To assess the Quality of Life (QoL) of children with Atopic Dermatitis (AD) and their families and the impact of the mothers’ illness perceptions on the family QoL.
Materials and methodsSeventy-five children with AD (54 infants and 21 children) and their mothers participated in the study. The following questionnaires were administrated: 1. Brief Illness Perception Questionnaire (Brief IPQ); 2. Infant's Dermatitis Quality of Life Index (IDQOL); 3. Children's Dermatology Life Quality Index (CDLQI); 4. Dermatitis Family Impact Questionnaire (DFIQ) and 5. The Severity Scoring of Atopic Dermatitis (SCORAD).
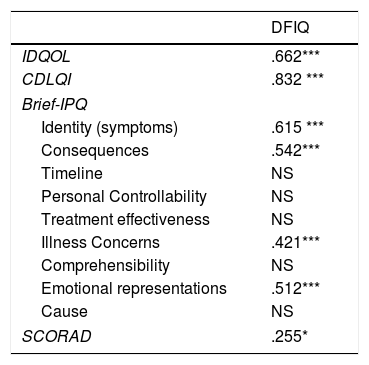
ResultsAtopic dermatitis had a moderate impact on the QoL of the infants (6.67±5.30), children (7.86±7.19) and their families (9.42±7.03). The DFIQ was associated with certain dimensions of the Brief IPQ, specifically, with Illness Identity (greater symptom burden) (r=0.615, p=0.000), beliefs about the Consequences of the illness (r=0.542, p=0.000), the Concerns (r=0.421, p=0.000) and the Emotional Representations (r=0.510, p=0.000). Correlation was demonstrated between IDQOL and DFIQ (r=0.662, p=0.000) and between CDLQI and DFIQ (r=0.832, p=0.000), and a weaker correlation between SCORAD and DFIQ (r=0.255, p=0.035). The chronicity of the AD showed negative association with DFIQ (p<0.001).
ConclusionsThe QoL of families with a child with AD is associated with the mother's illness perceptions about AD, the children's QoL and with both the severity and the chronicity of the disease. Therefore, clinicians should pay attention not only to the clinical characteristics of the children, but also to the parents’ beliefs and emotions, to improve the family QoL.
Atopic dermatitis (AD) is a chronic, inflammatory, relapsing skin disease. Its severity ranges widely and it may present frequent exacerbations.1 Up to 20% of children are estimated to be affected by AD, but its epidemiology is difficult to be assessed because of lack of uniformity in the definitions used and absence of a universally accepted approach. Its prevalence is constantly increasing, especially in urban areas, and association has been identified with the western lifestyle.2 Both genetic and environmental factors appear to contribute to the pathophysiology of AD.1,2 The clinical manifestations of AD include itching, skin pain, poor sleep quality, low self-confidence and social distress. These symptoms may constitute a considerable burden, often unrecognized and underestimated, not only for the affected child, but for the whole family unit, with profound effects on the quality of life (QoL) of both the child and other family members.3,4
Apart from the characteristics of the illness, a variety of psychosocial factors can affect the QoL of families with a child suffering from AD, including parenting and relationship characteristics (e.g., parental stress, parental locus of control, cohesion) and the family life-style.5,6 The parental perceptions about the chronic illness of a child may have a significant impact on the QoL of the whole family. Studies on families with a child who has a chronic illness have demonstrated that the Common Sense Model (CSM)7,8 provides a dynamic theoretical framework for understanding the process by which a parent/caregiver develops cognitive and emotional representations, which then lead to the adoption of specific coping strategies.9,10
According to the CSM, the beliefs of the patients and their parents/caregivers about a disease and its treatment determine the ways in which they manage and adapt to the new situation created by the diagnosis. Patients and their caregivers modify their beliefs, feelings and behaviors in ways that may affect the illness outcome, their compliance with treatment and their QoL.11,12 Individuals build cognitive and emotional representations of a disease through the information they receive, previous personal medical experiences and cultural influences. Concerning AD in infants and children, there is a lack of studies investigating the parents’ illness perceptions and their effects on the QoL of the family. Previous studies have focused mainly on the parenting behavior and parent-child relationship.12–14
Therefore, the present study aimed at estimating the QoL of children with AD and their families and the impact of the mothers’ illness perceptions about AD on the family QoL. The choice of mothers as the respondents in the study was based on the fact that they were the main caregivers of the study children with AD. The gender of the parent who estimates the QoL of the child and the family has been shown not to affect the results substantially, because family members tend to influence each other's illness perceptions and to present similar responses, reflecting shared experiences, information and social-emotional environment.12,13
Material and methodsParticipantsThe sample consisted of 75 children with AD and their mothers, who provided a written informed consent to participate in the study. The diagnosis of AD had been made according to the criteria of Hanifin and Rajka.15 One of the major criteria of AD is chronicity, a term used in the present study, which is defined as duration of at least four weeks of symptoms in the course of six months of monitoring.16 The infants and children in the study were recruited during follow-up in an outpatient hospital clinic. Inclusion criteria were: a confirmed diagnosis of AD in the child, absence of other systemic illness, ability of the mother to understand and complete the questionnaires, and provision of the mother's written informed consent. No participant withdrew from the study.
