The system that protects body from infectious agents is immune system. On occasions, the system seldom reacts with some foreign particles and causes allergy. Allergies of the ear, nose and throat (ENT) often have serious consequences, including impairment and emotional strain that lowers the quality of life of patients. This is further responsible for the common cold, cough, tonsillitis, dermal infection, chest pain and asthma-like conditions which disturb one's day to day life. The present review enlightens some common ENT allergies which one can suffer more frequently in one's lifetime, and ignorance leads to making the condition chronic. Information regarding pathophysiology and the management of ENT allergy by this review could help clinicians and common people to better understand the circumstances and treatment of ENT allergy.
The inappropriate immune response to an allergen is known as allergy.28 Broadly speaking, the inordinate reaction of the immune system against fungi, parasites, foreign particles like foreign organisms, organic molecules, dust, chemicals etc. leads to allergy. These foreign particles enter through the respiratory tract and react abnormally to the body cells.70 This may also be defined as the hypersensitivity reactions of our body cells initiated by specific immunological mechanisms against particular particles. Hypersensitivity is a reproducible symptom or signs rudiment by exposure to a powerful stimulus at a dose tolerated by an individual.36 The type-1 hypersensitivity reactions are encouraged by the non-scrounging antigen, i.e. allergen, in atopic individuals. In this type of hypersensitivity response cells like tissue mast cells and blood basophils are sensitised by the interaction with a Fc receptor of an IgE antibody produced in opposition to allergen. When the same allergen exposes again then crosslinking of bound IgE on sensitised cells occurs and this results in the degranulation of the allergen. The active mediators like histamines, leukotrienes and prostaglandins results in the contraction and vasodilation in smooth muscles and nearby tissues.3 In developed countries, allergic diseases are of great public health concern.55 The organs that show localised allergic symptoms after the entry of any type of allergens are eyes, nose, throat and lungs. In lungs, inhaled allergen triggers increased production of mucus and bronchoconstriction which also leads to coughing and shortening of breath, respectively.3
