Design a care protocol to restart scheduled surgical activity in a Urology service of a third level hospital in the Community of Madrid, in a safe way for our patients and professionals in the context of the SARS-CoV-2 coronavirus epidemic.
Material and methodsA multidisciplinary group reviewed the different recommendations of the literature, national and international health organizations and scientific societies, as well as their application to our environment. Once scheduled surgery has restarted, the patients undergoing surgery for complications related to COVID-19 are being followed up.
ResultsSince the resumption of surgical activity, 19 patients have been scheduled, of which 2 have been suspended for presenting COVID-19, one diagnosed by positive PCR for SARS-CoV-2, and another by laboratory and imaging findings compatible with this infection. With a median follow-up of 10 days (4–14 days), no complications related to COVID-19 were detected.
ConclusionsPreliminary results indicate that the protocol designed to ensure the correct application of preventive measures against the transmission of coronavirus infection is being safe and effective.
Diseñar un protocolo asistencial para reiniciar la actividad quirúrgica programada en un servicio de Urología de un hospital de tercer nivel de la Comunidad de Madrid, de manera segura para nuestros pacientes y profesionales en el contexto de la epidemia por coronavirus SARS-CoV-2.
Material y métodosConstituimos un grupo multidisciplinar que se encargó de analizar las diferentes recomendaciones de la literatura, organizaciones sanitarias nacionales e internacionales y sociedades científicas, así como de su aplicación a nuestro medio. Una vez reiniciada la cirugía programada, se está llevando a cabo un seguimiento de los pacientes intervenidos en cuanto a complicaciones relacionadas con COVID-19.
ResultadosDesde el reinicio de la actividad quirúrgica se han programado 19 pacientes, de los cuales 2 han sido suspendidos por presentar COVID-19, diagnosticado uno por PCR positiva para SARS-CoV-2, y otro por alteraciones analíticas y radiológicas compatibles con esta infección. En el seguimiento realizado no se han detectado complicaciones relacionadas con COVID-19, con una mediana de seguimiento de 10 días (4-14 días).
ConclusionesResultados preliminares indican que el protocolo diseñado para asegurar la correcta aplicación de medidas de prevención de transmisión de la infección por coronavirus está siendo seguro y efectivo.
The Community of Madrid is the most affected in our country with 71,503 cases of COVID-19 (diagnosed by PCR and antibody tests) and 8826 deaths caused by this disease (data as of May 15, 2020).1 This situation has forced us to change our usual practice in order to adapt to the population needs.
Thus, the Hospital Universitario 12 de Octubre, one of the most complex hospitals in the Community of Madrid, with 1256 hospital beds, has dedicated most of its resources to the care of COVID-19 patients, in the same way as other health centers.
The Urology department, as all surgical services in our country,2 has had to cope with this situation, in such a way that the scheduled surgical activity was suspended as of March 16, 2020, with only urgent surgery being performed. Meanwhile, urologists have been included in dedicated teams to provide healthcare for COVID-19 patients (hospitals and medicalized hotels), and have carried out telephone consultations for urologic patients.
Currently, the overcoming of the peak of the SARS-CoV-2 coronavirus pandemic indicates the need to restart scheduled surgical activity, since for many patients the delay in their intervention may be associated with a worse prognosis or severe quality of life damage.3–5 This requires adequate planning to ensure safe and high-quality healthcare, minimizing the risk of SARS-CoV-2 infection, which can be associated with significant complications.6
With this objective, we set up a work group to develop a healthcare protocol, which adapts the main recommendations of scientific societies to our framework. We will present the main recommendations gathered in this protocol, as well as an analysis of its initial results.
The main objective of the protocol is to organize surgical activity and adjust it to the therapeutic needs of the patients, trying to avoid delays that could imply a poor evolution of their pathology and reducing the risk of SARS-CoV-2 coronavirus infection in our patients and healthcare professionals. The specific objectives are to normalize the performance guidelines in the Surgical Services, adapting them to the best scientific evidence possible, to resource availability and to the optimization of their use, as well as to analyze the postoperative evolution of our patients.
