To reduce unnecessary biopsies (Bx) in an opportunistic screening programme of prostate cancer.
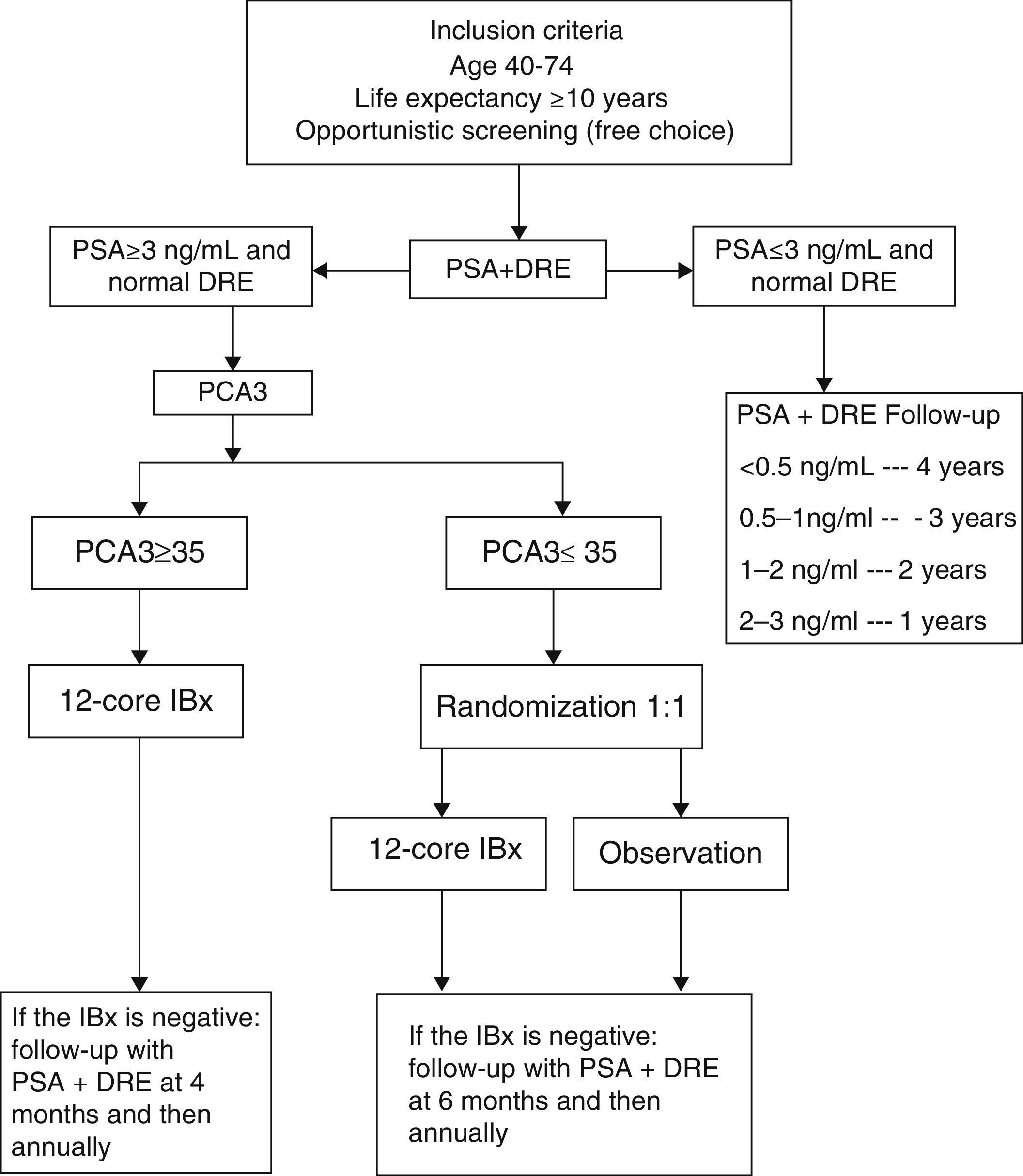
Material and methodsWe performed a prospective evaluation of PCA3 as a second-line biomarker in an opportunistic screening for prostate cancer (PCa). From September 2010 until September 2012, 2,366 men, aged 40–74 years and with >10 years life expectancy, were initially screened with PSA/digital rectal examination (DRE). Men with previous Bx or with recent urine infections were excluded. Men with abnormal DRE and/or PSA>3ng/ml were submitted for PCA3. All men with PCA3≥35 underwent an initial biopsy (IBx) —12cores—. Men with PCA3<35 were randomized 1:1 to either IBx or observation. Re-biopsy (16–18 cores) criteria were PSA increase>.5ng/ml at 4-6months or PSAv>.75ng/ml/year.