Antigen is a molecular substance that triggers a cascade of immunological reactions to a healthy person. This develops mainly a sensitisation response that specifically synthesises the IgE antibody. Aero allergens trigger sensitisation in the respiratory system and food allergens trigger that in the digestive system.47 These are air and food borne in nature: pollens and dust particles are air borne, and milk, animal proteins, drugs etc. are food borne. The allergic determinants in allergens are mostly proteins, allergens such as pollens are water soluble proteins or glycoproteins released from cytoplasm of pollens via diverse mechanisms.3,34 Chemically, most allergens are proteins, having molecular weight ranges between 3 and 80kDa. However, the chemical, structural and functional characteristics of these allergens have not been clearly identified to explain stimulating response of IgE antibody.47
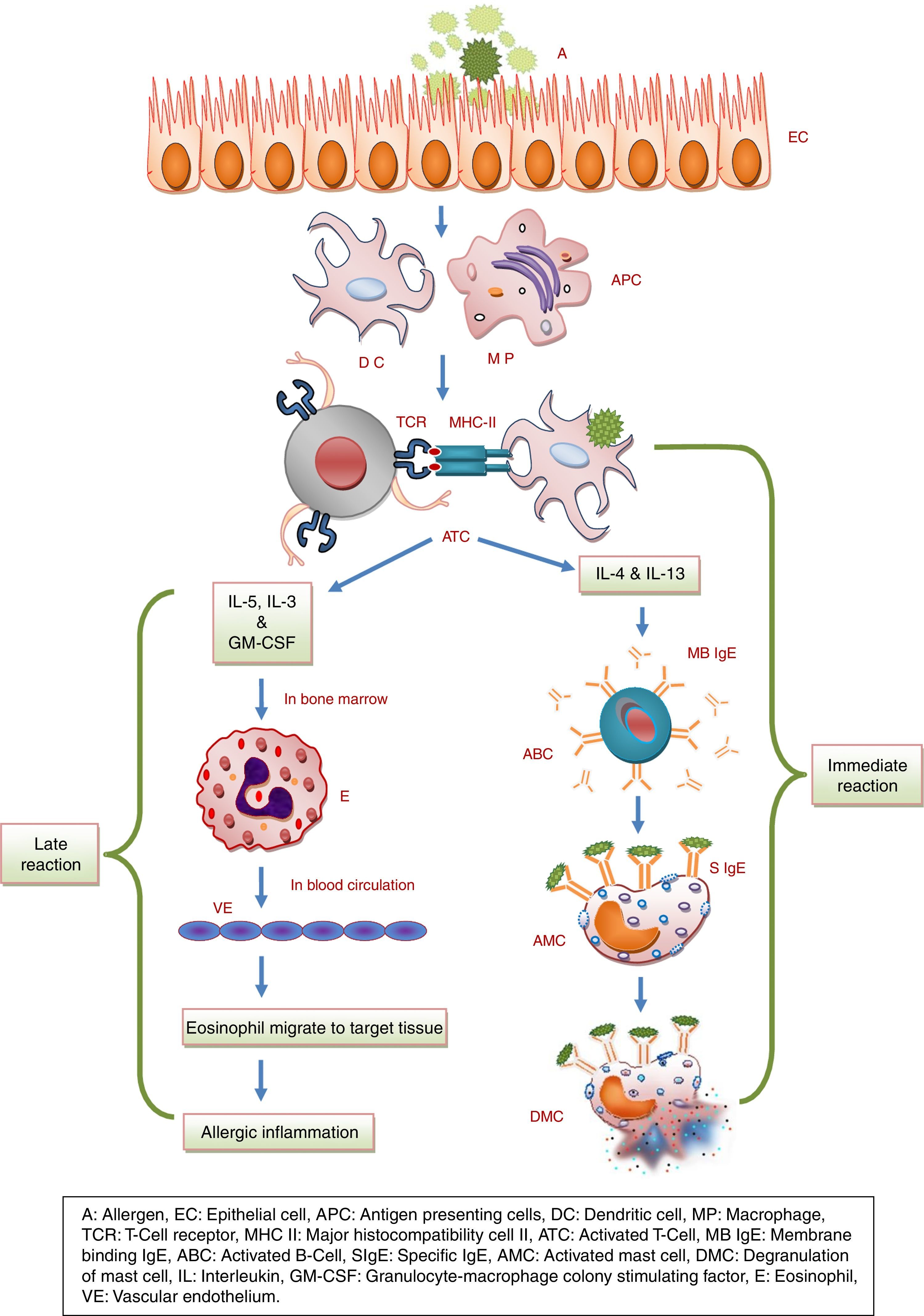
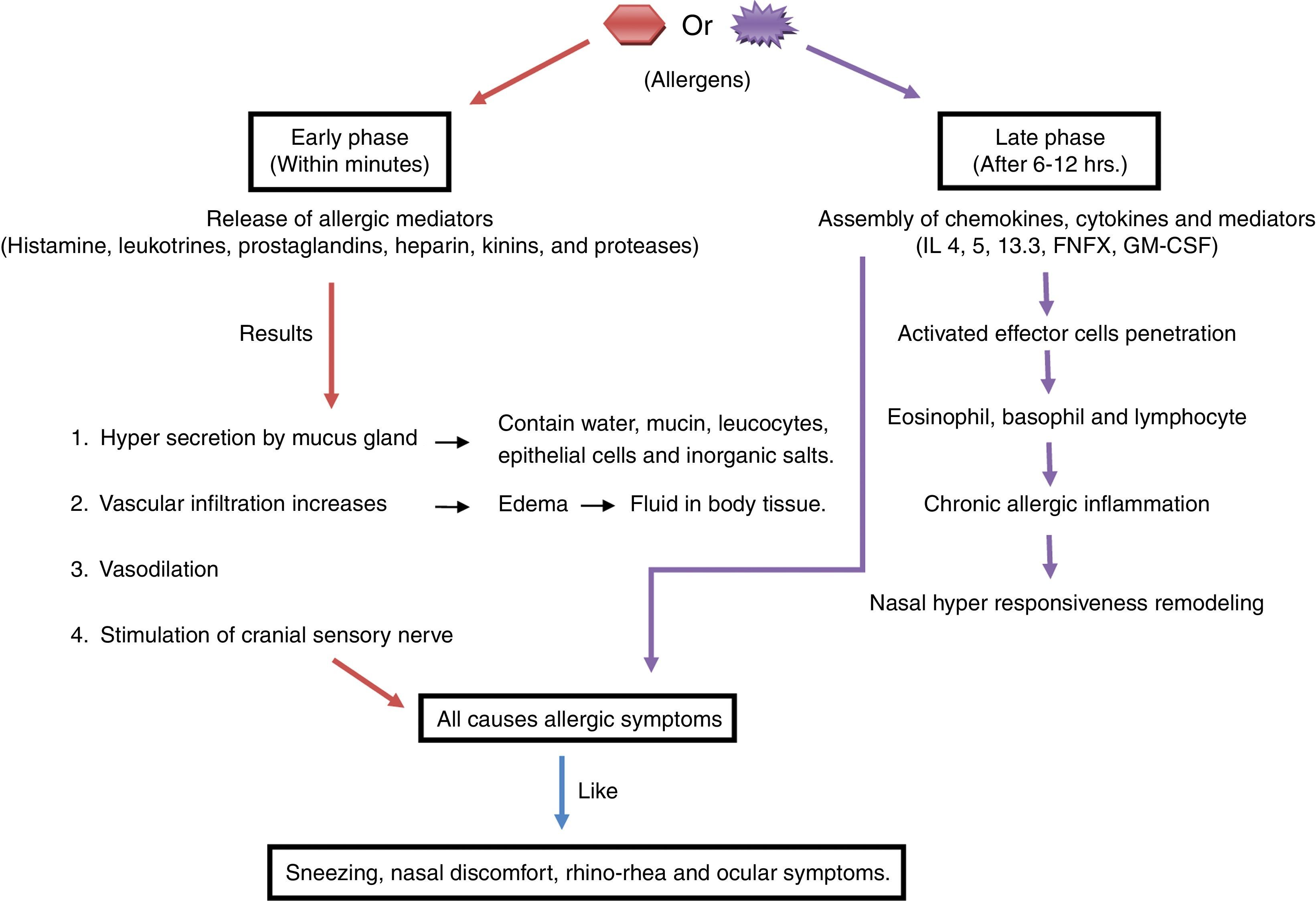
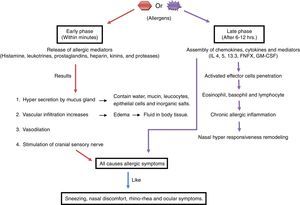
Pathophysiology of allergyThe immune response to allergy can be antibody-mediated or cell-mediated.36 During an allergic immune response, the antigen presenting cell (APC) reacts with allergen and presents peptides to the T cell. The activated T cell produces IL4 resulting in the activation of B cell. Mast cell and IgE antibodies are produced from activated B cell, which leads to phase reactions such as bronchospasm, sneezing and itching in the tissues by the chemicals such as histamine, leukotrienes and prostaglandins29,70 as shown in Fig. 1.69 Moreover, phase reaction includes two phases i.e. early and late: the early phase reaction starts within minutes after the exposure to allergen, consecutively cytokine secretion such as TNF-α, and IL-4 occurs hours later in late phase reaction9 as shown in Fig. 2. Beside this, cells like eosinophils, neutrophils and macrophages are also in the body that are involved in protection from foreign particles. On the basis of the sources of allergens, allergy can be divided into categories as food allergy, chemical