Material and methodsProtocol designThe Urology department, together with the Quality Management Unit, developed an initial workplan proposal, which was later joined by representatives of the Anesthesiology and Resuscitation services, Admissions, Preventive Medicine, Occupational Risk Prevention, Microbiology, Radiology, Clinical Analysis, Infectious Diseases Unit and members of the Medical and Nursing Directorate as well as Continuity of Care and Patient Assistance. In order to apply this protocol to all Surgical Services, representatives from all surgical specialties were involved.
We reviewed the main recommendations of the surgical and anesthesiological societies (in the case of Urology we used those of the European Association of Urology7), as well as the main databases (Medline and Embase), using as keywords “COVID-19”, “SARS-CoV-2” and “Surgery”. No language or publication date restrictions were applied, and the bibliographic references of the articles and documents included were reviewed.
Based on the analysis performed, a consensus document was proposed and subsequently approved by the Management Committee of our hospital.
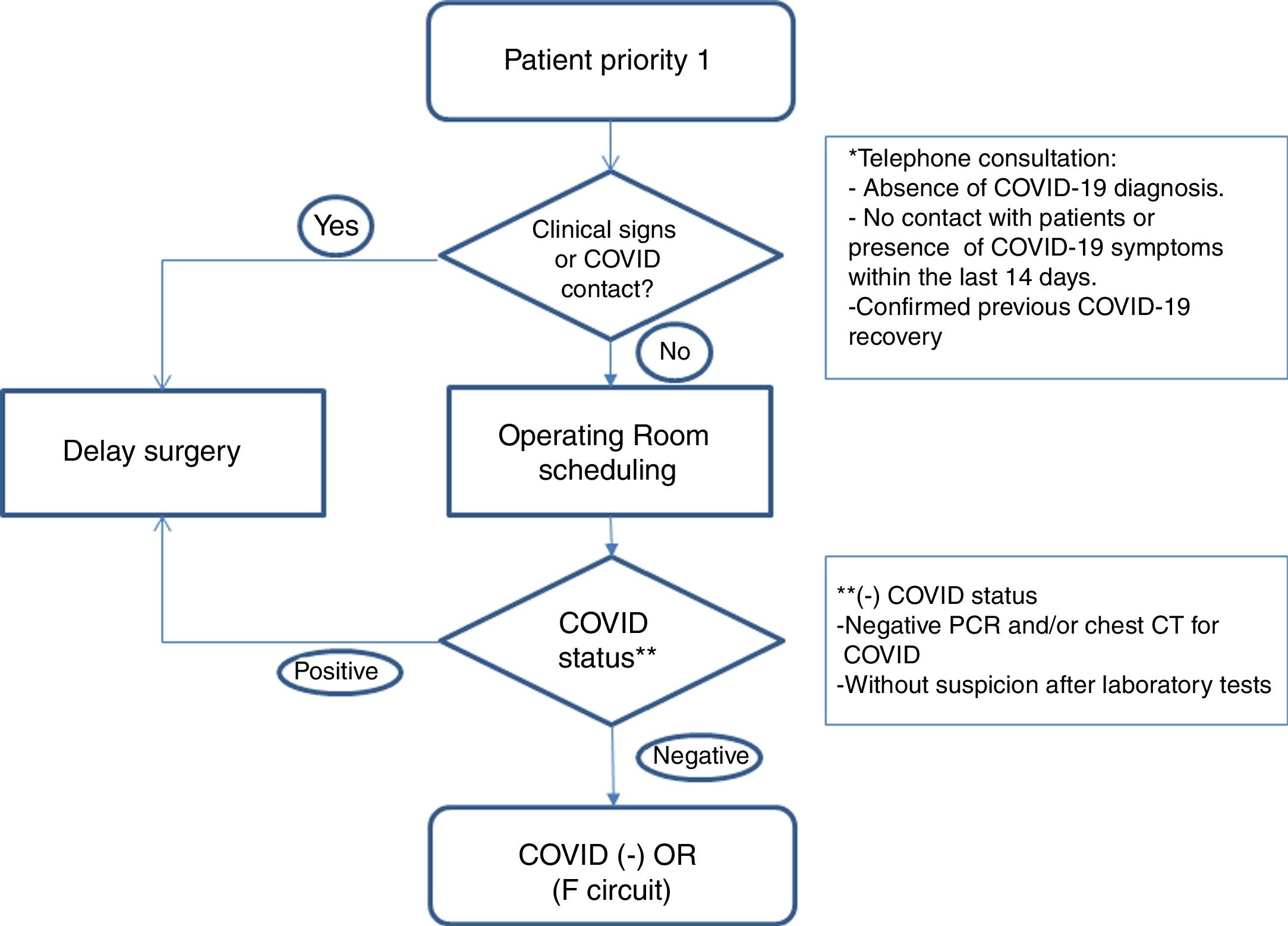
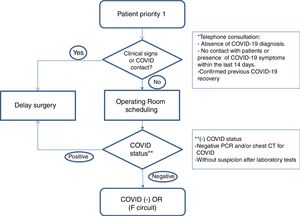
Healthcare planWe establish action guidelines according to the different stages of the process, which are summarized in Fig. 1.
