust, satisfaction, and improved outcomes.
With the aim of helping to identify the possible clinical scenarios in this group of patients and to establish therapeutic recommendations for each scenario, a multidisciplinary group of experts was created, consisting of the project coordinators and some of the authors of GEMA and POLINA guidelines from the specialties of allergology, pulmonology and otorhinolaryngology. After several meetings, this group established and agreed the possible scenarios to be considered. Then, they were subdivided into several committees that collected information from those guidelines and made preliminary proposals about the corresponding therapeutic strategies for each scenario. It should be noted that, due to the lack of evidence in this regard, it is not possible to establish a level of evidence and degree of recommendation for each proposal. The present publication is the result of this work. No approval was required by any ethics committee, since it is an expert consensus without patient participation. At the time of writing, preparations were underway for reaching a consensus using Delphi methodology with another 40 experts who did not participate in the drafting of this work.
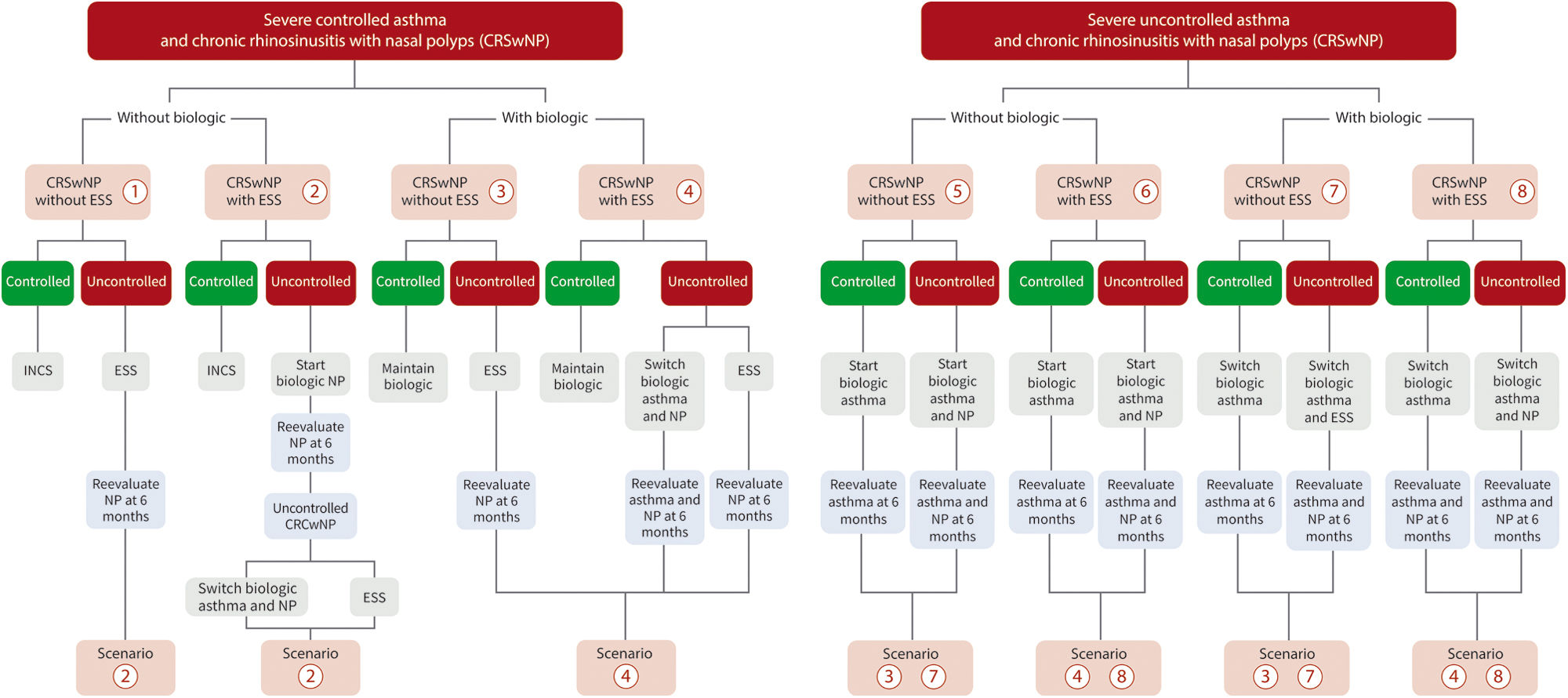
The expert group identified eight scenarios according to the control status of both severe asthma and CRSwNP (see Fig. 1 for details):
- (1)
Patient with severe asthma controlled without biologic treatment and CRSwNP (controlled or uncontrolled) without previous endoscopic sinus surgery (ESS): asthma treatment should be continued. For controlled CRSwNP, treatment with intranasal corticosteroids (INCS) should be maintained. For uncontrolled CRSwNP, ESS should be performed. Biologics approved to treat CRSwNP (omalizumab, mepolizumab and dupilumab) are indicated if the control is not achieved by ESS after 6 months.
