Sepsis and septic shock associated with tuberculosis are such critical diseases that are life-threatening to the patient. TB bacteremia has been associated with sepsis and septic shock, especially in immunosuppressed patients with high mortality. In this sense, it has been seen that 1 in 4 patients infected with human immunodeficiency virus (HIV) hospitalized for sepsis had TB bacteremia. Acute forms of tuberculosis can progress in severity and between 1 and 3% of cases require an Intensive Care Unit (ICU) and high mortality. Given this scenario, the authors consider it pertinent to carry out this Review article, which allows a diagnostic and therapeutic approach to this acute form of TB to provide key aspects of the disease that are decisive in saving the lives of patients in critical condition.
La sepsis y el shock séptico asociados con la tuberculosis (TB) son enfermedades críticas que ponen en peligro la vida del paciente. La bacteriemia por TB se ha asociado con sepsis y shock séptico, especialmente en los pacientes inmunodeprimidos con alta mortalidad. En este sentido, se ha visto que uno de cada 4 pacientes infectados por el virus de la inmunodeficiencia humana (VIH) hospitalizados por sepsis presentaba bacteriemia tuberculosa. Las formas agudas de TB pueden progresar en gravedad y entre el 1 y el 3% de los casos requieren una unidad de cuidados intensivos (UCI) y una alta mortalidad. Ante este escenario, los autores consideran pertinente realizar este artículo de revisión, que permita un abordaje diagnóstico y terapéutico de esta forma aguda de TB para brindar aspectos claves de la enfermedad que son decisivos para salvar la vida de los pacientes en estado crítico.
Sepsis is a clinical condition that causes a large burden of mortality around the world and is considered a challenging problem for global public health. In this sense, it has been estimated that approximately 11 million deaths are caused by sepsis1; insofar, as sepsis is responsible for 20% of global mortality.1 By 2017, the burden of the disease approaches 48.9 million cases.1 Tuberculosis (TB) is a well-recognized pathology, described as one of the oldest infectious diseases of humanity. However, it remains one of the main causes of mortality and a public health problem, due to its excessive morbidity and mortality. According to the World Health Organization (WHO), in the period 2000–2021, about 1.4–2 million people have died from TB each year.2 For the year 2021, a disease burden of 1.64 million and 2 million deaths have been reported among HIV-negative and positive patients.2 More than 500,000 cases of multidrug-resistant TB are reported each year.3 According to the US MMWR, TB has a prevalence of 2.64 million and a mortality of 1.5 million, being classified as one of the most feared infectious diseases with the highest mortality.4
TB bacteremia has been associated with sepsis and septic shock, especially in immunosuppressed patients.5,6 In this sense, it has been seen that 1 in 4 patients infected with human immunodeficiency virus (HIV) hospitalized for sepsis had TB bacteremia.7 Acute forms of tuberculosis can progress in severity and between 1 and 3% of cases require an Intensive Care Unit (ICU).8 A systematic review included 35 studies with 1815 patients hospitalized for TB; of which, 3.4% required admission to the ICU and had a mortality of 48% [95%; IQ: 1.6–5.7%].9 On the other hand, it has been revealed that mortality in patients admitted to the ICU for TB who require mechanical ventilation is close to 33–67%.10–12 Likewise, Kethireddy et al.,13 report that sepsis and septic shock associated-tuberculosis (SSTB) is 1% of cases.13 In this context, SSTB is uncommon in clinical practice and is therefore a rule out diagnosis, other more common bacterial causes are taken into consideration at the time of diagnosis, which determines that TB sepsis is barely described and addressed. Therefore, SSTB is a clinical condition that threatens the patient's life if it is not treated adequately and promptly, and delay in starting therapy may be conditioned by poor prognosis.14 Furthermore, due to the time required to identify Mycobacterium tuberculosis and nonspecific presentations, patients often die after receiving little or no antimycobacterial agents.8,15–20 In this context, SSTB represents a challenge because the pathogenesis has not yet been completely clarified, the difficulty in taking samples for diagnostic confirmation, and the poor absorption of anti-tuberculosis drugs in critically ill patients.21
Given this scenario, the authors consider it pertinent to carry out this Review article, which allows a diagnostic and therapeutic approach to this acute form of TB to provide key aspects of the disease that are decisive in saving the lives of patients in critical condition.
