COVID-19 pneumonia results in an impairment of the diaphragmatic musculature that influences the development of respiratory failure during the patient's hospitalization. Diaphragmatic ultrasound is a useful, non-invasive, and accessible tool for measuring the function of this muscle.
ObjectiveAssessing the morphological and functional ultrasound status of the diaphragm in patients admitted within the first 24h for COVID-related pneumonia and its association with hospital morbidity and mortality (NCT05805579).
Material and methodsObservational, prospective cohort study that included 68 patients admitted for COVID-19 pneumonia with respiratory failure. Diaphragmatic ultrasound was performed within the first 24h of admission to the pulmonology ward. Clinical, analytical, and ultrasound variables were collected: excursion, thickness, and diaphragmatic shortening fraction (DSF). DSF<20% was used to define diaphragmatic dysfunction (DD). Patients who showed favorable progression and were managed on the ward (HCONV) were compared to those who required admission to the respiratory monitoring unit (RMU).
ResultsA total of 68 patients were included, of which 22 (32.35%) were admitted to the RMU. Diaphragmatic excursion at maximum volume was higher in the HCONV group compared to the RMU group (58.41±17.83 vs. 50.03±16.23; p=0.123). Diaphragmatic dysfunction (DD) was observed in 21 (30.88%) patients, with a higher prevalence in the RMU group than in the HCONV group (15 (68.18%) vs. 6 (13.04%); p=0.0001). In the multivariate analysis, age and DSF at admission were the best predictors of failure to discharge.
ConclusionsPerforming diaphragmatic ultrasound to assess mobility and DSF within the first 24h of admission for COVID-19 pneumonia proves valuable in determining short-term progression and the need for admission to a respiratory monitoring unit.
La neumonía por COVID-19 provoca un deterioro de la musculatura diafragmática que influye en la aparición de insuficiencia respiratoria durante la hospitalización del paciente. La ecografía diafragmática es una técnica no invasiva accesible y útil para medir la función de este músculo.
ObjetivoEvaluar mediante ecografía el estado funcional y morfológico del diafragma en pacientes con neumonía por COVID durante las primeras 24 h de su ingreso y su asociación con la morbimortalidad intrahospitalaria (NCT05805579).
Material y métodosSe realizó un estudio prospectivo y observacional de una cohorte compuesta por 68 pacientes ingresados por neumonía por COVID-19 con insuficiencia respiratoria. La ecografía diafragmática se practicó durante las 24 h siguientes al ingreso en la planta de neumología. Se recopilaron variables clínicas, analíticas y ecográficas: desplazamiento, grosor y fracción de acortamiento diafragmático (FAD). Se utilizó una FAD < 20% como definición de disfunción diafragmática. Se comparó a los pacientes que evolucionaron favorablemente y recibieron tratamiento en planta (hospitalización convencional) con los pacientes que tuvieron que ser ingresados en la unidad de monitorización respiratoria (UMR).
ResultadosSe incluyó en el estudio a un total de 68 pacientes, de los cuales 22 ingresaron en la UMR (el 32,35%). El desplazamiento diafragmático con el volumen máximo fue más alto en el grupo de hospitalización convencional que en el grupo ingresado en UMR (58,41 ± 17,83 frente a 50,03 ± 16,23; p = 0,123). Presentaron disfunción diafragmática 21 pacientes (30,88%) y la prevalencia fue más alta en el grupo ingresado en UMR que en el de hospitalización convencional: 15 pacientes (68,18%) frente a 6 (13,04%); p = 0,0001. En el análisis multivariable, la edad y la FAD al ingreso son los factores que mejor predicen la imposibilidad del alta.
ConclusionesLa ecografía diafragmática para evaluar la movilidad y la FAD en las primeras 24 h del ingreso por neumonía por COVID-19 resulta valiosa para determinar la evolución a corto plazo y la necesidad de ingreso en una unidad de monitorización respiratoria.
In December 2019, dozens of cases of atypical pneumonia of unknown origin were diagnosed, with a possible suspected zoonosis in Wuhan, China.1 The pathogen was subsequently identified as a new coronavirus (SARS-Cov-2) with low lethality (2–3%) and a high infection rate. The onset of signs and symptoms of this disease occurs 5–6 days after contracting the infection, and the incubation period ranges from 1 to 14 days.2
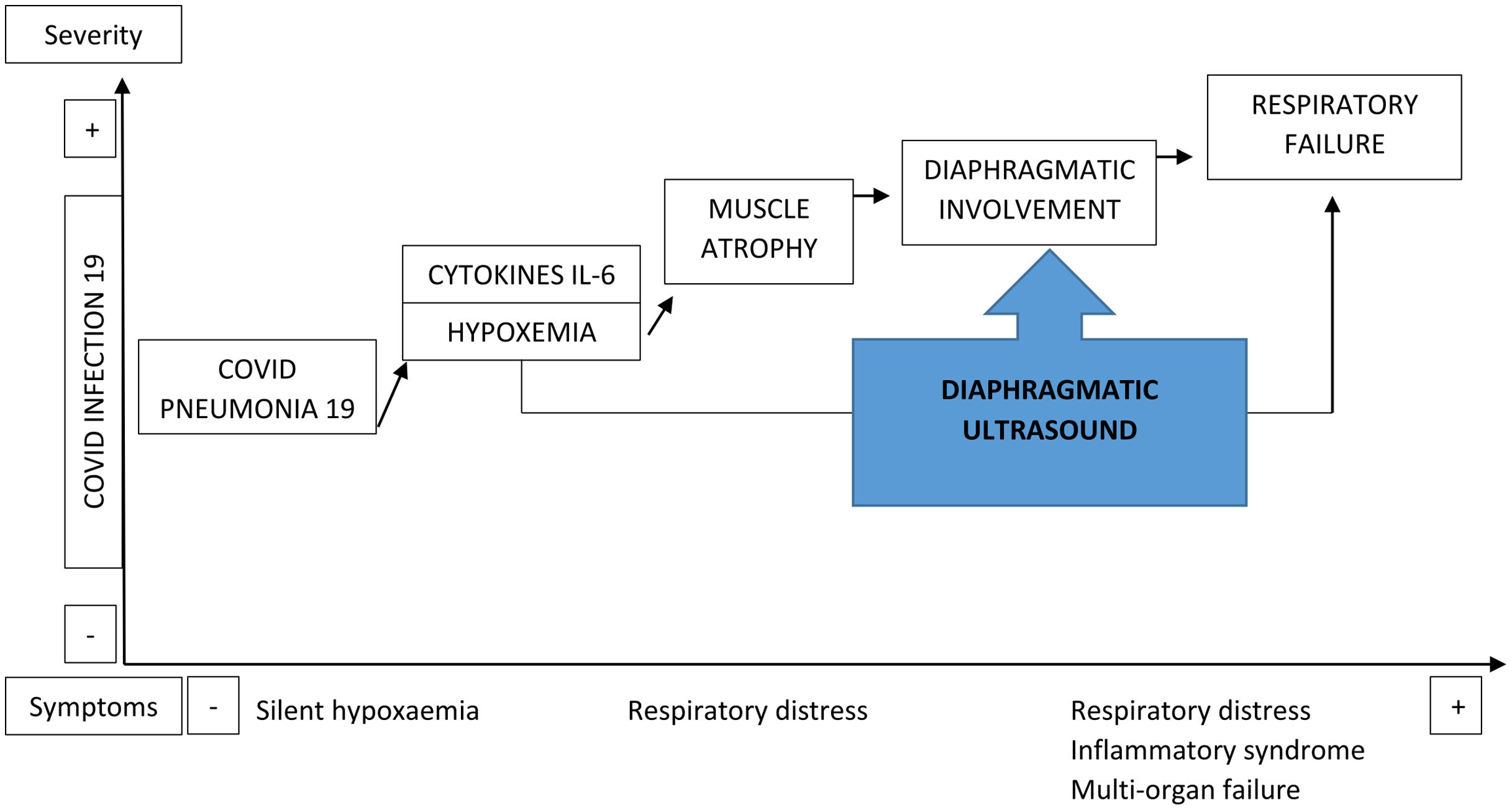
The entry of SARS-CoV-2 into the cell is mediated by the angiotensin-converting enzyme 2 receptor, which is highly abundant in the lungs and musculoskeletal tissue.3 However, the virus affects not just the lung parenchyma, given that the release of inflammatory mediators (interleukin-6),4 along with other not well-known mechanisms, triggers the involvement of skeletal muscles, producing myalgia, fatigue, weakness and atrophy (present in 25–50% of patients hospitalized with this infection).5