- 1.
Patient selection: we included patients with priority 1 on the surgical waitlist (Table 1), taking into account comorbidities that could increase surgical risk8 (COPD, HBP, immunodeficiencies, obesity, diabetes, neoplasms and cardiovascular disease). We reached them by phone to rule out infection or symptoms related to COVID-19 and contacts with patients in the last 14 days. We informed them of the possible risk of complications related to this infection. If there was no suspicion of disease, and the patient accepted the risks involved in the procedure, surgery was scheduled.
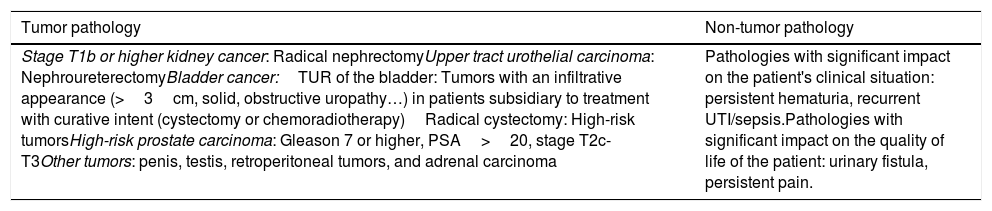
Table 1.Pathologies included in priority 1.
Tumor pathology Non-tumor pathology Stage T1b or higher kidney cancer: Radical nephrectomyUpper tract urothelial carcinoma: NephroureterectomyBladder cancer:TUR of the bladder: Tumors with an infiltrative appearance (>3cm, solid, obstructive uropathy…) in patients subsidiary to treatment with curative intent (cystectomy or chemoradiotherapy)Radical cystectomy: High-risk tumorsHigh-risk prostate carcinoma: Gleason 7 or higher, PSA>20, stage T2c-T3Other tumors: penis, testis, retroperitoneal tumors, and adrenal carcinoma Pathologies with significant impact on the patient's clinical situation: persistent hematuria, recurrent UTI/sepsis.Pathologies with significant impact on the quality of life of the patient: urinary fistula, persistent pain. - 2.
Preoperative evaluation: in addition to the preanesthetia evaluation, patients’ covid status was determined with a PCR test, 24–72h before the intervention, and surgery was suspended if the result was positive.
- a.
PCR for SARS-CoV-2 coronavirus.
- b.
Blood count, LDH, C-reactive protein and ferritin analysis and coagulation test. In case of finfings suggestive of COVID-19 with negative PCR, patients were submitted to clinical and radiological evaluation.
- c.
Computed tomography (CT) of the chest: it is performed in cases of high suspicion of COVID-19 in laboratory tests, symptomatic patients with negative PCR, and in patients with high anesthetia and/or surgical risk.
- a.
- 3.
Establishment of differentiated pathways: patients are included in the negative COVID-19 circuit (F circuit), with dedicated inpatient units, operating and recovery rooms for positive patients or those under evaluation.
- 4.