allergy, seasonal allergy, pet allergy, drug allergy, dust allergy and cosmetic allergy etc.70 A variety of allergens contain activating properties for the epithelium, such as house dust mites have protease activity. In addition to proteases and oxidases, pollen extract contains associated lipid mediators or adenosine, low molecular weight molecules that have capability to induce and modulate immune cells.26
Diagrammatic representation of the pathophysiology of allergy. A, Allergen; EC, Epithelial cell; APC, Antigen presenting cells; DC, Dendritic cell; MP, Macrophage; TCR, T-Cell receptor; MHC II, Major histocompatibility cell II; ATC, Activated T-Cell; MB IgE, Membrane binding IgE; ABC, Activated B-Cell; SIgE, Specific IgE; AMC, Activated mast cell; DMC, Degranulation of mast cell; IL, Interleukin; GM-CSF, Granulocyte-macrophage colony stimulating factor; E, Eosinophil; VE, Vascular endothelium.
Manifestations of allergy cover a broad area of phenotypes, by combining with almost every organ of the body and producing a wide range of possible symptoms.2 Allergies of ear, nose and throat significantly affect the daily life of adults as well as children. Diseases such as allergic rhinitis, otitis media, otitis externa and mould allergy have serious consequences such as retarded growth and development in academics among children in developing countries.54 The most common childhood infection, otitis media, leads to mortality of over 50,000 children under five years of age.51,54 Inflammation, irritation and ultimately infection in ear, nose and throat is directly due to an increase in air pollutants in the environment.22
Allergic rhinitisThe inflammation of nasal mucosa by the immune response mediated by immunoglobulin (IgE) antibodies results in allergic rhinitis.16 The term allergic rhinitis simply explains the clinical areas in which allergy is the specific cause of rhinitis that gives rise to inflammation of nasal mucous membranes. When itching, sneezing, increased secretion and blockage like hypersensitivity symptoms are mediated by an IgE antibody then the term IgE-mediated allergic rhinitis is used.36 This is the most frequent allergic disease and also common of all chronic conditions in children.23 This is due to a seasonal or perennial response