The diaphragm is characterized by resistance to fatigue, thanks to aerobic metabolism that uses type 1 or slow-twitch muscle fibers, which are highly prevalent in its structure.6 Hypoxia impedes the ability to obtain energy, initiating anaerobic metabolism, which in turn results in reduced contraction strength of the diaphragm and increases its fatigue.7 Diaphragmatic weakness, either by mechanical, inflammatory or infectious factors, causes dyspnea.8 The combination of these two factors (hypoxemia and dyspnea), along with muscle involvement, is present in most patients hospitalized with COVID-19 after a few days of contracting the disease.9 Patients with this infection undergo an inflammatory storm that significantly affects the skeletal muscles, including the diaphragm.10
Ultrasonography performed at the bedside (known as point-of-care ultrasound) has been shown to be a highly useful tool in monitoring patients with COVID-19 pneumonia.11,12 Diaphragm ultrasound (DUS) is a non-invasive technique by which the patient can be examined at the bedside with no risk. The usefulness of DUS has been demonstrated in intubated critical patients in predicting the success of withdrawing the invasive mechanical ventilation (IMV).13 DUS has also been employed for patients with chronic obstructive pulmonary disease (COPD) to predict the failure risk of noninvasive mechanical ventilation (NIMV) in exacerbations and to analyze the prognostic factors for the success of NIMV.14
Under the hypothesis that infection by COVID-19 produces a multisystem involvement that includes the diaphragm (Fig. 1), we proposed a study to determine the morphological and functional ultrasound condition of the diaphragm of patients admitted for COVID-19 pneumonia during their first 24h and its relationship with hospital morbidity and mortality.
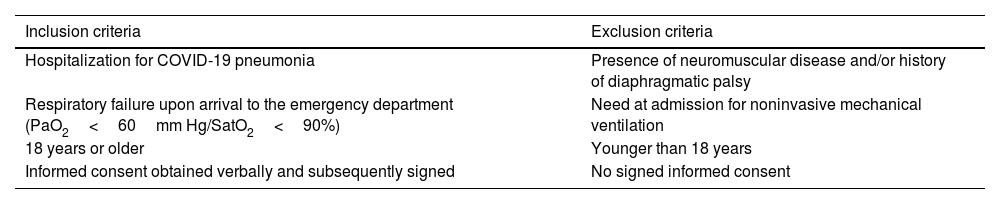
Material and methodsStudy designAn analytical, observational, prospective study was conducted on a patient cohort hospitalized in the pulmonology department of a general hospital due to COVID-19 infection with pneumonia and/or associated respiratory failure. The enrollment of patients, with the criteria listed in Table 1, was extended by 4 months (from 01/Feb/2021 to 20/Jun/2021). The study was approved by the Ethics and Clinical Research Committee of Aragon on 13/Jan/2021, and the protocol was registered in the Clinicaltrials.gov database (NCT05805579).
Selection of patients.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Hospitalization for COVID-19 pneumonia | Presence of neuromuscular disease and/or history of diaphragmatic palsy |
| Respiratory failure upon arrival to the emergency department (PaO2<60mm Hg/SatO2<90%) | Need at admission for noninvasive mechanical ventilation |
| 18 years or older | Younger than 18 years |
| Informed consent obtained verbally and subsequently signed | No signed informed consent |
For each patient, we collected demographic and anthropometric factors (weight, height, BMI), comorbidities (with the Charlson index), clinical, analytical and radiological factors and, lastly, the destination on discharge from the pulmonology unit (Table 1S). The number of patients to enroll was set at 70, considering the differences observed in the diaphragm thickening fraction (TfDi) of patients with COPD exacerbation upon their arrival to the emergency department, according to the study by Marchioni et al.14 As the endpoint, we used the destination at hospital ward discharge: discharge, respiratory monitoring unit (RMU) and intensive care unit (ICU). We also used the vital state at discharge: living or dead.
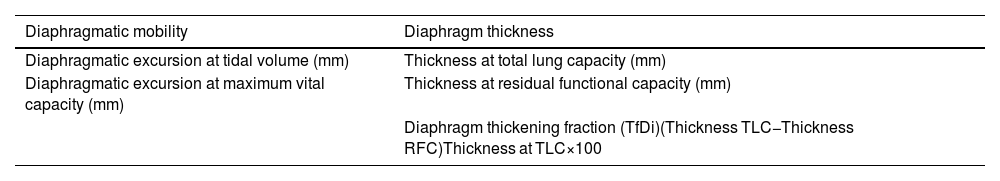
Ultrasound endpointsThe DUS was performed during the first 24h since the patient's admission to the pulmonology ward. A SonoStar UProbe-C5PL portable ultrasound scanner was employed, which consists of 2 heads that allow the user to select either a 7.5/10MHz linear, a 3.5/5MHz convex or a 3.5/5MHz phased-array transducer. We examined the right diaphragm due to its ease of access, thereby streamlining the tests. The analyzed endpoints are shown in Table 2. For the measures of diaphragmatic mobility and thickness, we used the convex and linear heads, respectively.
Study endpoints in the diaphragm ultrasound.
| Diaphragmatic mobility | Diaphragm thickness |
|---|---|
| Diaphragmatic excursion at tidal volume (mm) | Thickness at total lung capacity (mm) |
| Diaphragmatic excursion at maximum vital capacity (mm) | Thickness at residual functional capacity (mm) |
| Diaphragm thickening fraction (TfDi)(Thickness TLC−Thickness RFC)Thickness at TLC×100 |
TLC, total lung capacity; RFC, residual functional capacity.