Protective measures for the patient: preventive measures are maximized, including restricted visitors and companions (telephone information), admission to single-person rooms, limited patient movement and use of a surgical mask at all times. As for professionals, we must perform hand hygiene, wear gloves, surgical facemask and disposable gowns.
- 5.
Operating room procedures: there is a high probability of finding COVID-19 patients without apparent symptoms and with a negative PCR result. Aiming to reduce the chances of infection among healthcare personnel, droplet and airborne transmission precautions are taken in risky procedures, such as those performed on the airway (intubation, suctioning, …), as well as in surgical interventions with aerosol-generating procedures or risk of blood or body fluids splashes. These measures include recommendations on personal protective equipment (PPE).
- 6.
Teaching activity: the participation of resident doctors as main surgeons or assistants is considered according to the procedure and the risk of the patient. In case they participate, personal protection precautions are maximized performed under adequate supervision.
Prospective, descriptive study of the first patients operated after the implementation of measures included in the “Protocol for surgical activity during the transition phase of the SARS-CoV-2 coronavirus pandemic” of the Hospital Universitario 12 de Octubre.
Several variables and indicators have been collected for the evaluation of the protocol, including demographic data, diagnostic and preoperative features and indicated procedure, results of clinical, epidemiological and analytical studies prior to surgery, postoperative complications, appearance of COVID-19 in the immediate postoperative period and evolution of these patients.
Patient consent was requested for data collection and analysis, and it meets the requirements of the Ethics Committee for Research with medicinal products (ECRmp) of the Hospital Universitario 12 de Octubre.
ResultsOn May 4, 2020, elective surgery activity was restarted. By then, there were 43 patients on the priority 1 waitlist. Of them, 23 presented oncological pathologies.
During the first 2 weeks we were provided with one operating room daily, since part of the surgical services was still dedicated to the care of patients with COVID-19.
Twenty-three patients were evaluated; none of them had a history of COVID-19, suspicious symptoms, or had been in close contact with COVID-19 patients. Of these, 4 were not scheduled due to different reasons: one was treated at another hospital (radical prostatectomy), another for recent diagnosis of pulmonary embolism (laser adenomectomy), and another 2 were undergoing chemotherapy (adrenal tumor and bladder neoplasm).
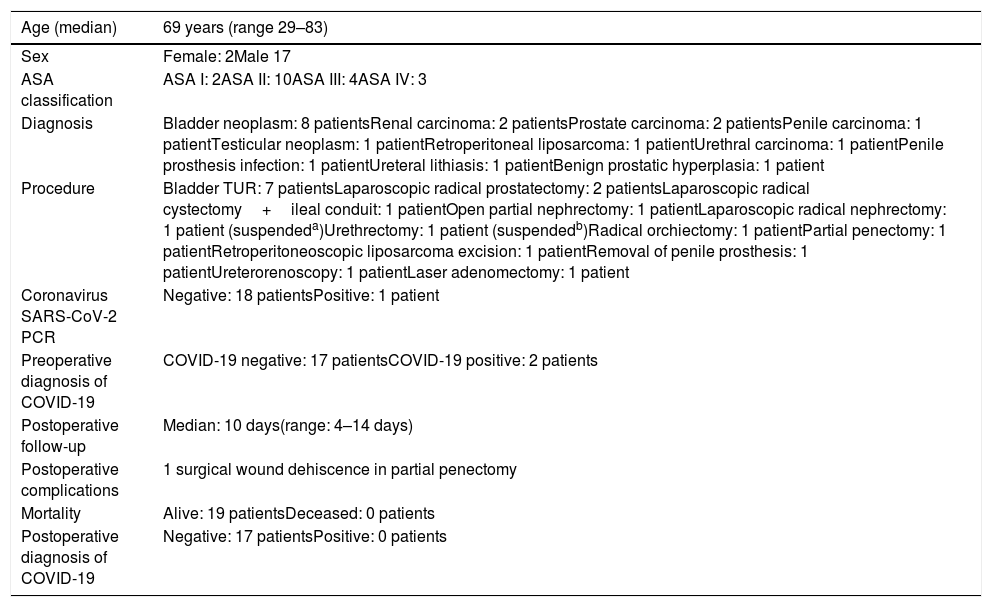
The 19 patients scheduled (all of them asymptomatic) underwent PCR for SARS-CoV-2 coronavirus and blood test according to protocol (Table 2). Two patients were suspended, one for positive PCR (urethral tumor scheduled for urethrectomy), and the other (renal tumor scheduled for radical nephrectomy) for presenting abnormal laboratory findings (lymphopenia, increased CRP and coagulopathy). In the latter, despite the negative PCR for coronavirus, a chest X-ray was performed showing the presence of a middle-field infiltrate of the left lung suggestive of pneumonia, possibly related to COVID-19.