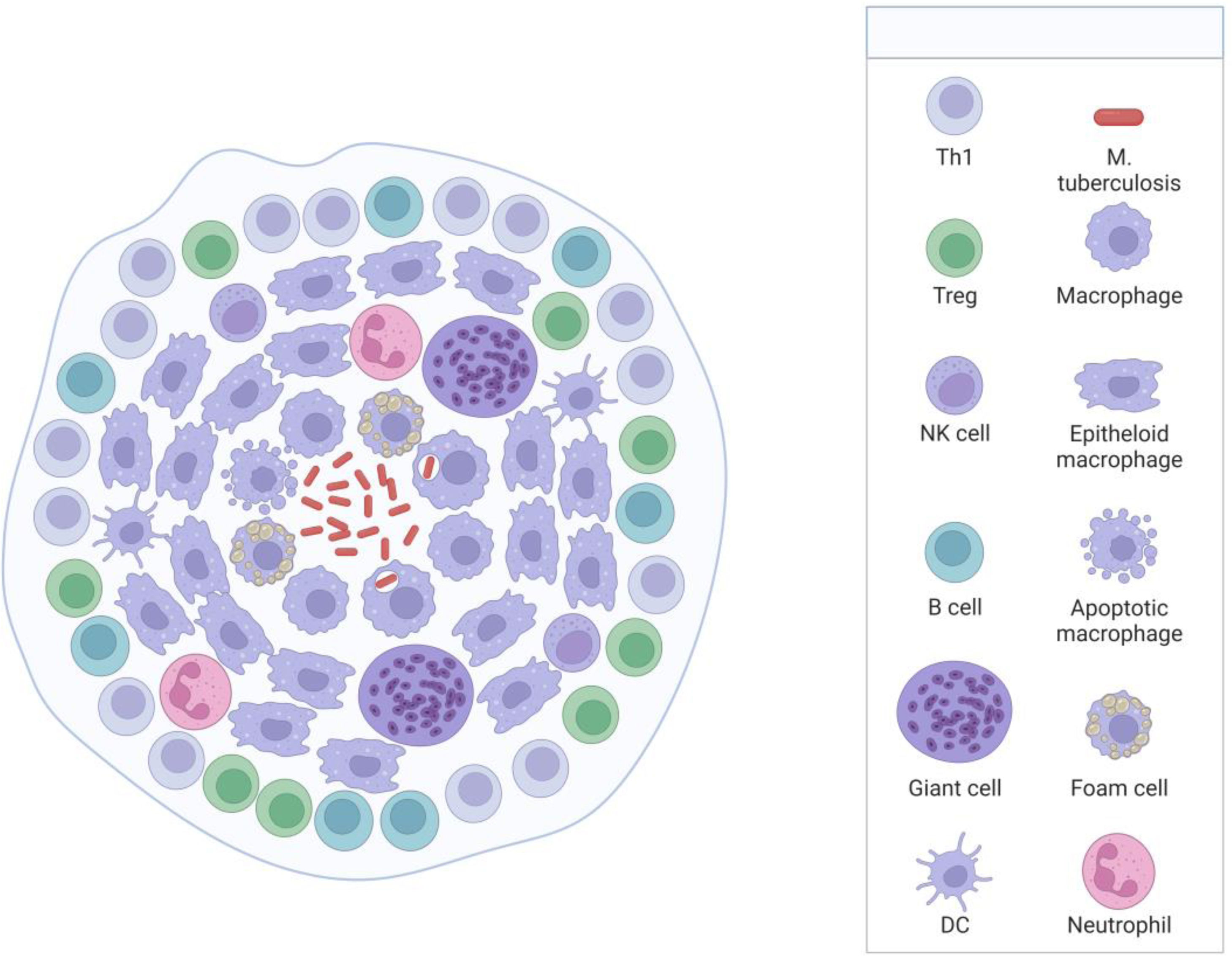
Brief pathophysiological of tuberculosis infectionM. tuberculosis is an acid-alcohol-resistant bacillus, whose predominant site of involvement is the lung; however, it can involve other body parts.22 The disease begins when the individual breathes in micro aerosols that are exhaled from the infected person when they cough, sneeze, or speak.23 Once the bacteria is in the body, it migrates to the lungs through the respiratory tract. In the lungs, in the presence of M. tuberculosis and the pathogen-associated molecular patterns (PAMPs) of its cell wall, alveolar macrophages are activated by pattern recognition receptors (PRRs),24 which interact and attempt to control its propagation by digesting the bacillus; however, without the necessary effectiveness to eliminate it.2 In this favorable environment, the bacillus takes advantage to multiply, they are released and are then phagocytosed by other alveolar macrophages, perpetuating the cycle.2 TB encompasses an aberrant immune response of lymphocytes, for which its control is partial and local. Consequently, granuloma formation has been considered the prominent finding of pulmonary TB,25 characterized by an amorphous and heterogeneous collection of macrophages and other immune cells surrounding the tubercle bacillus, whose objective is to stop its dissemination.22,26 Mycolic acids (MA) are the most abundant component of the cell wall of M. tuberculosis, which are essential for mycobacterial growth and survival.2,25,27 In immunocompetent individuals, the bacteria survive by preventing the phagolysosome from fusing to the granuloma, allowing it to survive for a long time in a slowly replicating state.2,28 In this phase of the disease, known as “latent infection”, the individual is non-infectious and asymptomatic (Fig. 1).29 In a state of granuloma maturation, the chaperone protein DnaK of M. tuberculosis induces M1 macrophages to polarize to M2, which secrete arginine (Arg) and IL-10, which are immunosuppressive of the host's immunity, which leads to a decrease in its containment capacity.30 The nucleus of the granuloma can necrotize and be lysed by other immune cells and form the caseous granuloma.31 The caseous waste is used as a source of energy and nutrients that promote the growth of the bacillus.2,32 The rupture of the granuloma after a long time causes the progression of TB and its spread to other parts of the body, which is known as the reactivation of the disease or “secondary phase.” The reactivation state has been described mainly in immunocompromised patients, such as in HIV coinfection, malnutrition, immunosuppressive drugs, alcohol or drug abuse, chronic kidney disease, and malignancy.28 Under these conditions of immune weakness, the bacillus takes advantage to replicate with greater vigor and aggression, causing lichenification and cavitation.2,22,23,33M. tuberculosis spreads to other regions of the lung and leaks into capillaries, resulting in transmission to other individuals and hematogenous dissemination to distant tissues.2,32 The pathology becomes highly infectious and symptomatic, and the development of granulomas can occur in different phases; As the disease progresses, three types have been recognized; solid, caseous, and necrotic,2,31,34 further favoring its replicative and infectious capacity and given by the degree of limited response of the immune system.