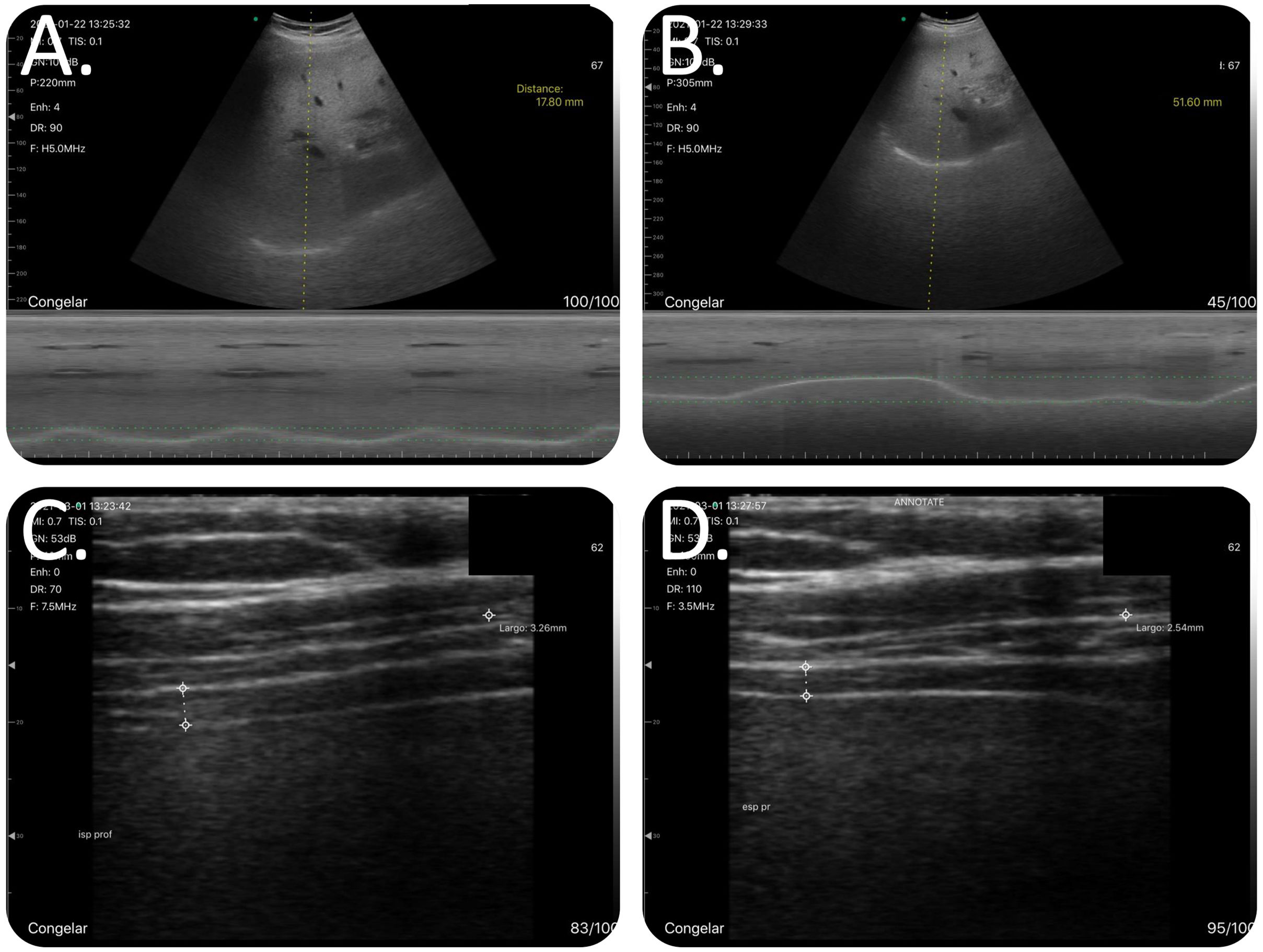
To measure diaphragmatic mobility, the patients were placed in supine decubitus, with the bed angled at 30–45°. We used the convex probe in B-mode to examine the right diaphragm, with the ultrasonic beam in the cephalic-dorsal direction, with the ultrasound probe below the costal arch in the midclavicular line. Once the diaphragm had been located, the ultrasound was changed to M-mode, and the patients were given indications so as to perform measurements at tidal volume and during maximum inspiration and expiration (Videos 1 and 2).
To measure the diaphragm thickness, the patients were placed in the seated position, with the shoulders at 90° and the linear probe in B-mode. In this measurement, the ultrasonic beam was aimed in the craniocaudal direction, perpendicular to the direction of the muscle fibers, including the pleural and peritoneal membrane (Fig. 2). Patients were asked to breathe at tidal volume; once the zone of apposition had been located, they were requested to breathe at tidal volume (Video 3), perform a forced inspiration until total lung capacity had been achieved (Video 4) and then perform a forced expiration (Video 5). All images were collected in jpeg format and on video. To diagnose the diaphragmatic dysfunction (DD), we used the TfDi in forced maneuvers, with a cutoff<20% according to literature reviewed.15
Methodology for examining the diaphragm. To measure diaphragmatic mobility, the patients were placed in supine decubitus, with the bed angled at 30–45°, with the ultrasound probe below the costal arch in the midclavicular line and with the ultrasonic beam in the cephalic-dorsal direction, pointing toward the right shoulder. The diaphragm thickness was measured with the patient seated and the shoulder at 90°. The ultrasonic beam was aimed in the craniocaudal direction, perpendicular to the direction of the muscle fibers, including the pleural and peritoneal membrane. (A) Combined B/M-mode study with convex probe during a maneuver at tidal volume. In M-mode, we observed the sinusoidal image that represented the diaphragm's movement at tidal volume. (B) Combined B/M-mode study with convex probe during a forced maneuver. In M-mode, the wave is no longer sinusoidal; however, there is a large amplitude in its movement. The highest part of the line corresponds to the deep inspiration and the lowest part corresponds to the maximum expiration. (C) B-mode study with linear catheter during a forced inspiration maneuver. We can see the diaphragm's typical morphology as a structure with 2 parallel hyperechogenic lines and a third line between the two lines, with lower intensity hyperechogenicity that is not always visible. At the end of the inspiration, the diaphragm reaches its maximum thickening. (D) B-mode study with linear catheter during a forced expiration maneuver. At the end of the forced expiration, the diaphragm reaches its maximum narrowing.
The statistical analysis was performed with SPSS version 20.0 (IBM), Epidata 3.1 and graph representation with GraphPad Prism 7 (Graphpad Software). The database was subsequently reviewed, and the outliers were removed by applying the ROUT (regression and outlier removal) test, with a q-value of 1% (Graphpad Prism 7).
Initially, a description of the sample was performed, using numbers (n) and percentages (%) to indicate the frequency of the qualitative variables; the quantitative variables were expressed as the mean and standard deviation. As stated above, we used a TfDi value of 20% as the cutoff to define DD. The Kolmogorov–Smirnov test was performed to compare the normality of the quantitative variables and determine whether the hypothesis test should be performed with parametric or nonparametric tests.