Scheduled patients.
| Age (median) | 69 years (range 29–83) |
|---|---|
| Sex | Female: 2Male 17 |
| ASA classification | ASA I: 2ASA II: 10ASA III: 4ASA IV: 3 |
| Diagnosis | Bladder neoplasm: 8 patientsRenal carcinoma: 2 patientsProstate carcinoma: 2 patientsPenile carcinoma: 1 patientTesticular neoplasm: 1 patientRetroperitoneal liposarcoma: 1 patientUrethral carcinoma: 1 patientPenile prosthesis infection: 1 patientUreteral lithiasis: 1 patientBenign prostatic hyperplasia: 1 patient |
| Procedure | Bladder TUR: 7 patientsLaparoscopic radical prostatectomy: 2 patientsLaparoscopic radical cystectomy+ileal conduit: 1 patientOpen partial nephrectomy: 1 patientLaparoscopic radical nephrectomy: 1 patient (suspendeda)Urethrectomy: 1 patient (suspendedb)Radical orchiectomy: 1 patientPartial penectomy: 1 patientRetroperitoneoscopic liposarcoma excision: 1 patientRemoval of penile prosthesis: 1 patientUreterorenoscopy: 1 patientLaser adenomectomy: 1 patient |
| Coronavirus SARS-CoV-2 PCR | Negative: 18 patientsPositive: 1 patient |
| Preoperative diagnosis of COVID-19 | COVID-19 negative: 17 patientsCOVID-19 positive: 2 patients |
| Postoperative follow-up | Median: 10 days(range: 4–14 days) |
| Postoperative complications | 1 surgical wound dehiscence in partial penectomy |
| Mortality | Alive: 19 patientsDeceased: 0 patients |
| Postoperative diagnosis of COVID-19 | Negative: 17 patientsPositive: 0 patients |
Of the 17 patients who underwent surgery, 14 were diagnosed with neoplasms. Only 3 patients had a non-oncological diagnosis (a ureteral lithiasis, a penile prosthesis infection and one benign prostatic hyperplasia).
During postoperative follow-up, with an interval of 4 to 14 days (median 10 days), no patients presented data on SARS-CoV-2 coronavirus infection. As relevant complications, we only found a surgical wound dehiscence in a penectomy.
DiscussionAccording to the epidemiological data, the deceleration phase of the COVID-19 pandemic began in mid-April. This translates into less human and material resources dedicated to this pandemic, which enables resumption of surgical activity, at least partially.
To this end, we must guarantee patient and healthcare personnel safety, following the recommendations of the health care authorities and scientific societies, adopting the best practices available in our framework.9,10 Based on these premises, we developed our care protocol, which highlights an adequate selection of patients (according to priority criteria), a prior rigorous study of their situation with respect to the COVID-19, and the establishment of pathways and procedures that guarantee, as far as possible, the safety of patients and professionals.
We need an adequate prioritization of our patients, not only in terms of oncological or non-oncological pathologies, but also those with possible associated complications.2,7,10,11 We must consider that delaying surgery in oncological patients may imply a worse prognosis, but it may also increase complications in non-oncological patients.3–5 In our case we have chosen to classify according to priorities, which includes not only tumoral pathology but also patients with potential complications with significant clinical impact and loss of quality of life.