Overview pathogenesis of SSTBThe approach to the pathophysiology of sepsis is broad and complex; this section does not intend to provide an exhaustive review, but rather to offer the basic foundations necessary to understand the pathology. Therefore, to delve deeper into this topic, the following references are suggested.35,36
Sepsis is defined as organ dysfunction that threatens the patient's life due to an uncontrolled immune response,36,37 with physiological, and biochemical alterations of the host.36 It is conceived as the aberrant and severe systemic inflammatory response, which causes the collapse of the cellular and metabolic machinery, favoring dysfunction of vital systems in the face of an exogenous or endogenous stimulus.35–37
In SSTB, the immune state plays a transcendental role in the pathogenesis of the disease. However, how the immune status influences the host–pathogen interaction is complex and not fully understood.20 HIV is the most important factor in the development and progression of SSTB; some clinical studies have provided evidence to support that people living with HIV and M. tuberculosis have 20–30 times increased risk of developing active tuberculosis, compared to people without HIV infection,38 since it favors an immunosuppressive microenvironment for M. tuberculosis due to the critical role played by LT and the modulation of the innate and adaptive immune system, which contribute substantially in preventing the spread of M. tuberculosis to other body parts; Furthermore, the development of active TB is significantly higher during the early phase of HIV infection.39,40 This can be supported by the rapid and advanced clinical course, given by the deficient cellular response that generates a reduced inflammation with the presence of some granulomas, more intense necrosis, and a greater load of the bacillus.40 Similarly, it has been observed that DnaK induces the polarity of M1 macrophages to M2 macrophages, and these express Arg and IL-10, which suppress host immunity.30,41
Disseminated TB manifests as very severe tuberculosis sepsis, which is characterized by septic shock and severe multiple organ failure.20 SSTB occurs more frequently in immunosuppressed patients that progress to multiple organ failure16 but has also been described in immunocompetent patients.42 SSTB is more strongly associated with miliary TB, but cases of tuberculosis sepsis without miliary TB have also been reported (Fig. 2).43
It has been suggested that septic shock could be triggered by tumor necrosis factor-α (TNF-α), which is stimulated by lipoarabinomannan, a glycolipid from the cell wall of M. tuberculosis that attracts leukocytes and modulates the immune response.44,45 TNF-α is a cytokine that is present in various physiological and pathological processes of the immune response. It is recognized for its participation in most infectious processes as an important proinflammatory and pyrogenic molecule.
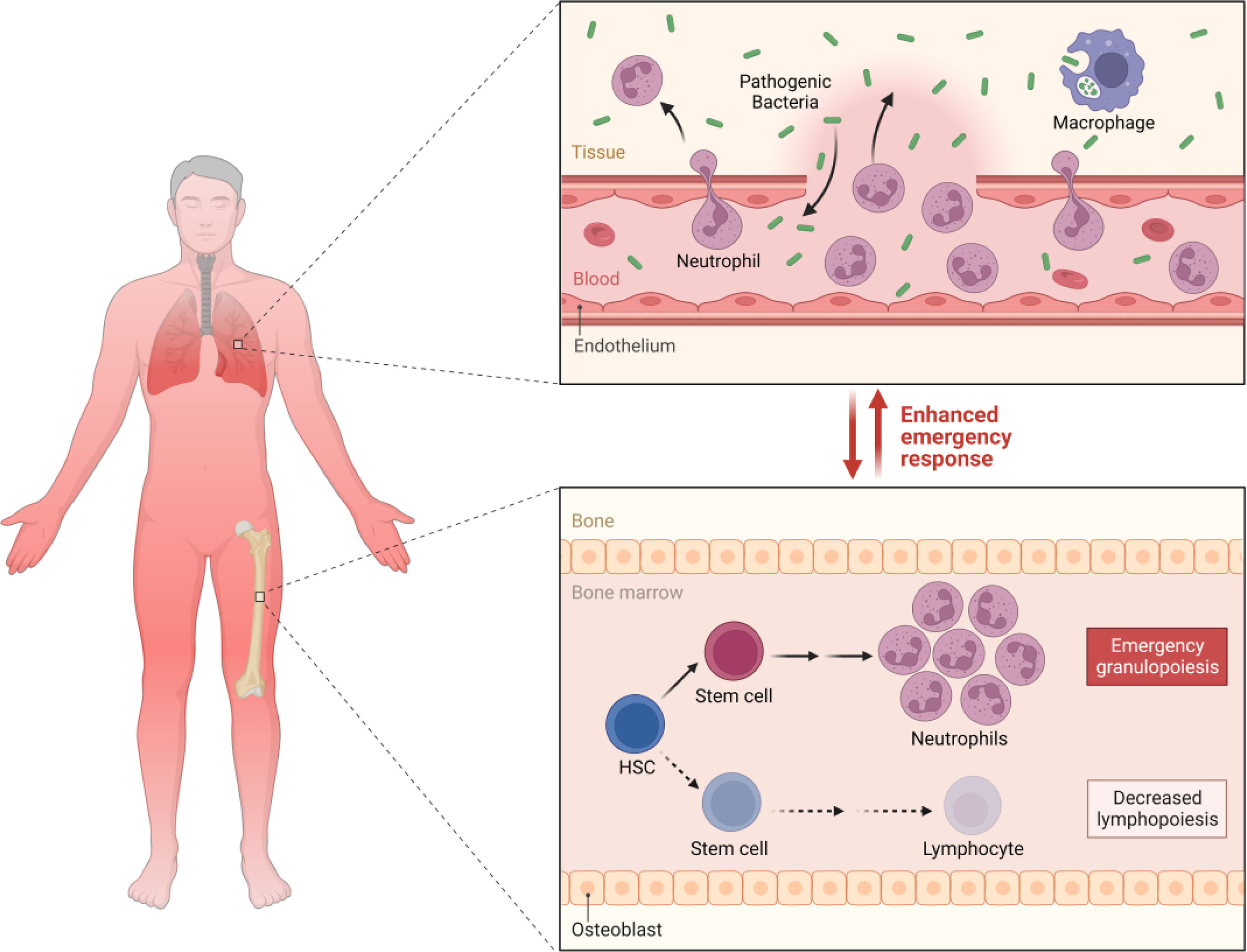
M. tuberculosis produces lipopolysaccharides that can cause sepsis, through a mechanism like Gram-negative bacteria, although the exact mechanism is not yet well established.46 It is known that lipopolysaccharides (LPS) are found in the outer membrane of the cell wall of Gram-negative bacteria and are powerful microbial mediators that contribute to the pathogenesis of sepsis and septic shock. When they enter the bloodstream, they bind to mononuclear cells and trigger the production of proinflammatory mediators such as IL-1, IL-6, and TNF-α, which stimulates subsequent reactions in the endothelial adhesion of neutrophils, which leads to the transmigration and diapedesis of these cells to the inflammatory site and the activation of the coagulation system that ends with the formation of microthrombi.16,47 For their part, in TB, in a study, they were able to demonstrate that LPS levels in severely ill patients were positively associated with plasma concentrations of IL-6, IFN-γ, and IL-1β, while IL-6 had a positive correlation with the cortisol/DHEA ratio. Higher levels of circulating LPS during progressive TB may emerge as a contributing factor to the persistence of the increased immunological endocrine imbalance distinctive of advanced disease, which could suggest a vicious cycle between LPS, inflammation, and endocrine imbalance.47 It has also been proposed that mycobacterial septic shock is triggered by the release of cytokines induced by systemic inflammation of the host in response to tuberculous antigens.48
Toll-like receptors (TLRs) are the most distinguished PRRs in the response to infectious microorganisms, but their expression on the surface of macrophages does not contribute enough to host defense.24,49 In this sense, TLRs could be involved in a decreased response of the immune system and favor the development of sepsis. At the very least, TLR2 and TLR4 have been shown to contribute to innate resistance against M. tuberculosis,49 but the evidence is still inconclusive.