To determine the factors’ power to predict the need for RMU admission, we opted for a univariate binary logistic regression analysis. We selected those factors with statistical significance (p<0.05) for entry into a multivariate model.
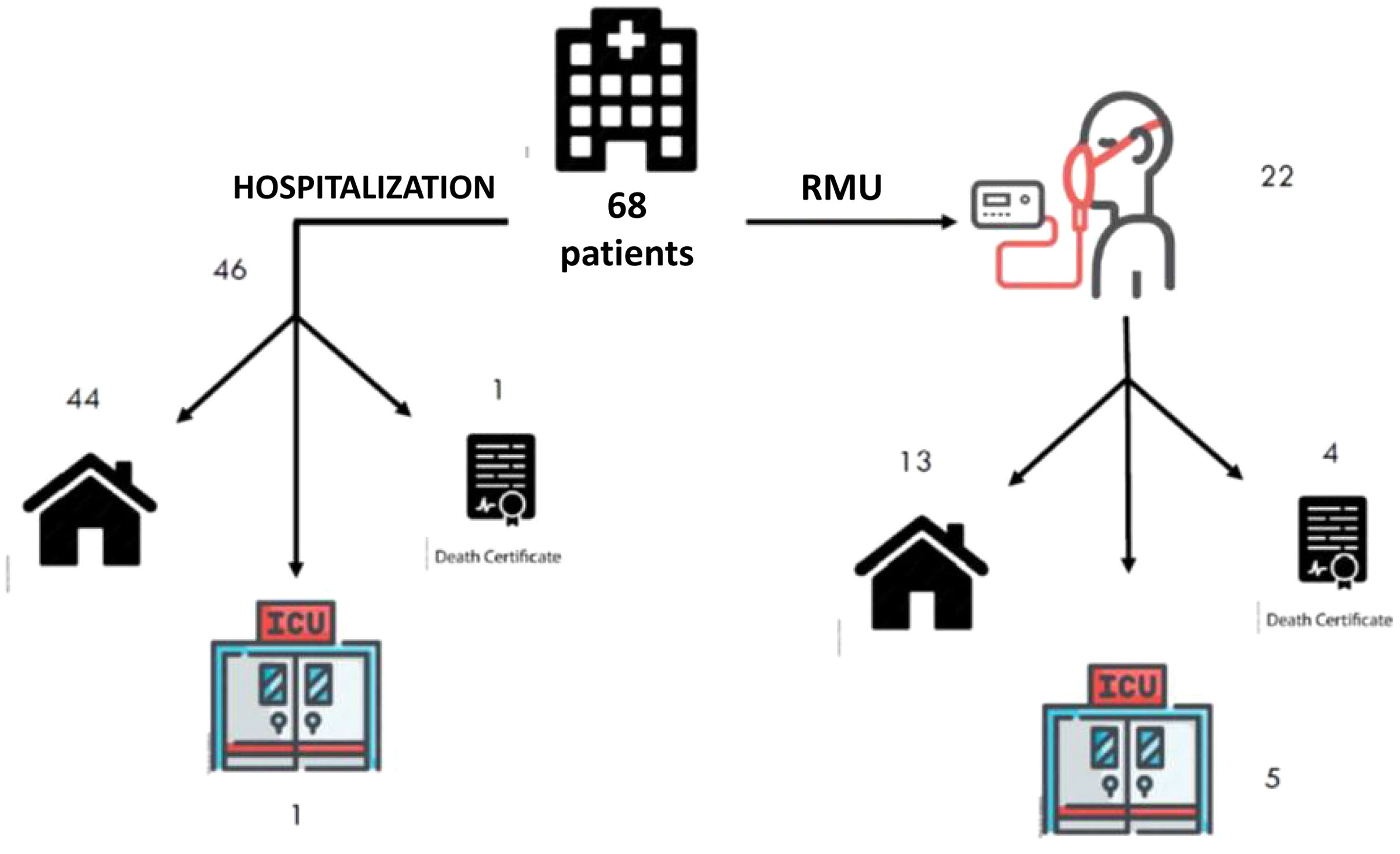
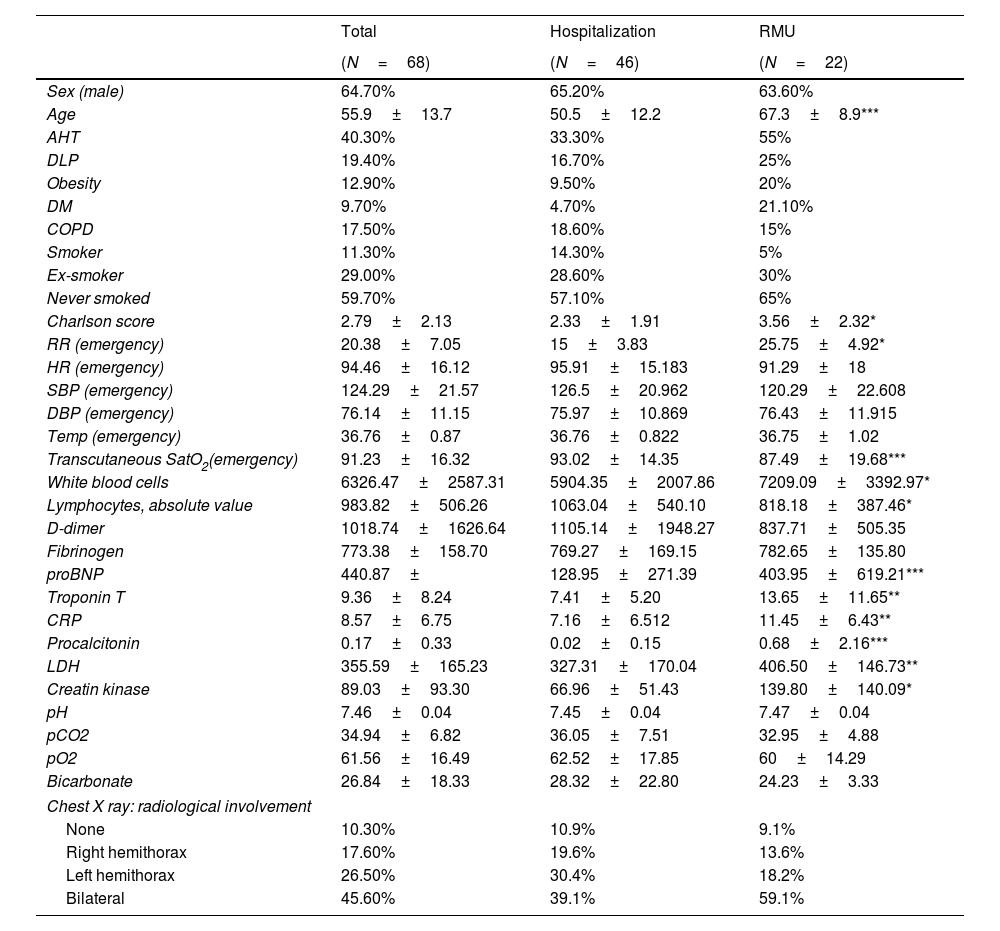
ResultsDescription of the sampleThe study included 72 patients, 4 of whom were excluded for not granting their consent. The analyzed sample consisted of 68 patients, 46 (67.64%) of whom were admitted to the hospital ward without going through the RMU; the remainder were admitted to the RMU. The mean age of the patients who were admitted to the RMU was 67.3±8.9 years, compared with 50.5±12.2 years for those who were not (p=0.0001) (Table 3). The patients who required RMU admission had a higher incidence of comorbidities, except for COPD, which was higher for the patient group who did not require RMU admission. The Charlson index was higher for the patient group who was admitted to the RMU (3.56±2.32) than for the group who did not require RMU admission (2.33±1.91; p=0.036).
