Estramustine, an agent with both hormonal and non-hormonal effects in men, is supposed to be effective in treating castration-resistant prostate cancer. However, previous studies have reported conflicting results. We conducted this meta-analysis to evaluate the efficacy and toxicity of additional estramustine to chemotherapy.
MethodsData sources including PubMed, Medline, EMBASE, and Cochrane Controlled Trials Register were searched to identify potentially relevant randomized controlled trials. Prostate specific antigen (PSA) response, overall survival, and grades 3–4 toxicity were analyzed.
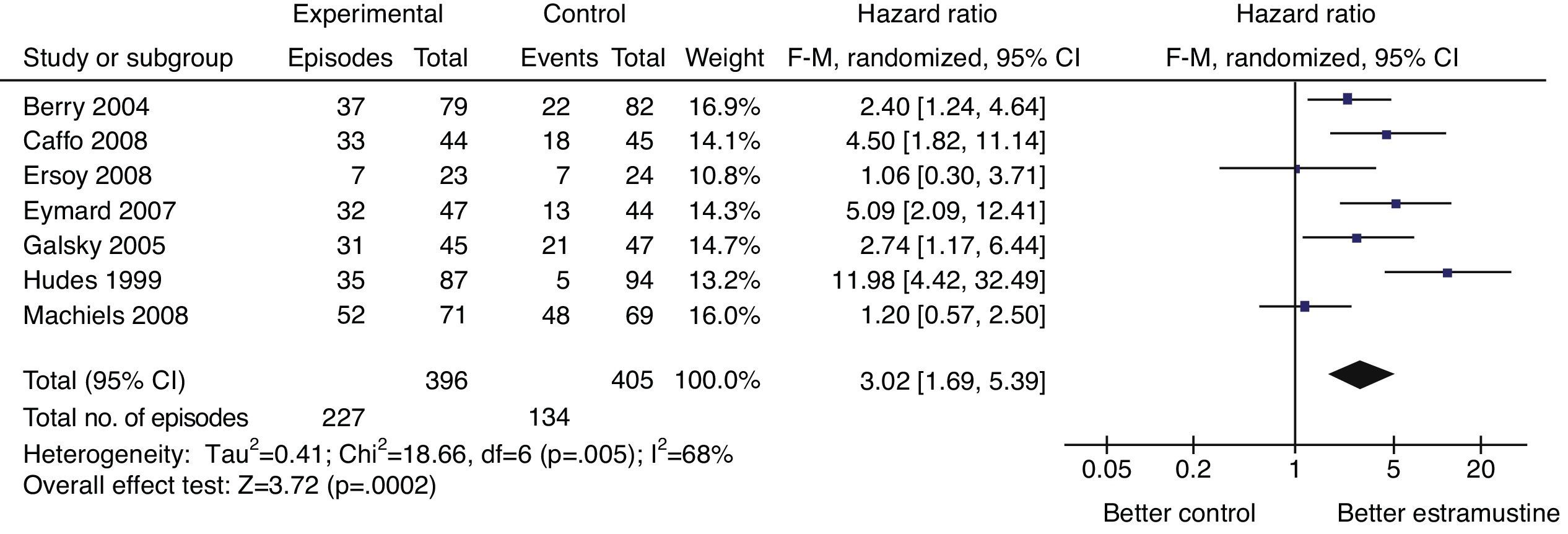
ResultsSeven randomized controlled trials, a total of 839 patients, were enrolled. The pooled odds ratio for PSA response was 3.02 (95% CI=1.69–5.39, p=.0002); the pooled hazard ratio for overall survival was .95 (95% CI=.80–1.14, p=.58); the pooled odds ratio for nausea/vomiting and cardiovascular toxicity were 3.90 (95% CI=1.05–14.45, p=.04) and 2.22 (95% CI=1.15–4.30, p=.02). No significant difference was detected for neutropenia, anemia, thrombocytopenia, diarrhea, fatigue, or neuropathy (p>.05).
ConclusionsAccording to this meta-analysis, chemotherapy with additional estramustine increased the PSA response rate. However, it increased the risk of grade 3 or 4 adverse effects such as nausea/vomiting and cardiovascular events, and the overall survival was not improved for castration-resistant prostate cancer patients.
La estramustina, un agente con efectos tanto hormonales como no hormonales en los hombres, se supone que es eficaz en el tratamiento de cáncer de próstata resistente a la castración. Sin embargo, estudios previos han notificado resultados contradictorios. Hemos llevado a cabo este metaanálisis para evaluar la eficacia y la toxicidad de estramustina adicional a la quimioterapia.
MétodosSe realizaron búsquedas en las bases de datos, incluyendo PubMed, Medline, EMBASE y Cochrane Controlled Trials Register, para identificar los ensayos controlados aleatorizados potencialmente relevantes. Se analizaron la respuesta del antígeno prostático específico (PSA), la supervivencia global y la toxicidad de grado 3 a 4.
ResultadosSiete ensayos controlados aleatorizados, un total de 839 pacientes, fueron incluidos. La odds ratio combinada para la respuesta de PSA fue de 3,02 (IC 95%=1,69-5,39, p=0,0002); el cociente de riesgos instantáneos agrupado de supervivencia global fue de 0,95 (IC 95%=0,80-1,14, p=0,58); la odds ratio combinada para náuseas/vómitos y toxicidad cardiovascular fue de 3,90 (IC 95%=1,05-14,45, p=0,04) y 2,22 (IC 95%=1,15-4,30, p=0,02). No se detectó ninguna diferencia significativa para neutropenia, anemia, trombocitopenia, diarrea, fatiga o neuropatía (p>0,05).
ConclusionesSegún este metaanálisis la quimioterapia con estramustina adicional aumentaba la tasa de respuesta del PSA. Sin embargo, aumentaba el riesgo de efectos adversos de grado 3 o 4 como náuseas/vómitos y los episodios cardiovasculares, y la supervivencia global no mejoró en los pacientes con cáncer de próstata resistente a la castración.