Recently, it was shown that C-type lectin receptors (CLRs) are important in the recognition of PAMPs.50 In this sense, Mincle-1 is one member and is present on the surface of macrophages and its function is to recognize the glycolipid trehalose-6,6′-dimycolate from M. tuberculosis to produce nitric oxide (NO) and is necessary to induce an immune response in Th1.41,50,51 In the pathogenesis of sepsis, it has been seen that deregulation in the exaggerated production of NO leads to cardiovascular dysfunction, bioenergetic failure, and cellular toxicity52; furthermore, it generates severe vasodilation, causing hypotension and permeability of the capillaries, which leads to leakage of extracellular fluid into the third space, which forms edema and ascites.53
For its part, Dection-2 is also a CLR present in macrophages, which can recognize the mannose-covered lipoarabinomannan of M. tuberculosis, exerting its bactericidal effect by stimulating the secretion of proinflammatory cytokines by macrophages.54 It has been observed that lipoarabinomannan is a chemoattractant for leukocytes. However, its role in the pathogenesis of severe TB is not completely clear.
In the severity of tuberculosis and the human leukocyte antigen (HLA), it has been proven that the susceptibility of TB is mediated by HLA-I and HLA-II.55 In this sense, peptides that bind more slowly to class I alleles; for example, HLA-A*0201, were associated with a low frequency of CD8+ T cells in PBMC from tuberculosis patients. HLA-B alleles showed rapid off-rates in peptide binding and restricted high numbers (up to 6%) of antigen-specific CD8+ T cells in patients with pulmonary tuberculosis.56 Despite this, more research is still necessary to understand the role of HLA in SSTB.
Likewise, it has been suggested that TB may be related to endothelial injury, through an autoimmune process followed by chronic infection, triggered by the production of antibodies against mycobacterial hot shock proteins (HSP65).57 However, this may result in a cross-reaction against human HSP65 proteins, culminating in endothelial injury and atherogenesis.58 HSP65 is a superfamily of proteins that respond to a stress stimulus. In animal models, it has been shown that the inhibition of HSP65 affects IL-10 and the activity of paraoxonase-1, which increases the expression of IFN-γ and myeloperoxidase activity.58 Likewise, it is presumed that the pulmonary inflammatory state, in general, can induce the systemic inflammatory response, endothelial dysfunction, and destabilization of atheroma plaques, which have also been related to an increased cardiovascular risk in TB.59 Serum C-reactive protein has been correlated with TB load in sputum, being associated with prognostic evaluation and with a high risk of mortality.60–62 TB increases the risk of thrombosis since macrophages express tissue factor (TF) playing an essential role in coagulation and inflammation.63 TF is a transmembrane receptor that can bind to Factor VII/VIIa and triggers the extrinsic coagulation pathway when there is vascular injury.64–68 Likewise, TF