to allergens.82 Medically, the symptoms included are sneezing, rhinorrhoea, nasal obstruction and nasal membrane, pharynx, or soft palate itching. Ocular symptoms are also sometimes associated with bronchial asthma.8,16 In allergic rhinitis, the provocation of allergen leads to an inflammatory response in both upper and lower airways, which supports the fact that asthma exists simultaneously with rhinitis. This is proven by the fact that the upper (nose, nasal cavity, paranasal sinuses, pharynx and larynx) and lower (trachea, bronchial tubes, bronchioles and lungs) respiratory tracts are interrelated physiologically, functionally and immunologically. This represents a combined inflammatory disease of allergic rhinitis and asthma.72
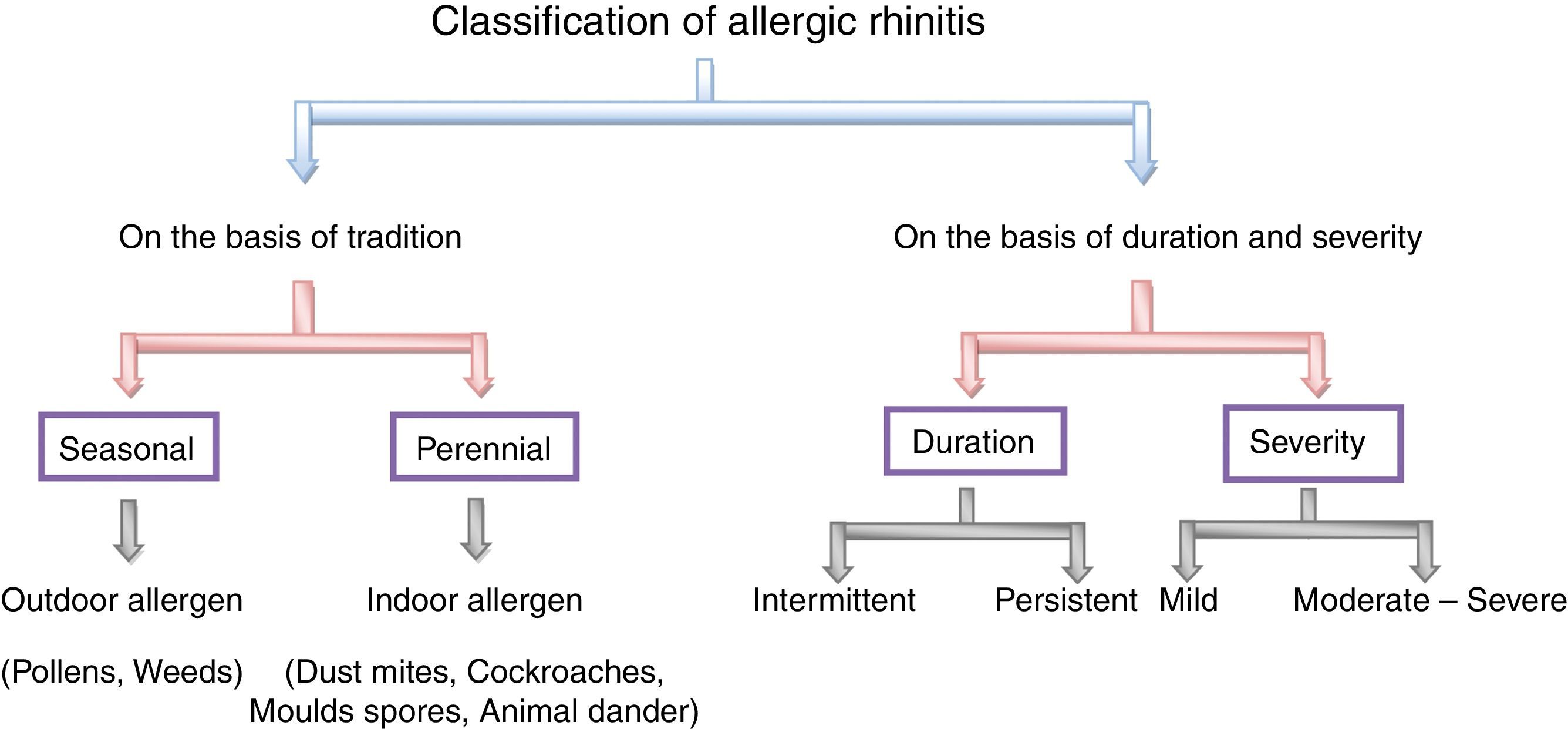
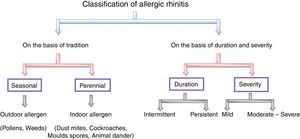
Classification of allergic rhinitisTraditionally, allergic rhinitis is classified into the two classes, namely seasonal and perennial. The former occurs during a specific season at a particular time of year and the later describes the symptoms to allergens that persist throughout the year.41,42,72 The pollen from trees, grasses and weeds are the causes of seasonal allergic rhinitis while indoor allergens like dust mites,79 cockroaches,71 mites,47,61 moulds spores79 or animal dander52 refer to perennial allergic rhinitis.41 The best example of seasonal allergic rhinitis is pollens20 induced allergic rhinitis in temperate climates but this may be perennial allergic rhinitis in warmer climates.42 To avoid this confusion, a new classification is suggested according to the duration and severity of symptoms. It may be intermittent or persistent in duration and mild, moderate and severe in severity.7,73 When the duration of inflammation is less than six weeks and when it persists throughout the year then it is said to be intermittent and persistent allergic rhinitis, respectively. Symptoms of mild allergic rhinitis include normal body performance and are usually intermittent, whereas the moderate or severe condition significantly affects the normal day-to-day living activity and is considered to be troublesome. The classification on the basis of duration and severity of symptoms helps in the management of individual patients,73 as shown in Fig. 3.