The response to surgical stress is associated with relevant changes in patient immunity.12 These alterations require extreme precautions to be taken in surgical patients, taking into account that a 20% mortality rate has been reported in asymptomatic patients scheduled for surgery during the incubation period of COVID-19.6 In addition, we must take into account that most of these patients present oncological pathologies, which seems to be associated with a greater vulnerability, among other infections, to the COVID-19.13 This is why we determine the patient's COVID-19 status before surgery by means of a brief epidemiological survey; in case it is negative, we carry out the PCR for coronavirus SARS-CoV-2 as well as blood tests in search of alterations that suggest COVID-19.14 Given the possibility of a false negative in the PCR for coronavirus, in case of suspicion due to abnormal laboratory test results, a chest CT scan would be indicated to rule out COVID-19.15,16 Systematic chest CT could be considered in all patients prior to surgery; however, we must take into account the capacity of each center in relation to the volume of surgeries performed. One of our first patients presented abnormal laboratory findings suggestive of COVID-19, with negative PCR for coronavirus. In this case, a chest CT scan was not performed, since the chest X-ray diagnosed pneumonia.
Once the negativity of the patient for COVID-19 has been reasonably established, we must ensure the necessary conditions for a safe intervention and postoperative period.9,10 To this effect, we establish a negative COVID-19 circuit (F circuit), different from the one for patients diagnosed with COVID-19 and pending evaluation. It includes various in-patient units, operating rooms and resuscitation/ICU beds. Likewise, extreme measures must be taken to avoid contagion during admission, through hand hygiene, as well as with the use of disposable gloves, masks and gowns by hospital personnel. We must restrict visitors, use private rooms, and limit patient movement. In order to avoid patient isolation, we can provide a tablet to make video calls to family or friends, and prioritize quick recovery measures so that discharge is as early as possible.10
The protection of healthcare personnel has to be a priority. In the specific case of operating rooms, the established measures recommend minimum personnel in the operating room, limited traffic, procedures performed by experienced surgeons, and reduced teaching work.17,18 The use of protective equipment in Urology stands on the performance of aerosol-generating procedures, as well as on the risk of splashing (risk of significant bleeding and contamination with gastrointestinal contents19). Despite the fact that disease transmission through urine has not been demonstrated yet, the persistence of SARS-CoV-2 nucleic acid in urine has been reported, and preventive measures are advisable.7 These measures include waterproof gown and shoe covers, face shields or goggles and type IIR (splash resistant) masks.
The laparoscopic approach provides our patients with better recovery, but it could be associated with a higher risk of contagion through surgical smoke. We must keep pneumoperitoneum pressure as low as possible, and be careful to avoid leakage by using trocars of the smallest possible caliber or the use of suction before removing trocars and for specimen retrieval.7,17,20,21
Study limitations may include the fact that it is a small series regarding the number of patients, and the short follow-up (although all patients exceed the mean COVID-19 incubation period). However, we believe that the actions carried out for the development of this protocol, as well as the recommendations comprised in it, could be valuable to other groups who are currently designing their strategy to restart elective surgery.
Compliance with these measures should be monitored continuously, as well as our patients’ outcomes. The detection of cases of COVID-19 healthcare-associated infections would require an assessment of patient selection measures, preoperative evaluation and clearly, the functioning of the negative COVID-19 circuit.
ConclusionsThe progressive deceleration of the COVID-19 epidemic brings us to a new stage in which elective surgery activity must be restarted. It must be done in a way that the safety of our patients is guaranteed, without forgetting the safety of the health professionals. The protocolization of procedures provides us with quality care for our patients, minimizing the risk related to infection by the SARS-CoV-2 coronavirus. Preliminary results indicate that the protocol designed to ensure the correct implementation of preventive measures for the transmission of coronavirus infection is being safe and effective.
FundingThis study has not received any funding.
Conflicts of interestThe authors of this study state that they have no conflicts of interest.
Please cite this article as: Tejido-Sánchez A, González-Díaz A, García-Rojo E, Santos-Pérez de la Blanca R, Varela-Rodríguez C, Ruiz-López P, et al. Diseño de un protocolo asistencial para el reinicio de la cirugía urológica programada en periodo de epidemia COVID-19. Actas Urol Esp. 2020;44:597–603.