also promotes the activation of protease-activating receptors (PAR), which have the power to mediate direct or downstream cell signaling in the generation of thrombin.69,70 TF Aberrant expression and activation of coagulation is a major cause of mortality associated with infection and inflammation.71 For direct action, M. tuberculosis through cell wall components can induce TF expression in macrophages.72–74 At this point, it has been shown that M. tuberculosis infection in vivo (H37Rv) markedly increases TF expression and procoagulant activity in macrophages and endothelial cells.63,75 Furthermore, the deposition of fibrin in the granuloma and the increase of cytokines such as IFN-γ, TNF-α, IL-6, and IL-3β in the lung tissue.63 Likewise, the relationship between disseminated intravascular coagulation (DIC) and deep vein thrombosis is well known,76,77 due to the hypercoagulable state.77,78
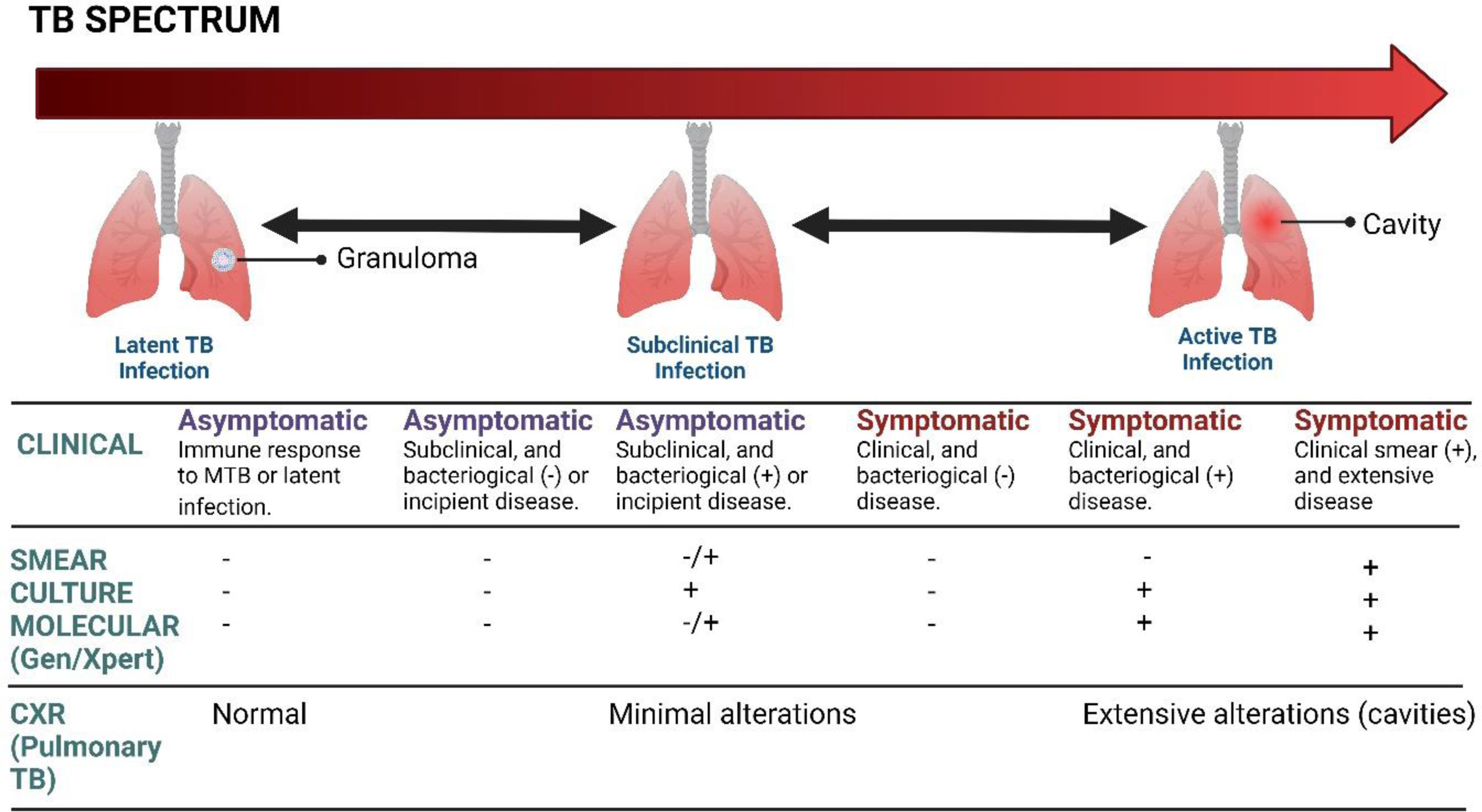
Clinical manifestationsTB has been established as a chronic disease, whose natural history course of the disease has been demonstrated by the formation of granuloma secondary to the immune response that contains the progression of the disease, as has been previously described in the pathophysiology of TB. When found in the lungs, at least three outcomes can be observed, such as eradication, primary infection, or latent infection. Acute TB can arise as a primary infection or the reactivation of a latent infection.20
The symptoms and signs of sepsis are nonspecific and can mimic a wide spectrum of other diseases.79–82 Although there are some acute forms of TB, through which it manifests itself; they have a very broad and flowery presentation, depending on the organic commitment. The acute forms that should be considered are miliary TB, cardiac TB, tuberculous meningitis, abdominal TB, cardiac tamponade secondary to tuberculous effusion, and SSTB.20 During SSTB, critically ill patients are the result of the progression of acute forms, requiring intensive care in 1–3% of cases.8 Pulmonary TB is the most frequent with a mortality that reaches 25–33% and around 70% requiring mechanical ventilation.15,83 Patients with constitutional and respiratory compromise develop acute respiratory distress syndrome (ARDS) concomitantly associated with TB, which progresses to septic shock in the absence of another cause for sepsis, becoming part of the multisystem disease.20 According to Chiu et al.,84 the most common clinical and laboratory manifestations were: fever (80%), lymphopenia (75%), and pulmonary symptoms (58%). Additionally, other symptoms such as weight loss, diaphoresis, cough, dyspnea, and hemoptysis may also occur.
Disseminated TB leads to multiple organ failure or very severe tuberculous sepsis which progresses rapidly to death. DIC has been associated with many forms of disseminated TB, however, SSTB or ARDS has a very high incidence of DIC.18,19,85 Patients with TB that induces DIC have a high frequency of hemophagocytic syndrome,76 with hematological complications being those with the greatest morbidity and mortality in SSTB.17,86