Pathophysiology of allergic rhinitisIn allergic rhinitis after the exposure of the inciting allergen viz. air borne dust mites, faecal particles, cockroach residues, animal dander, moulds and pollens, numerous inflammatory cells along with mast cells, CD4+ T cells, B cells, macrophages and eosinophils permeate the nasal lining. In the mucosal surface, peptides of allergens are processed by dendritic cell (APC) and presented to the major histocompatibility complex (MHC) class II molecule. This triggers the activation and production of allergen-specific T helper (Th) cells. Principally Th cells lie within the nasal mucosa which releases cytokines like interleukin (IL)-3, IL-4, IL-5 and IL-13, these IL's promote plasma cells to produce IgE. The cytokines from activated Th2 cells induce isotype switching of B cells which also produce specific IgE, eosinophils proliferation, neutrophils and mast cell.48 With this response, mediator as histamine and leukotrienes are released. Symptoms like itching, rhinorrhoea, mucus secretion, smooth muscle contraction, arteriolar dilation and increased vascular permeability are triggered.17,73 The cytokines and mediators are released during the early phase of an immune response; after that the late phase reaction includes cellular inflammatory response within four to eight hours showing the frequently appeared symptoms.42,72
ManagementFor the treatment of allergic rhinitis, several factors like nasal anatomy, airflow dynamics, muco-ciliary dynamics, non-allergic nasal inflammatory diseases, the nasal cycle, temperature regulation and humidity control issues, exercise, age, external pollutants and toxins are taken into account for the response to allergic rhinitis treatment.23
Pharmacologically it is treated by a stepwise approach depending upon the duration and severity of the allergic condition. The treatment is based on ARIA (allergic rhinitis and its impact on asthma) guidelines 2008, namely:
- A.
Antagonists of leukotriene receptor can be used in all types of allergic rhinitis.
- B.
Antihistamines of second generation are more preferable over the first-generation antihistamines.
- C.
The most effective drug for adult and paediatric allergic rhinitis is topical steroids.
Oral antihistamines,14 intranasal antihistamines,5 intranasal corticosteroid,6 anti IgE antibody,7 leukotriene receptor antagonists,33 immunotherapy like sublingual immunotherapy13 are the treatments to be taken for curing allergic rhinitis.48 The categorisation of phenotypes of allergic rhinitis is needed, which helps to improve the perceptiveness of environmental and occupational factor that determines the severity of disease. It may also help to understand the endogenous and exogenous factors that develop symptoms from rhinitis to asthma.57
Otitis media with effusion (OME)The common name of this disease is glue ear and is a widespread disease in childhood. Hearing loss is the most common symptom in children acquired by OME.40 The disease is characterised and defined by fluid containing inflammation in the middle ear space with the absence of acute symptoms.11,25 The disease may be associated with hearing loss, delayed language development and typically following a preceding upper respiratory illness with or without otalgia, if remain unmanaged.64 Behavioural, developmental and cognitive difficulties are also found to be associated with OME, which sometimes persist in late childhood and also in early teens.11
The reasons regarding OME development are not very clear but it was found that increased receptiveness to local airway infections or allergic inflammatory response leads to blocking the opening of the Eustachian tube and adenoidal contagion or hypertrophy.11 This is due to the nasal cavity and Eustachian tube both being lined by the same type of epithelium, i.e. respiratory type ciliated mucus producing epithelium. The epithelium becomes pseudostratified and thickened by disorganised dedifferentiation in chronic inflammation and in efficiency of this de-ciliated disorganised type and sub-mucosal inflammation results in the retention of secretions in the middle ear space.64 Viral and bacterial infections have been found in children with OME. Reflux of gastric contents into nasal-pharynx and Eustachian tube, allergy and cigarette smoke irritation also leads to OME.11,64
In another case, a chronic condition of otitis media was found showing eosinophilic enriched secretions with characteristic highly viscous oedematous pink mucosa. The condition is termed as eosinophilic otitis media (EOM) as the effusion contains many eosinophils despite the presence of type I allergy.35
PathophysiologyMore often in OME, middle ear and tympanic membrane functioning decreases due to the presence of fluid, this leads to occasional pain and pressure changes.78 The disproportionate ratio of pro-inflammatory cytokines and inhibitors may result in the acute to chronic condition of otitis media. In OME, due to the imbalance of the above factors, the middle ear is consistently having activated inflammatory cells. This also sometimes synchronised with upregulation of mucin genes when OME is due to bacterial infection.43 Immune modulation and upregulation of mucin genes proliferates mucous producing goblet cell and mucous gland density.12 There were a significant number of allergy competent cells and cytokines found in the middle ear of OME patients, implicating allergic process in OME also.81
Management of OME- A.