Description of the sample.
| Total | Hospitalization | RMU | |
|---|---|---|---|
| (N=68) | (N=46) | (N=22) | |
| Sex (male) | 64.70% | 65.20% | 63.60% |
| Age | 55.9±13.7 | 50.5±12.2 | 67.3±8.9*** |
| AHT | 40.30% | 33.30% | 55% |
| DLP | 19.40% | 16.70% | 25% |
| Obesity | 12.90% | 9.50% | 20% |
| DM | 9.70% | 4.70% | 21.10% |
| COPD | 17.50% | 18.60% | 15% |
| Smoker | 11.30% | 14.30% | 5% |
| Ex-smoker | 29.00% | 28.60% | 30% |
| Never smoked | 59.70% | 57.10% | 65% |
| Charlson score | 2.79±2.13 | 2.33±1.91 | 3.56±2.32* |
| RR (emergency) | 20.38±7.05 | 15±3.83 | 25.75±4.92* |
| HR (emergency) | 94.46±16.12 | 95.91±15.183 | 91.29±18 |
| SBP (emergency) | 124.29±21.57 | 126.5±20.962 | 120.29±22.608 |
| DBP (emergency) | 76.14±11.15 | 75.97±10.869 | 76.43±11.915 |
| Temp (emergency) | 36.76±0.87 | 36.76±0.822 | 36.75±1.02 |
| Transcutaneous SatO2(emergency) | 91.23±16.32 | 93.02±14.35 | 87.49±19.68*** |
| White blood cells | 6326.47±2587.31 | 5904.35±2007.86 | 7209.09±3392.97* |
| Lymphocytes, absolute value | 983.82±506.26 | 1063.04±540.10 | 818.18±387.46* |
| D-dimer | 1018.74±1626.64 | 1105.14±1948.27 | 837.71±505.35 |
| Fibrinogen | 773.38±158.70 | 769.27±169.15 | 782.65±135.80 |
| proBNP | 440.87± | 128.95±271.39 | 403.95±619.21*** |
| Troponin T | 9.36±8.24 | 7.41±5.20 | 13.65±11.65** |
| CRP | 8.57±6.75 | 7.16±6.512 | 11.45±6.43** |
| Procalcitonin | 0.17±0.33 | 0.02±0.15 | 0.68±2.16*** |
| LDH | 355.59±165.23 | 327.31±170.04 | 406.50±146.73** |
| Creatin kinase | 89.03±93.30 | 66.96±51.43 | 139.80±140.09* |
| pH | 7.46±0.04 | 7.45±0.04 | 7.47±0.04 |
| pCO2 | 34.94±6.82 | 36.05±7.51 | 32.95±4.88 |
| pO2 | 61.56±16.49 | 62.52±17.85 | 60±14.29 |
| Bicarbonate | 26.84±18.33 | 28.32±22.80 | 24.23±3.33 |
| Chest X ray: radiological involvement | |||
| None | 10.30% | 10.9% | 9.1% |
| Right hemithorax | 17.60% | 19.6% | 13.6% |
| Left hemithorax | 26.50% | 30.4% | 18.2% |
| Bilateral | 45.60% | 39.1% | 59.1% |
RMU, respiratory monitoring unit; AHT, arterial hypertension; DLP, dyslipidemia; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; RR, respiratory rate; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; SatO2, baseline oxygen saturation; proBMP, pro B-type natriuretic peptide; CRP, C-reactive protein; LDH, lactate dehydrogenase; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