For its part, it has also been described that SSTB can present as acute abdomen that includes fever, diffuse abdominal pain, weight loss, and constipation; associated with clinical signs such as hepatosplenomegaly, pleural effusion, and ascites accompanied by retroperitoneal lymphadenopathy.48
Hypovolemic shock may be increased by adrenalitis associated with adrenal insufficiency is observed,18,20 or massive hemoptysis due to the rupture of Rasmussen aneurysm.87Cardiogenic shock is due to the involvement of myocarditis, endocarditis, pericarditis, or cardiac tamponade.20 In fulminant presentations of TB, it is essential to consider respiratory failure.19 It must also be considered that the progression of very serious tuberculous sepsis and death even occur; in patients receiving adequate antimycobacterial therapy, without a known immunodeficiency.42,85
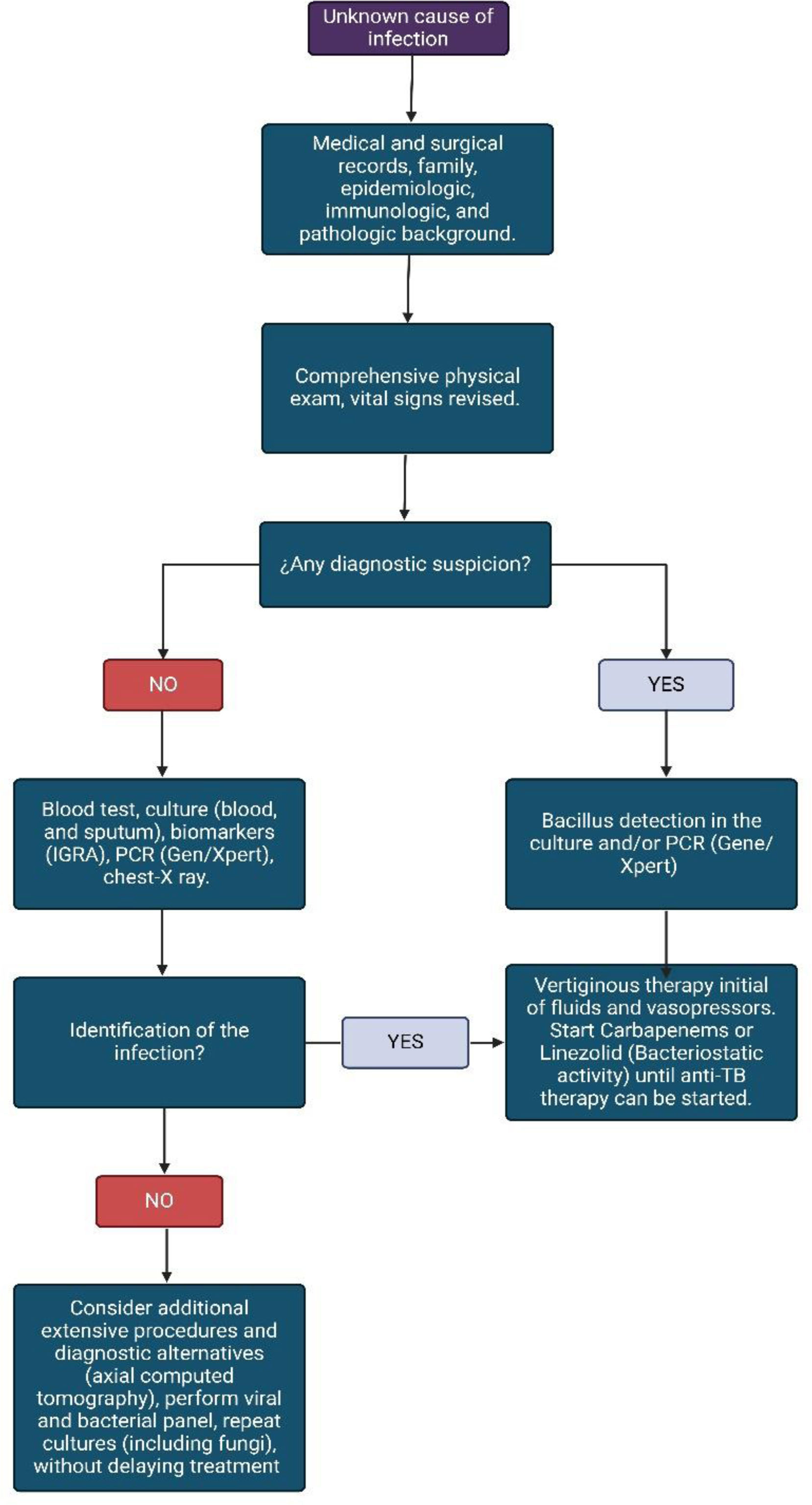
Diagnostic approachThe preparation of an exhaustive and complete clinical history, the anamnesis, and a detailed physical examination will allow us to clinically suspect the pathology in the context of septic shock. Initially, the patient must be approached in an active and rapid search for the source of sepsis.88 Although SSTB is a differential diagnosis to rule out, in the event of a torpid patient evolution, it should be considered early (see Fig. 3). It must be considered that the pathology is more likely to manifest in patients with risk factors that contribute to the development of active primary disease such as age (with a peak between 15 and 29 years), coinfection with HIV, size of the tuberculin, and exposure time.89 Risk factors linked to TB reactivation include HIV, insulin-dependent diabetes, transplants, chronic kidney disease, age, and duration of latent infection.90
During the initial physical examination, adequate taking of vital signs is essential; that allows us to clinically confront the patient's hemodynamic status. Subsequently, the rigorous search for clinical signs such as edema, paleness, decreased pulses, and S2 splitting or presence of S3. Patients over 65 years old and coinfected with HIV, may present some or few classic clinical symptoms and signs of TB, showing fewer signs of neurological system involvement and laboratory findings, such as alterations in cerebrospinal fluid, serum lymphocytes, and extrapulmonary disease. Likewise, the positivity of microbiology is diminished.91 Pancytopenia is coincident with DIC and SSTB but can be confused with the immune status in an immunocompromised patient.
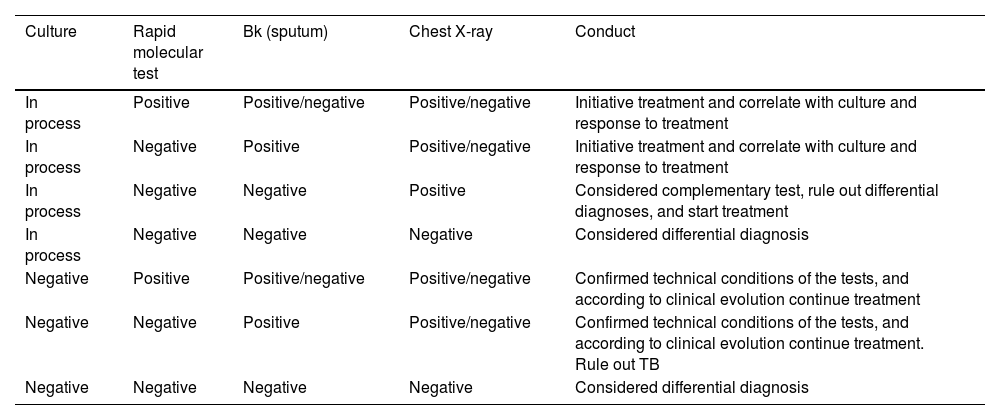
Sputum and blood cultures can take time to come back positive, GeneXpert/TB may be useful in this setting as it can reduce the timely to provide a result. In extrapulmonary TB, a recent systematic review concludes that Xpert/Ultra and Xpert MTB/RIF can be useful in diagnosis.92 In this sense, GeneXpert/Ultra and Xpert MTB/RIF provide a suggested diagnostic in critical conditions when sputum and blood cultures are expected (Table 1).92
TB test and therapeutic option.