Hearing aids: when the OME is associated with hearing impairment then hearing tactics and aids are very useful. Hearing aids may be an effective alternative to surgery.32
- B.
Auto-inflation: Pressure is provided in the nasal-pharynx, which cause the Eustachian tube to open and allowing effusions in the middle ear to drain. Auto-inflation is helpful in the management of OME, but is not simple to perform and not really practiced in young children.64
- C.
Ventilation tube: Insertion of ventilation tube improves the hearing for some time. Adenoidectomy in addition with ventilation tube gives better improvement and benefit over ventilation tube insertion alone. There was an increase in 6–12db in mean hearing threshold by using ventilation tube. Long term insertion sometimes gives high complication rates and adverse effects.18,63
- D.
Surgical treatment: to reduce Eustachian tube dysfunction and blockage, surgery such as adenoidectomy, tonsillectomy and adenotonsillectomy are undertaken to improve OME. By adenoidectomy one can able to improve hearing and behaviour along with perfection in nasal and upper respiratory health, but no improvement in symptoms of ear infection was observed.59,64,78
- E.
Oral steroids such as prednisolone given along with antibiotic were found to be effective for a short period of time. In long term cases, steroids were found ineffective.65 Intranasal steroids have the same effect as oral one.11
Fungi are ubiquitous eukaryotic organisms different from prokaryotes, plants and animals. They exist as unicellular (yeast) as well as multi-cellular, forming hyphal network called mycelium. Spores are produced by them via both asexual and sexual processes. Phyla like Zygomycota, Ascomycota and Basidiomycota represent the certain allergenic fungi.58 Certain fungi (moulds) cause adverse human health effects. Harmful immune response can be mechanised via allergic reaction by mould and sometime their by-product or secondary metabolites consists of a complex mixture of organic compounds that effects largely by producing toxic irritants. During winter, 24-h mean outdoor spore concentration ranges 50spores/m3 and in summer it reaches up to 50,000spores/m3. Dry air spores of genus Cladosporium and Alternaria peak in afternoon hours during low humidity; on the other hand wet air spores like ascospores and basidiospores of mushrooms and puffballs reach their peak during high humid pre-dawn hours. Temperature and dew point are the crucial meteorological factors affecting the presence and absence of spores.10 As per the allergen database, 189 fungal species produce allergens, consisting of 61 protein families having 132 allergens approximately. The sensitisation of mould allergy is both IgE-mediated and non-IgE-mediated.74 However, all IgE binding molecules produced by moulds are not equally.76 The clinical allergy by moulds includes asthma, bronchopulmonary aspergillosis, fungal sinusitis and hypersensitivity pneumonitis.10
Allergic fungal rhinosinusitis (AFRS)It is an immunological disease, a well-documented type of chronic rhino sinusitis and most probably non-tissue-invasive disease, representing allergic hypersensitivity response.24 AFRS can largely be defined by the presence of extramucosal fungi and mucin within sinus cavity, showing thick, persistent, eosinophilic secretion with significant histological characters.30 Microscopically mucin is very similar to that found in patients with allergic bronchopulmonary aspergillosis (ABPA).27
The primary criteria for diagnosing AFRS are IgE-mediated type I hypersensitivity, nasal polyposis, marked CT results, mucin along with eosinophilic mucus and positive fungal smear and culture. Whereas the same secondary criteria were also considered with patients having AFRS these are: asthma, unilateral preponderance, radiological osteoporosis, type of fungal culture, charcot leyden crystals and serum eosinophilia.30
PathophysiologyThe pathogenesis of AFRS is always a matter of controversy but a combined form of all views are discussed here. As the disease is mostly persistent with chronic rhino sinusitis, severe allergic inflammation directed against fungal colonisation is very common.31 A research by Luang et al., confirmed that the peripheral blood lymphocytes of patients with AFRS show production of Th2 cytokine (IL-5) in vitro when induced with fungal antigens of genus Cladosporium and Alternata, whereas healthy control subjects do not give such type of production.46 The AFRS patients are completely indistinguishable from allergic rhinitis and fungal allergy patients, in terms of fungal specific serum IgE level related to total serum IgE measurements.56 The Th2 cytokines played a very important role in accounting eosinophilic inflammation in AFRS, as the eosinophil accumulation and eosinophil mediated attack on fungal hyphae in mucus is due to the involvement of Th2 derived IL-5 and IL-13 components.31 Further it is cleared that AFRS is non-infectious and an immunologically mediated process because in AFRS patients eosinophilic mediators predominated over neutrophil-derived mediators, while the ratio is equal in normal persons as control.27