In terms of the factors observed in the emergency department, there was a faster respiratory rate in the patients who subsequently required RMU admission (25.75±4.92 vs. 15±3.83; p=0.028), as well as a lower oxygen saturation (SatO2) (87.49±19.68 vs. 93.02±14.35; p=0.0001). There were no significant differences in the other factors analyzed upon arrival at the emergency department.
The mean stay for the entire sample was 7.98±6.36 days. The patients who required treatment in the RMU remained hospitalized for longer than those who did not require it (12.90±8.65 vs. 5.63±2.78 days; p=0.0001). Only 1 patient (2.17%) in the group who were not admitted to the RMU died, while 4 patients (18.18%) in the group who were admitted to the RMU died, and 5 (22.72%) required ICU admission (Fig. 3).
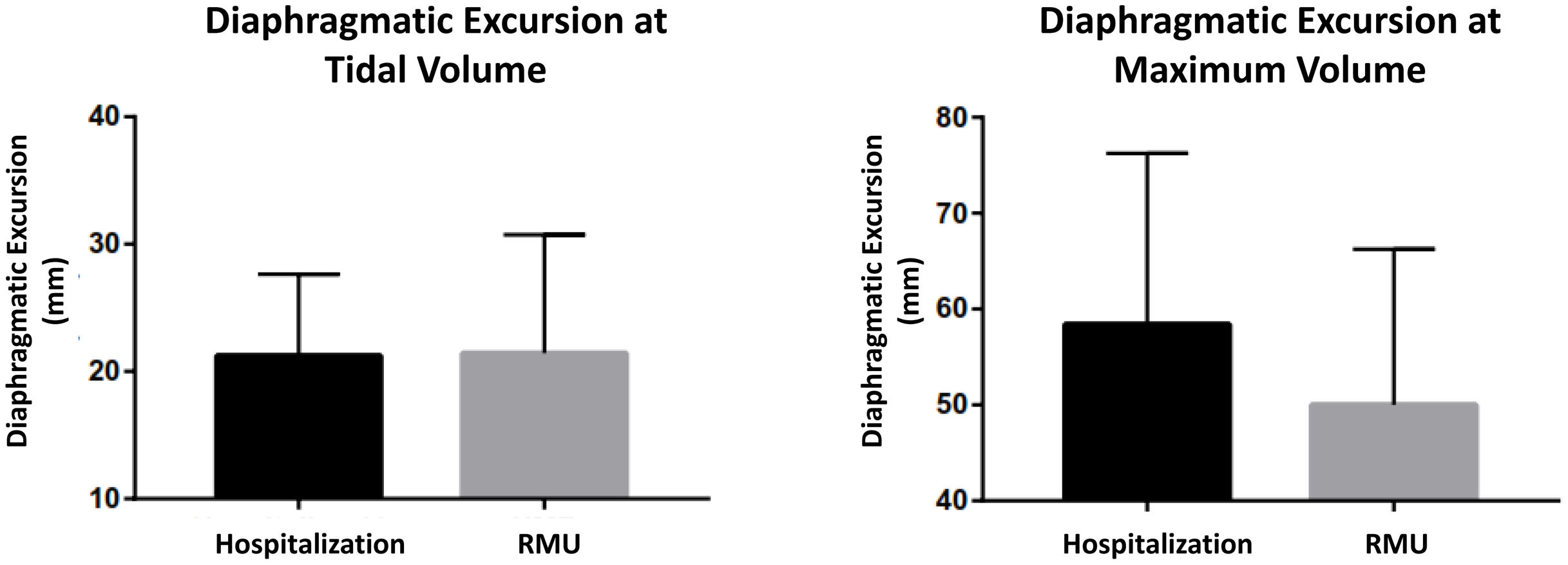
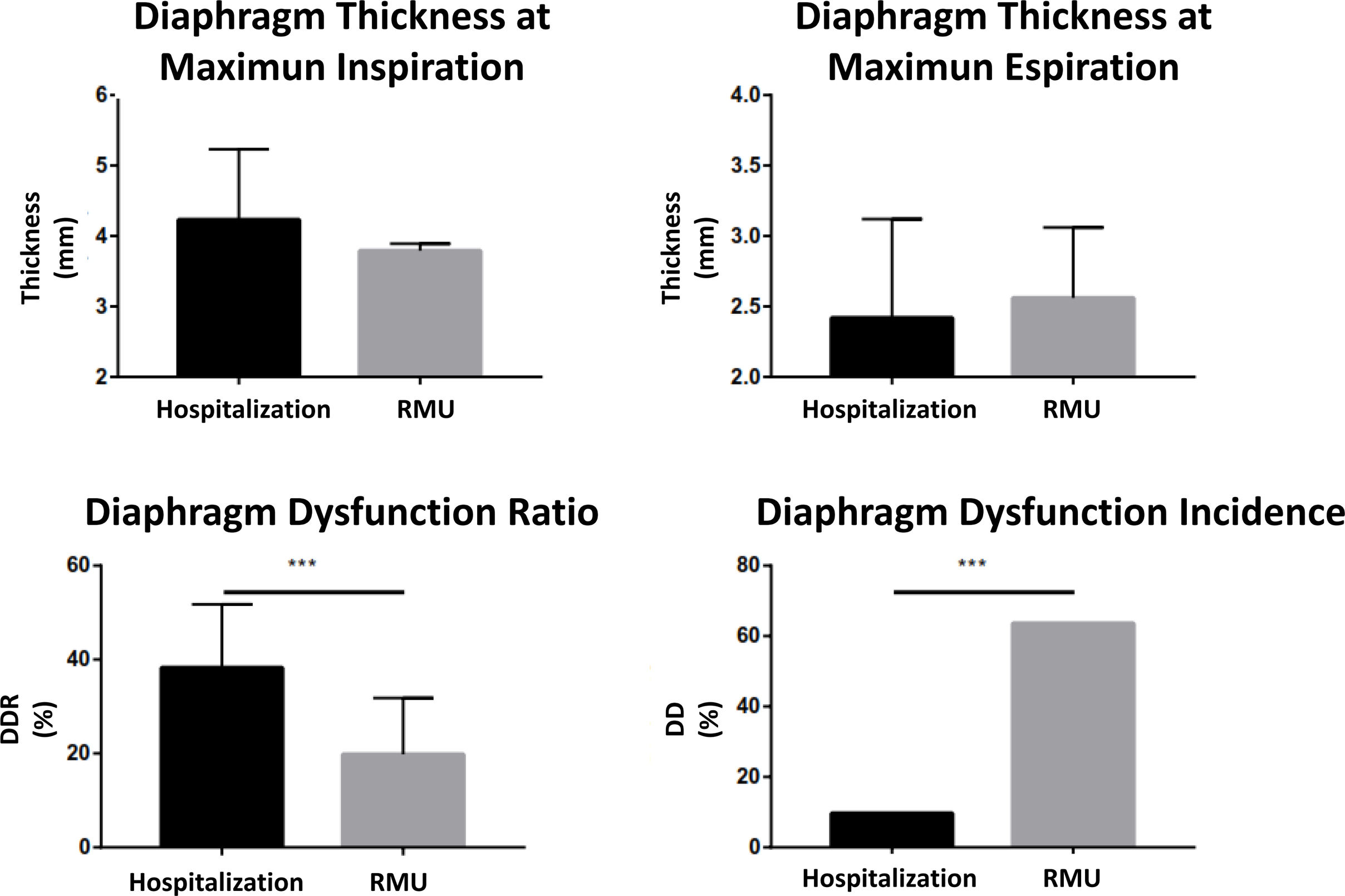
The ultrasound scan was performed in the first 24h after admission, analyzing both diaphragm mobility and thickness, observing differences in both types of measures. In the entire sample, the diaphragmatic excursion at tidal volume was lower than the measure at maximum volume (21.31±7.41 vs. 56±17.64mm). The excursion at tidal volume was similar for both groups (21.24±6.41mm in the hospitalized group vs. 21.44±9.30mm in the RMU group; p=0.931) (Fig. 4). Diaphragmatic mobility at maximum volume was greater in those treated in conventional hospitalization than in those who required RMU admission (58.41±17.83 vs. 50.03±16.23mm; p=0.123).
Diaphragm thickness was measured at forced inspiration and expiration (Fig. 2). The inspiratory thickness was greater in the patient group admitted to conventional hospitalization than in those who required RMU admission (4.23±1 vs. 3.79±0.10mm; p=0.192). In expiration, the diaphragm thickness showed similar results between the two groups (2.42±0.75 vs. 2.42±0.76mm; p=0.765). With these parameters, we calculated the TfDi for both groups. The TfDi was lower for the patient group who ultimately were admitted to the RMU (19.80±12 vs. 38.3±13.48%; p=0.0001). In the entire sample, DD was diagnosed (TfDi<20%) in 21 patients (30.88%), with a greater presence in the patients who subsequently were admitted to the RMU than in those who were not (15 [68.18%] vs. 6 [13.04%]; p=0.0001) (Fig. 5).