| Culture | Rapid molecular test | Bk (sputum) | Chest X-ray | Conduct |
|---|---|---|---|---|
| In process | Positive | Positive/negative | Positive/negative | Initiative treatment and correlate with culture and response to treatment |
| In process | Negative | Positive | Positive/negative | Initiative treatment and correlate with culture and response to treatment |
| In process | Negative | Negative | Positive | Considered complementary test, rule out differential diagnoses, and start treatment |
| In process | Negative | Negative | Negative | Considered differential diagnosis |
| Negative | Positive | Positive/negative | Positive/negative | Confirmed technical conditions of the tests, and according to clinical evolution continue treatment |
| Negative | Negative | Positive | Positive/negative | Confirmed technical conditions of the tests, and according to clinical evolution continue treatment. Rule out TB |
| Negative | Negative | Negative | Negative | Considered differential diagnosis |
However, sensitivity may vary depending on the sample collected, that is, in tuberculous meningitis the Xpert/Ultra has high sensitivity, but lower specificity than culture. It should be noted that Xpert/Ultra and Xpert MTB/RIF have similar sensitivity and specificity for detecting rifampicin resistance.92 Pleural, ascitic, and cerebrospinal fluid should be evaluated for cell count, protein, glucose, and ADA levels.93
Regarding diagnostic images, a chest X-ray is useful for an initial diagnostic approach, allowing the definition of lung pathologies such as pneumonia, bronchiectasis, neoplasms, cysts, and pleural effusion.94 However, in the critical scenario, it does not contribute significantly to confirming the clinical suspicion.95 Faced with this challenge, high-resolution computed axial tomography (HR-CT) or magnetic resonance imaging (MRI) is the imaging tool that provides the most information to support the diagnosis93 (Fig. 4).
In a study conducted by Jacob et al.,7 independent factors associated with TB bacteremia include male sex, tachycardia, low CD4 count, HAART absence, fever, hyponatremia, and low hemoglobin.
TreatmentImmediate management corresponds to hemodynamic and circulatory resuscitation of the patient, with the administration of supplemental oxygen; if required, and fluids as soon as possible.80.96 Intravenous crystalloids or Ringer's lactate are the choice and should be started within the first hour.80,96 Empirical broad-spectrum antibiotic coverage is considered to cover the most common pathogens in the event of coinfection with other microorganisms. The indication for starting vasopressors is reserved for patients who do not respond to adequate initial fluid resuscitation and remain hypotensive. The first-line vasopressors that should be considered are norepinephrine, second-and third-line epinephrine or dobutamine, and vasopressin, depending on availability and reduced cardiac output.80,96
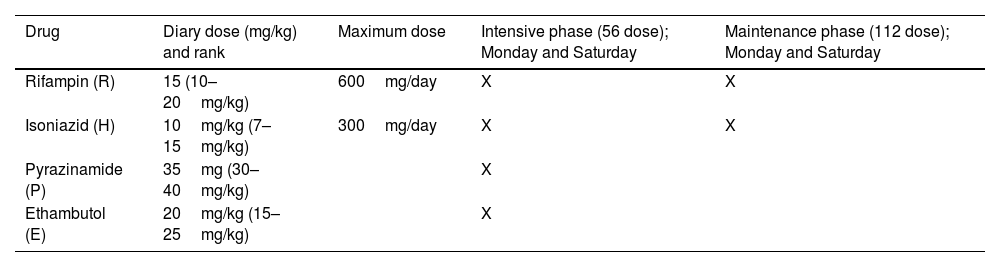
Empirically, anti-TB treatment should be started as soon as possible while waiting for diagnostic confirmation, especially if the clinical suspicion is very strong, due to the clinical symptoms and signs that suggest the pathology and through consistent imaging findings (Table 2).97 It has been suggested in the scenario of critically ill patients that the doctor should not wait for the sputum culture results to begin anti-tuberculosis treatment, since the delay in the start of treatment significantly affects the outcome.13 The parenteral route must be prioritized for the administration of drugs in disseminated TB and SSTB, given that it has been determined that the oral route has unpredictable absorption, probably due to the poor splanchnic circulation in shock, which can lead to poor results, and unfavorable in the patient.13.93 In this sense, it has been determined that antimycobacterial therapy is more complex in critically ill patients because of unpredictable absorption, altered pharmacokinetics, increased side effects, and limited first-line intravenous agents. Second- and third-line agents are frequently used, with little clear data or consensus on their use.98 Therefore, some investigators have proposed monitoring serum drug levels instead of standard dosing schedules.20,99 In this context, carbapenems (ertapenem, imipenem, meropenem) are used to treat multidrug-resistant (MDR) and extensively drug-resistant tuberculosis (XDR-TB) which is an alternative option for critical SSTB patients. A systematic review by Sotgiu et al. showed that treatment success was higher than 57% in five studies with culture conversion rates between 60% and 94.8%, suggesting that safety and tolerability is very good.100,101 Also, linezolid has anti-TB bacteriostatic activity in XDR-TB and is an excellent option in this scenario.102
Treatment in SSTB.
| Drug | Diary dose (mg/kg) and rank | Maximum dose | Intensive phase (56 dose); Monday and Saturday | Maintenance phase (112 dose); Monday and Saturday |
|---|---|---|---|---|
| Rifampin (R) | 15 (10–20mg/kg) | 600mg/day | X | X |
| Isoniazid (H) | 10mg/kg (7–15mg/kg) | 300mg/day | X | X |
| Pyrazinamide (P) | 35mg (30–40mg/kg) | X | ||
| Ethambutol (E) | 20mg/kg (15–25mg/kg) | X |
Treatment of pulmonary and extrapulmonary TB; including miliary TB and SSTB without coinfection with HIV, consists of 2 months of rifampicin, isoniazid, pyrazinamide, and ethambutol, followed by the 4-month phase.97 When there is coinfection with HIV, treatment is challenging,103 since drug interactions can modify the dose, safety, and response. Furthermore, the condition changes radically, and in this scenario, the evidence and development of new regimes may provide an option103 (Fig. 5).