- (2)
Patient with severe asthma controlled without biologic treatment and CRSwNP (controlled or uncontrolled) with previous ESS: Asthma treatment should be continued. For controlled CRSwNP, treatment with INCS should be maintained. For uncontrolled CRSwNP, biologic approved to treat CRSwNP should be started. If the control is not achieved after 6 months, a new ESS could be performed or the biologic could be switched to one of the other two alternatives.
- (3)
Patient with severe asthma controlled with biologic treatment and CRSwNP (controlled or uncontrolled) without previous ESS: Asthma treatment should remain unchanged whether CRSwNP is controlled or not. For controlled CRSwNP, the biologic should be maintained. For uncontrolled CRSwNP, the biologic of asthma should be maintained and ESS should be considered as the first option for CRSwNP.
- (4)
Patient with severe asthma controlled with biologic treatment and CRSwNP (controlled or uncontrolled) with previous ESS and recurrence of nasal polyps: Asthma biological treatment should be continued. For controlled CRSwNP, biologic established for asthma and INCS should be maintained. For uncontrolled CRSwNP, and in case of inadequate surgery, a reintervention should be considered. If the previous ESS was correct and recurrence is observed after 6 months, a change to another biologic approved to treat CRSwNP is indicated. As an alternative, surgical reintervention may be considered.
- (5)
Patient with severe uncontrolled asthma with maintenance treatment in step 5 of GEMA, candidate for biologic and CRSwNP (controlled or uncontrolled) without previous ESS: Asthma biological treatment should be considered. For controlled CRSwNP, the biologic established for asthma should be maintained. CRSwNP should be assessed 6 months after starting biologic. If uncontrolled CRSwNP continues, surgery is indicated before considering a switch to another biologic.
- (6)
Patient with severe uncontrolled asthma with maintenance treatment in step 5 of GEMA, candidate for biologic and CRSwNP (controlled or uncontrolled) with previous ESS: Asthma biological treatment should be considered. For controlled CRSwNP, the biologic should be maintained. For uncontrolled CRSwNP, biologic approved to treat CRSwNP should be started, with a review performed after 6 months. If control is not achieved, a new ESS could be performed or switch to another biologic.
- (7)
Patient with severe uncontrolled asthma with poor response to biologic treatment and CRSwNP (controlled or uncontrolled) without previous ESS: Switch to another biologic approved to treat both asthma and CRSwNP should be considered. For controlled CRSwNP, biologic should be maintained. If poor control of CRSwNP continues after 6 months with the new biologic for asthma, ESS is indicated before considering a swich to another biologic.