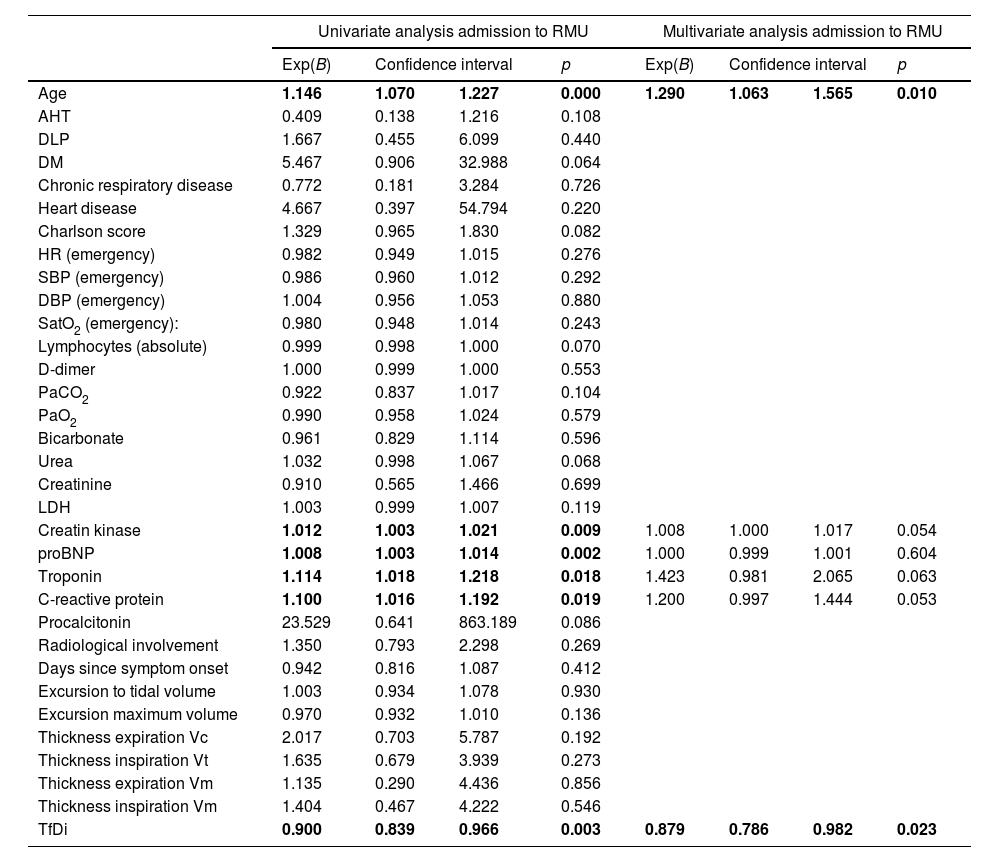
A logistic regression analysis was performed to determine the predictive power of the various factors for RMU admission. In the univariate analysis: age, proBNP, troponin, C-reactive protein and TfDi demonstrated this power (Table 4). Of all the factors included in the multivariate study, age and TfDi were shown to be good predictors of RMU admission (Table 4).
Univariate and multivariate analysis of the risk factors for RMU admission.
| Univariate analysis admission to RMU | Multivariate analysis admission to RMU | |||||||
|---|---|---|---|---|---|---|---|---|
| Exp(B) | Confidence interval | p | Exp(B) | Confidence interval | p | |||
| Age | 1.146 | 1.070 | 1.227 | 0.000 | 1.290 | 1.063 | 1.565 | 0.010 |
| AHT | 0.409 | 0.138 | 1.216 | 0.108 | ||||
| DLP | 1.667 | 0.455 | 6.099 | 0.440 | ||||
| DM | 5.467 | 0.906 | 32.988 | 0.064 | ||||
| Chronic respiratory disease | 0.772 | 0.181 | 3.284 | 0.726 | ||||
| Heart disease | 4.667 | 0.397 | 54.794 | 0.220 | ||||
| Charlson score | 1.329 | 0.965 | 1.830 | 0.082 | ||||
| HR (emergency) | 0.982 | 0.949 | 1.015 | 0.276 | ||||
| SBP (emergency) | 0.986 | 0.960 | 1.012 | 0.292 | ||||
| DBP (emergency) | 1.004 | 0.956 | 1.053 | 0.880 | ||||
| SatO2 (emergency): | 0.980 | 0.948 | 1.014 | 0.243 | ||||
| Lymphocytes (absolute) | 0.999 | 0.998 | 1.000 | 0.070 | ||||
| D-dimer | 1.000 | 0.999 | 1.000 | 0.553 | ||||
| PaCO2 | 0.922 | 0.837 | 1.017 | 0.104 | ||||
| PaO2 | 0.990 | 0.958 | 1.024 | 0.579 | ||||
| Bicarbonate | 0.961 | 0.829 | 1.114 | 0.596 | ||||
| Urea | 1.032 | 0.998 | 1.067 | 0.068 | ||||
| Creatinine | 0.910 | 0.565 | 1.466 | 0.699 | ||||
| LDH | 1.003 | 0.999 | 1.007 | 0.119 | ||||
| Creatin kinase | 1.012 | 1.003 | 1.021 | 0.009 | 1.008 | 1.000 | 1.017 | 0.054 |
| proBNP | 1.008 | 1.003 | 1.014 | 0.002 | 1.000 | 0.999 | 1.001 | 0.604 |
| Troponin | 1.114 | 1.018 | 1.218 | 0.018 | 1.423 | 0.981 | 2.065 | 0.063 |
| C-reactive protein | 1.100 | 1.016 | 1.192 | 0.019 | 1.200 | 0.997 | 1.444 | 0.053 |
| Procalcitonin | 23.529 | 0.641 | 863.189 | 0.086 | ||||
| Radiological involvement | 1.350 | 0.793 | 2.298 | 0.269 | ||||
| Days since symptom onset | 0.942 | 0.816 | 1.087 | 0.412 | ||||
| Excursion to tidal volume | 1.003 | 0.934 | 1.078 | 0.930 | ||||
| Excursion maximum volume | 0.970 | 0.932 | 1.010 | 0.136 | ||||
| Thickness expiration Vc | 2.017 | 0.703 | 5.787 | 0.192 | ||||
| Thickness inspiration Vt | 1.635 | 0.679 | 3.939 | 0.273 | ||||
| Thickness expiration Vm | 1.135 | 0.290 | 4.436 | 0.856 | ||||
| Thickness inspiration Vm | 1.404 | 0.467 | 4.222 | 0.546 | ||||
| TfDi | 0.900 | 0.839 | 0.966 | 0.003 | 0.879 | 0.786 | 0.982 | 0.023 |
RMU, respiratory monitoring unit; AHT, arterial hypertension; DLP, dyslipidemia; DM, diabetes mellitus; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; SatO2, baseline oxygen saturation; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood; LDH, lactate dehydrogenase; proBMP, pro B-type natriuretic peptide; Vc, vital capacity; Vt, tidal volume; Vm, maximum volume; TfDi, diaphragm thickening fraction.
In bold, statistically significant values.