Management of AFRSThe most common way to manage AFRS is allergen avoidance and if it enters someway then corticosteroid nasal spray and antihistamines are the secondary ways to control.66 Sometimes oral corticosteroid is also helpful in treating it. The surgical removal of allergic mucin with sinus drainage followed by immunotherapy is very effective in treating the chronic conditions.27 The surgery results in complete removal of all mucin and debris to eliminate the antigenic provocation factor is also suggested. Antifungal therapy followed with surgical therapy is also sometimes favoured.31
Otitis externaWhen there was inflammation in the external auditory canal due to factors like bacterial infections, allergy and dermatological disease then the term external otitis or swimmer's ear or tropical ear is used. The most common cause of this condition is acute bacterial infection and allergic contact dermatitis. Various oto-topical medications like neomycin, benzocaine, propylene glycol and cosmetics, shampoos rudiment type IV delayed hypersensitivity reactions. The disease occurs in all age groups but more commonly in 10–14-year-old children. Due to increased outdoor water activity participation, the disease occurs more commonly in summer than in winter.38 Korres et al., first reported malignant otitis externa having Alternaria sp. as causative pathogen.39
PathophysiologyThe physiology of the ear is designed to fight foreign particles and infection. The outer portion is made up of cartilage and lined with hair follicles and cerumen gland. The continuous sloughing of cells allows the removal of keratin debris and cerumen from the lining of ear canal. The acidic and sticky nature of cerumen helps to trap particles and maintain the barrier. The collapsing of this skin-cerumen barrier leads to engender the pathogenesis of external otitis. Allergen like bacteria and sometimes fungus exposure initiates the inflammatory response, causing the acute inflammation of external ear canal involving pinna and tympanic membrane too with variable oedema.38,80
The most common pathogen to cause otitis externa is Pseudomonas aeruginosa (20–60% prevalence) and Staphylococcus aureus (10–70% prevalence). Polymicrobial infection is often very common with some gram-negative organisms. Factors like sweating, allergy and dermatological condition also encourage infections and implicate pathogenesis of acute otitis externa (AOE).38
ManagementManagement of otitis externa includes irrigating ear with 3% hypertonic saline and cleansing with 70% alcohol. For reducing inflammation and infection ofloxacin or ciprofloxacine-dexamethasonotic solution can be used. Sometimes neomycin drops or polymyxin with hydrocortisone is effective.50 In severe condition, additional surgical intervention may be indicated sometime.75 In a case report, a 29-day-old neonate suffers from acute otitis externa caused by methicillin susceptible Staphylococcus aureus and was successfully treated by intravenous cloxacillin with local antiseptic cleansing.67
TonsillitisThe lymphatic structure closest to nasal mucosa is adenoid and tonsils.49 Inflammation of tonsils is termed as tonsillitis.77 Palatine tonsils are the glands present on either side of the back of throat.4 These are the part of Waldeyer's lymphatic ring and responsible for the first line of defence of the immune system. They help the body to fight against infectious agents or allergens like bacterial spores and viral particles.4,62 This can occur occasionally or recur frequently depending on conditions. Common symptoms include sore throat, swollen red tonsils, swallowing pain, fever, cough, headache, tiredness, chills, swollen lymph nodes near throat and pain in ear and throat. Beyond this, sometimes some less common symptoms are also seen, these include nausea, stomach ache, vomiting, bad breath, voice change and difficulty in mouth opening.77 The histopathological and immunohistochemical studies of allergic tonsillitis validate the presence of APCs like dendritic cells and macrophages with these eosinophils and mast cells are also found to be involved and play important role.49
Viral, bacterial and fungal infection in the form of allergen leads to tonsillitis. Bacteria and viruses are the most common that cause a sore throat in tonsillitis.4 The inflammation and pain that causes tonsillitis is due to the synthesis of prostaglandins and bradykinin and the very common treatment includes the inhibitors of these mediators like ibuprofen. Nasal steroids and antihistamines manage and can help to reduce symptoms of adenoidal hypertrophy and nasal airway obstruction.49
DiscussionIn recent years, a variety of tests have been developed focusing on various mechanisms of allergic reactions. Most of the time clinicians always are confused when electing the appropriate allergy test for their patients and often do not get updated with the latest tests and guidelines for various disciplines.45 A combination of in vitro and in vivo techniques along with nasal provocation or acoustic rhinometry may be used for allergic diseases. It is mandatory for all otolaryngologists to have better knowledge of allergy testing and immunotherapy.15