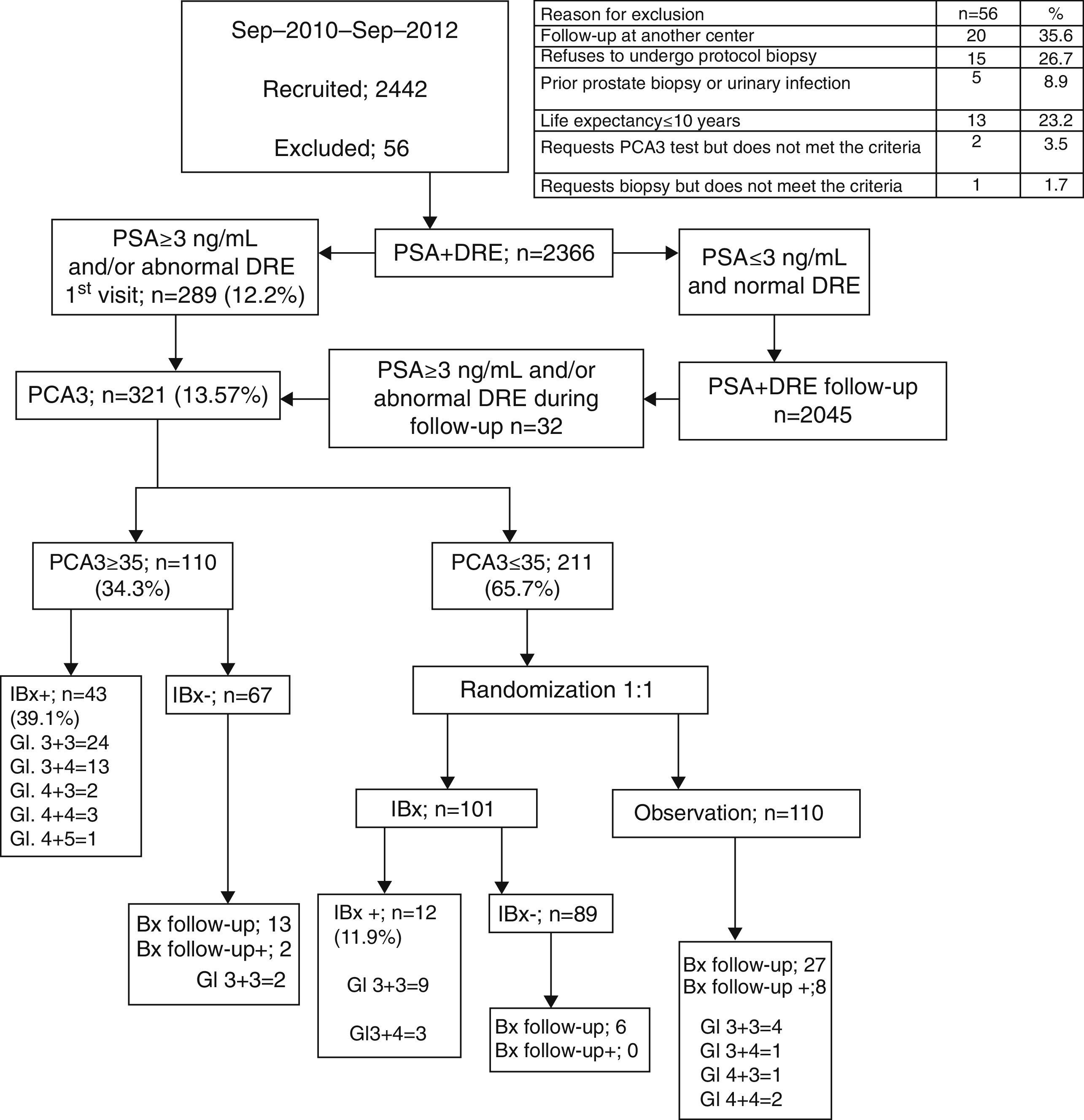
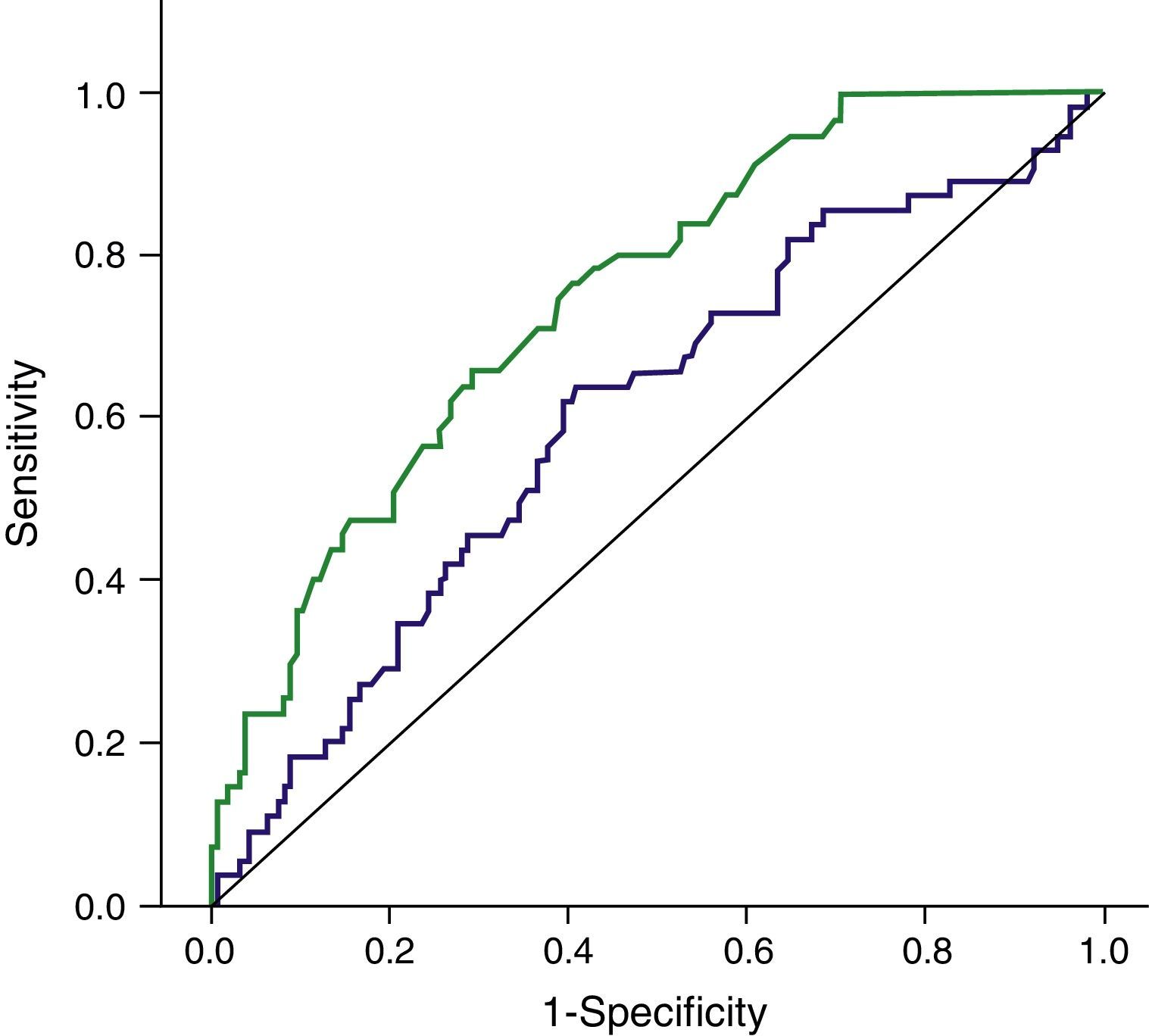
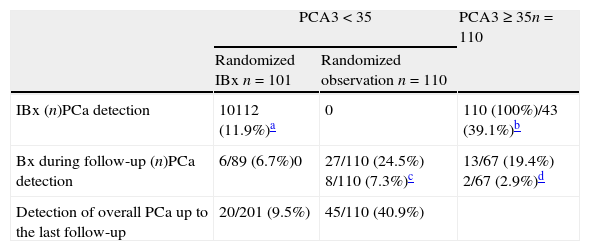
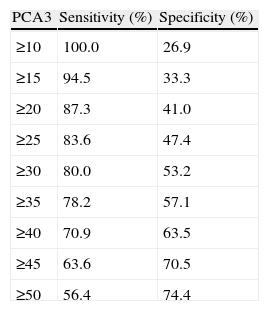
ResultsWith a median follow-up (FU) of 10.1months, PCA3 was performed in 321/2366 men (13.57%), 289 at first visit and 32 during FU. All 110 PCA3+ men (34.3%) were biopsied and PCa was identified in 43 men in IBx (39.1%). In the randomized arm, 110 were observed and 101 underwent biopsy, finding 12 PCa (11.9%), showing a statistically significant reduction of PCa detection rate in this cohort (p<.001). Global PCa detection rates were 40.9% and 9.5% for the PCA3+ and PCA3− branches, respectively (p<.001). Area under the curve for PSA and PCA3 were .601 and .74, respectively. This is an ongoing prospective study limited by its short follow-up period and still limited enrolment.