This prospective study demonstrated that the patients with COVID-19 pneumonia experienced more or less severe diaphragm function impairment and that the implementation of DUS at admission is able to predict short-term outcomes. The parameter that presented the best predictive power in our study was the diaphragmatic fractional shortening, with a cut-off of <20%. The study also provides more evidence for the hypothesis that this virus affects the diaphragm and that this involvement is maintained over time, causing diaphragmatic weakness that is maintained even months after the initial infection in those patients who continue with dyspnea, regardless of whether they required IMV or not.16,17
In our sample, the patients who subsequently presented poor outcomes, requiring RMU admission, had more cardiovascular comorbidities but not more respiratory diseases. However, the overall analysis of comorbidities using the Charlson index showed them to be greater in the patients who subsequently had poorer outcomes. Similarly, the group admitted to the RMU exhibited higher CK levels at the time of admission. These findings agree with other patient series reported in the literature.18,19
The respiratory function impairment of the hospitalized patients revealed the need for developing specialized units: intermediate respiratory care units (IRCUs) and RMUs for administering non-invasive respiratory therapies outside of the ICU.20,21 The patients who were admitted to the RMU experienced more severe pneumonia; therefore, their mortality rate was significantly higher than those who only went through conventional hospitalization. The creation of the RMU in our facility avoided ICU admission for 64.8% of the patients who were candidates for the ICU and for 44.2% of those who were not candidates for ICU.22 This type of unit has undergone a transformation to successfully respond to the COVID-19 pandemic. These units have incorporated new diagnostic techniques such as thoracic ultrasonography, which is present in 85% of these units.21,23
Thoracic ultrasonography for patients with COVID-19 has been shown to be a highly useful prognostic tool, given the complexity of managing these patients.24 The baseline values for diaphragmatic excursion in healthy individuals were already established by 2009.25 The right diaphragm mobility was greater in men than women, measuring 1.8±0.3cm versus 1.6±0.3cm for excursion at tidal volume and 7±1.1cm versus 5.7±1cm for excursion at maximum volume. The excursion at tidal volume in our study was above the normal limits. In contrast, the mobility at maximum volume was below normal limits, especially in the group who ultimately required RMU admission.
Diaphragmatic mobility has been employed to determine the outcome of patients hospitalized in an IRCU for undergoing NIMV due to hypercapnic respiratory failure.26 The study by Cammarota et al. observed that the mobility was significantly less in the patients for whom this therapy ultimately failed. Taking into account the 2 aforementioned studies,25,26 the diaphragmatic excursion in our sample's patients was significantly decreased, especially in the forced maneuvers, in those who presented more severe symptoms and required RMU admission.
As with diaphragmatic excursion, diaphragm thickness presented significant differences depending on sex (greater in the men than in the women) but was not affected by BMI or age.27,28 The normal range for diaphragm thickness is very wide, ranging from 1.3 to 3mm for expiration in women and 1.1 to 2.7mm for men; forced inspiration ranges from 2.8 to 5.9mm in men and from 2.4 to 5.4mm in women.27 Our study's results are within the normal range according to these ranges. Nevertheless, the diaphragm thickness in forced inspiration was smaller in those who ended up in the RMU. If we compare them with the mean thickness observed in healthy individuals, it was also smaller when taking the baseline values both for men (4.3mm) and for women (3.9mm).
COVID-19 infection produces muscle damage defined by generalized arthromyalgia and increased creatine kinase (CK) levels above 200U/L, which occur in more severe conditions of the disease.29 In our sample, there was no relationship between CK levels and diaphragmatic measurements (Table S2). Patients with COVID-19 pneumonia who require IMV experience rapid muscle impairment, including a significant reduction in diaphragm thickness at the seventh day of ICU hospitalization.30 In the DUS of patients with severe symptoms of COVID-19 pneumonia who required IMV, there was a higher incidence of DD than in other convalescents with other non-COVID-19 conditions.31 An important confounding factor is whether the location of parenchymal involvement influences on DD. In our sample, no differences were observed in the location of parenchymal involvement and the presence of DD.
DUS at emergency department admission due to COVID-19 infection is an easy and reproducible technique, although its findings have not been related to the 30-day prognosis.32 Our study confirmed the usefulness of this technique and shows its usefulness in the prognosis of patients hospitalized for this disease. TfDi had the best power in predicting poor outcomes in our study, unlike other studies such as the one conducted by Adolf Helmy et al. in which the reduction in forced diaphragmatic excursion at ICU admission was a predictor of mortality and the requirement for IMV.33 This study did not analyze the diaphragm thickness or its associated measures. However, the DD measured by TfDi was shown to be a predictor of continuous positive airway pressure treatment failure in 27 patients hospitalized in the ICU for respiratory failure due to COVID-19 pneumonia.34
Diaphragm thickness at the end of expiration has also been analyzed in patients admitted to the IRCU, observing that the reduction in this measure was an independent predictor of mortality and the need for IMV. This same study set 2.2mm as the cut-off below which the probability of a poor outcome increases.35 In our sample, the diaphragm thickness at the end of expiration was above this value (2.4mm); however, the DUS in our case was performed at ward admission, and the DUS in the Corradi et al. group was performed at IRCU admission. This difference could be explained by the severity of the infection, given that in our case, we excluded patients who initially went to the RMU.
It is worth noting the study by Formenti et al. who employed ultrasound to determine the prognostic role of the respiratory (intercostal and diaphragm) and skeletal muscles (rectus femoris) in 32 patients admitted to the ICU.36 In this case, the authors analyzed not only the muscle thickness but also its echogenicity. This parameter is measured using a greyscale and indicates the muscle quality, which is directly proportional to the fatty infiltration and of connective tissue (poorer quality). In their study, the authors concluded that the echogenicity of all the muscle groups, including the diaphragm, was greater in those who died. In contrast, only the thickness of the rectus femoris was reduced in those who died. This finding does not agree with our study's observations, although the analyzed factors are not the same and shows a new method for analyzing respiratory muscle function.
Our study has significant limitations that need to be considered when interpreting its results. The DUS was performed by a single operator; therefore, the interobserver variability of the examinations was not considered due to the difficulty entailed in its implementation. The examinations were performed at the patients’ point-of-care, with the examiners dressed in their individual protection equipment. We also did not compare the DUS with the gold standard, the stimulation of the phrenic nerve to confirm the DD.37 Another limitation was the absence of a control group of patients with COVID-19 who did not require hospitalization, as well as healthy individuals. We encountered difficulties in performing the point-of-care ultrasound for patients with high BMIs, given that the ultrasound probe had insufficient penetration to obtain accurate results. In certain patients undergoing high-flow therapy, the measurement could not be performed given that their physical limitations impeded collaboration, both when adopting appropriate postures for the taking of certain measures and in the collaboration when following the instructions to obtain others. Due to the technical difficulty in performing an ultrasound examination of the left diaphragm, combined with the clinical and epidemiological situation at the time of the fieldwork, the decision was made to only conduct a study of the right diaphragm.
In conclusion, the performance of a diaphragmatic ultrasound to determine the mobility and rate of diaphragmatic shortening in the first 24h of hospitalization for COVID-19 pneumonia was useful for determining the short-term outcomes and the need for admission to a respiratory monitoring unit. The DUS is a highly useful and accessible tool, but its use and its baseline values need to be standardized with prospective studies that include patients of differing ages, sex and body mass index.
FundingThis project was granted funding by the Sociedad Aragonesa de Patología del Aparato Respiratorio (SADAR) in their 2020 call for proposals.
Authors’ contributionsJLS, as the principal investigator, designed the study, conducted the ultrasounds, performed the statistical analysis, and drafted the final manuscript. MDA participated in performing the ultrasounds and in manuscript writing. PCM, MTR, MASL, LPG, TL, MZL, ABL, AH, and DN contributed to patient recruitment. MASL, SGS, and JAC reviewed and approved the manuscript once finalized.
Conflicts of interestNone.
To my colleagues for their collaboration and constant support. To the patients for their trust and willingness to participate during a period of uncertainty and loneliness, such as the early waves of the pandemic.