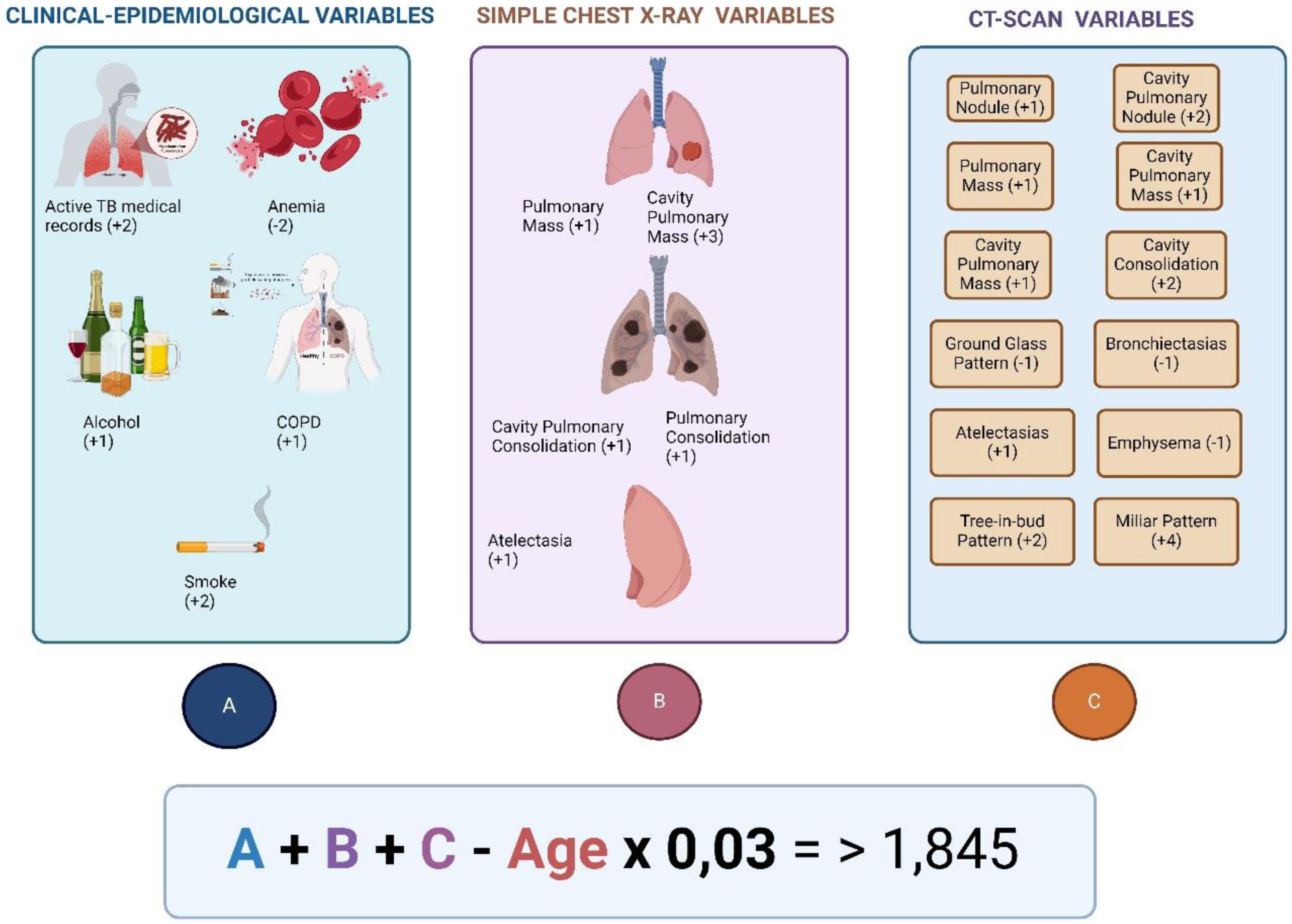
Risk prediction score for diagnostic of active TB. The model suggests the diagnosis of active TB in patients with a score higher than 1.845. The model obtained a sensitivity of 85.1%, and specificity of 83.6%. Besides, positive predictive value was 26.6%, and negative predictive was 98.7%.
Taken and adapted for Navarro-Ballester et al.102.
Steroids have been proposed for 6 weeks in patients with tuberculous meningitis, tuberculous pericarditis, and tuberculous adrenalitis, in those with persistent septic shock despite adequate fluid resuscitation.13,93 In a systematic review, glucocorticoid use was not associated with improved survival [OR 0.65, 95% CI: 0.27–1.57].9
Recombinant human-activated protein C has been used successfully, but its indications in the hospital context have not yet been defined.104
Remarks conclusionsSSTB has a wide variety of differential diagnoses, especially due to its broad and variable clinical manifestations, which are determined by multisystem organic involvement, in those with prolonged symptoms upon admission to the emergency room, negative cultures, and no response to the standard antimicrobial therapy.
It is a pathology with high mortality if it is not treated promptly, therefore, it is imperative to consider a high degree of diagnostic suspicion of acute TB, including a rigorous clinical history, and clinical, radiological, pathological, and laboratory findings that support the diagnosis. However, they should not delay the initiation of anti-tuberculosis treatment appropriately.
FundingNone declared.
Conflict of interestNone declared.