ConclusionsPCA3 as a second-line biomarker within an opportunistic dual screening protocol can potentially avoid 65.7% and 50.1% biopsies at first round and at median FU of 10.1months, respectively, just missing around 3.2% of high grade PCa.
Reducir el número de biopsias (Bx) innecesarias en un programa de cribado oportunista en cáncer de próstata (CaP).
Material y métodosEstudio prospectivo y aleatorizado evaluando el PCA3 como biomarcador de segunda línea. De septiembre de 2010 a septiembre de 2012 2.366 hombres con edad en rango 40–74 años, y más de 10 años de expectativa de vida, fueron estudiados mediante PSA y tacto rectal (TR), excluyendo los biopsiados previamente o con infección urinaria reciente. Ante un TR sospechoso y/o PSA>3ng/ml se les realizó un PCA3. A todos aquellos con PCA3≥35 se les realizó una Bx inicial (IBx) —12 cilindros—. Con PCA3<35 fueron aleatorizados 1:1 a IBx u observación. Los criterios de rebiopsia (16–18 cilindros) durante el seguimiento fueron un incremento de PSA>0,5ng/ml a 6meses o PSAv>0,75ng/ml/año.
ResultadosCon un seguimiento medio de 10,1 meses se testó el PCA3 en 321/2.366 hombres (13,57%), 289 en la primera visita y 32 durante el seguimiento. Entre los 110 hombres con PCA3+ (34,3%) se identificó CaP en 43 en IBx (39,1%). En el brazo aleatorizado 110 se observaron y 101 se biopsiaron, encontrando 12 CaP (11,9%), mostrando un reducción en la detección de CaP estadísticamente significativa en esta cohorte (p<0,001). Las tasas de detección global de CaP fueron de 40,9 y 9,5% para las ramas PCA3+ y PCA3− respectivamente (p<0,001). AUC para PSA y PCA3 fueron 0,601 y 0,74. Este es un protocolo abierto en este momento, limitado por su seguimiento insuficiente.
ConclusionesEl PCA3 como biomarcador de segunda línea en un programa de cribado oportunista podría potencialmente evitar un 65,7% de IBx y 50,1% a 10 meses de seguimiento, dejando de diagnosticar 3,2% de CaP de alto grado.