On the basis of allergic diseases, different tests can be broadly classified as IgE mediated allergy test or Type 1 or Immediate Hypersensitivity test and Non IgE mediated allergy test or Type III and IV or Delayed Hypersensitivity test.60 Most of the ENT allergies are IgE mediated.45 There is ambiguity in the management and line of action in immunological allergic disorders since no such practical information exists. Treatment of allergens on the basis of symptoms and avoidance are commonly recommended, as well as specific allergen immunotherapy (AIT).52 At present, allergen specific immunotherapy applied sublingually and subcutaneously is found to be more suitable for both children and adults for a variety of allergies including ENT too.1 There is no such remarkable difference between the patients treated with sublingual immunotherapy (SLIT) and subcutaneous immunotherapy (SCIT). The clinical effects and statistical analysis against native allergens in these patients are found to be the same.52
Saporta, in his study, reported that the effects and results of both SCIT and SLIT treatment improved considerably, still SLIT has been suggested as first choice therapy because it is found to be safer and favoured over SCIT, which can be a risky choice.68 In the USA, SLIT was officially permitted by the Food and Drug Administration (FDA) in 2014.19 In a study by Niederberg et al., they found that allergen-specific IgG antibodies are provoked by allergen specific immunotherapy, which further blocks the allergen to recognise IgE antibody. Patients having such IgG antibody experienced less IgE production against allergen and in the nasal secretion of these patients blocking IgG antibody could be detected. Consequently, IgE-allergen interaction was interfered by nasal IgG antibodies and furthermore these allergens can inhibit the IgE memory cells activation by allergens.53
Multiple organ affecting, IgE-mediated hypersensitivity disease engulfs more than 25% of the population.21,37 Linhart and Valenta, in their research concluded that vaccinations with allergen containing extracts that were launched in the past ten decades were clinically effectual, allergen specific, disease altering and long lasting form of therapeutic therapy. At present, with the help of recombinant DNA technology the majority of the disease causing allergens structure have been revealed. In order to reduce side effects during allergen specific immunotherapy, recombinant hypoallergenic allergen derivatives with lessen allergenic activity have been engineered. The in vitro characterisation and clinical trials of these engineered hypoallergens were done in experimental animal models and allergic patients, respectively.44
ConclusionIn the present scenario allergies of ear, nose and throat are very common showing large public health concern especially among adults and children. This is due to their high pervasiveness, coupled morbidity and societal costs. ENT allergies sometimes show substantial burden and potential risk to health services of industrialised and developing countries too. It is frequently found that clinicians always confronted towards the therapy that is best for their patients, which is less time consuming, more comfortable and safe. Clinical studies and advanced laboratory techniques have afforded much better techniques in understanding the pathophysiology of ENT allergies. To ameliorate and obstruct the allergic disorders, specific T-helper lymphocytes, associated cytokines and chemokines orchestrate and spurred the research of new potential agents. It is the demands of the future to highlight sublingual immunotherapy as it is safe, shows high acceptability rates and better adherence property. SLIT has been recommended as an effective and manageable form of therapy by World allergy organisation (WAO) for the treatment of allergic rhinitis, from the year 2010. In comparison to conventional SLIT medication, allergoid SLIT exhibits more biochemical advantages with advancement in immunotherapy. However, more frequent studies and random trials are needed to make this more convincing to the world.
Yet with all these research and potential agents the pace of ENT allergies increases multidimensionality, without satisfactory theories, therapies and strong animal models. Both systemic and topical antifungal agents are found to have a limited role in treatment and this area of ENT allergies needs further study in its pathophysiology and management. Further exploration in allergic diseases and tests will lead to novel pathways and targets that will likely help us to produce treatments for the patients.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors and co-authors agreed to publish this review paper in the Journal Allergologia et Immunopathologia and we do not have any type of potential conflict of interest.
The authors owe huge gratitude to Vice Chancellor, R.D. University Jabalpur. An eternal gratefulness to Head, Department of Biological Science, R.D. University, Jabalpur, India, for allowing completing this review and bringing it to the world.
This review did not receive any specific grant from funding agencies in the public, commercial or non-for-profit sectors.