- (8)
Patient with severe uncontrolled asthma and CRSwNP with previous ESS, with biologic treatment, who does not respond to treatment in either of the two pathologies: Switch to a biologic approved to treat both asthma and CRSwNP; if asthma control is not achieved, consider bronchial thermoplasty (if available) and, as a last option, systemic corticosteroids (always at the lowest effective dose and for the shortest possible time). In case of uncontrolled CRSwNP, a new ESS should be considered. Bronchial thermoplasty should only be considered in patients with phenotypes not subsidiary to treatment with biologic or in those who have failed, as long as there are no contraindications for the technique and is applied in centres with experience.
Algorithms for the therapeutic management of patients with severe asthma and CRSwNP. This scheme is assumed once the patient is under optimal treatment according to GEMA 5.3 and POLINA 2.0 guidelines. ESS with sinus opening according to POLINA 2.0 guideline. CRSwNP: chronic rhinosinusitis with nasal polyps; ESS: endoscopic sinus surgery; INCS: intranasal corticosteroids; NP: nasal polyps.
Since it is not the purpose of our article to discuss the different treatment options, only the options recommended by consensus in the current scientific literature have been taken into account. The recommendations for each of these eight scenarios will be validated with a panel of experts using a Delphi methodology throughout May 2024. Pulmonologists, allergists, otorhinolaryngologists, and hospital pharmacists will participate in this validation. Then, the final recommendations will be included in the next update of GEMA and POLINA guidelines.
FundingThis work has received sponsorship from AstraZeneca.
Authors’ contributionsAll authors have been involved in the creation of the text and algorithm.
Conflicts of interestVP in the last three years received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer-Ingelheim, Chiesi, Gebro, GSK, Luminova-Medwell, and Sanofi; received help assistance to meeting travel from AstraZeneca and Chiesi; and acted as a consultant for AstraZeneca, Chiesi, GSK, and Menarini.
CCH has received honoraria for consultancy, projects, advisory boards and talks from AstraZeneca, GlaxoSmithKline, Sanofi, CINFA.
MBA received honoraria for speaking at sponsored meetings and advisory from AstraZeneca, Chiesi, Gebro, GSK, TEVA, and Sanofi; and received help assistance to meeting travel from Faes, AstraZeneca, Chiesi, and Sanofi.
CC in the last five years received honoraria for speaking at sponsored meetings from Chiesi, AstraZeneca, GSK, Sanofi, Novartis, Mylan, MSD, Takeda, and Menarini; received help assistance to meeting travel from Novartis, AstraZeneca, Temofischer, and Sanofi; and acted as a consultant for Mylan and AstraZeneca.
JE in the last three years received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer-Ingelheim, GSK, Janssen, and Vertex; received help assistance to meeting travel from AstraZeneca and Pfizer; and acted as a consultant for AstraZeneca and Novartis.
NG in the last three years received honoraria for speaking at sponsored meetings from Bayer, Novartis, GSK, and Sanofi; received help assistance to meeting travel from PharmaMar and Pfizer; and acted as a consultant for AstraZeneca and GSK.
RGP In the last three years received honoraria for speaking at sponsored meetings from Allergy Therapeutics, AstraZeneca, Diater, Gebro, GSK, Inmunotek, Leo-Pharma, and Sanofi; and acted as a consultant for AstraZeneca and GSK.
JMS has received honoraria for consultancy, projects, advisory boards, and talks from AstraZeneca, GSK, MSD, Novartis, Sanofi, and FAES.
JGS in the last three years received honoraria for speaking at sponsored meetings from AstraZeneca, GSK, Gebro, and Sanofi; received help assistance to meeting travel from AstraZeneca and Sanofi; and act as a consultant for AstraZeneca, Gebro, and Sanofi.
IA has been consultant for GSK, Sanofi, AstraZeneca, Menarini, Salvat, Storz, Olympus, Metronic, Galenus Health, Roche, Novartis, Viatris, and Cinfa.
The authors wish to thank the Research Unit at Luzán 5 (Madrid) for the design and coordination assistance; and Fernando Sánchez Barbero PhD for the support on the preparation of this manuscript.