Procedure and study instrumentsThe study was conducted in accordance with Ethical Standards as formulated in the World Medical Association Helsinki Declaration (2002) and an IRB statement that IRB approval was unnecessary was received. The study was cross-sectional and questionnaire based. The following questionnaires were used:
- 1.
The Brief Illness Perception Questionnaire (Brief IPQ). This questionnaire provides a rapid quantitative assessment of the nature and strength of the individual's perceptions concerning nine dimensions. Specifically, five items cover cognitive representations: illness identity (perception of symptoms related to the illness), timeline (perception regarding the duration of the illness), consequences, personal control (refers to the concept of control of the disease by the individual), treatment control (refers to the perception of the effectiveness of treatment); two items cover emotional representations: concerns and emotions (the extent to which the illness results in symptoms of anxiety and depression) and one item is on illness comprehensibility (coherence) and refers to the degree of understanding of the illness by the individual. Responses are given on a scale of 0–10, and higher values are indicative of a stronger perception. The last item, on the perceived cause of illness, is an open-ended question requiring the participants to rank the three most important causal factors. The questionnaire completed by the mother, and minor changes were made in the wording of the items in order to assess the mothers’ perceptions about AD specifically.17
- 2.
Infant's Dermatitis Quality of Life Index (IDQOL). It is designed to be completed by parents to assess the impact of AD on the QoL of infants aged 0–4 years. It consists of 10 items covering the symptoms of AD, time taken to get to sleep, total time disturbed, playing or swimming, enjoying family's activities, mealtime, problems with treatment, uncomfortable dressing and problems at bath time. The answers are given on a Likert scale of 0–3 and the total score ranges from 0 to 30, with higher scores indicating more marked effects on QoL. The Greek language version of the questionnaire was used.18
- 3.
Children's Dermatology Life Quality Index (CDLQI). It is designed for children aged from 4 to 16 years and consists of 10 questions regarding the experience of the child in different aspects of life affected by AD. There is also a Cartoon version in which each question is illustrated by a cartoon based on the theme of the question. The CDLQI can be analyzed under six headings: symptoms and feelings (questions 1 and 2); leisure (questions 4, 5 and 6); school or holidays (question 7); personal relationships (questions 3 and 8); sleep (question 9); and treatment (question 10). Each question is scored on a four-point Likert scale, and the total score ranges from 0 to 30 with the following categorization: 0–1: no effect, 2–6: small effect, 7–12: moderate effect, 13–18: very large effect and 19–30: extremely large effect. The questionnaire was completed by the children, assisted by the interviewers, who gave the instructions and read the questions aloud when necessary. The Greek language version of the questionnaire was used.19,20
- 4.
Dermatitis Family Impact Questionnaire (DFIQ). This is a disease-specific questionnaire designed to assess the impact of a child's AD on the QoL of the family.21 The questionnaire consists of 10 items concerning the effects of the child's disease on different domains of family life, including extra housework, emotional distress, physical fatigue, other peoples’ reactions, social life, leisure activities, relationships, daily expenditure, time spent looking after the child, work or education. Each question is answered in a four-point Likert scale (0–3) and the total score ranges from 0 (=no impact) to 30 (=maximum effect).
- 5.
Severity Scoring of Atopic Dermatitis (SCORAD). It is a clinical tool for assessing the severity of AD by physicians (pediatricians, allergists). In the present study for each child two pediatricians completed the SCORAD. It is composed of three parts: 1. The extent of involvement uses the rule of nines; 2. Six intensity items (erythema, oozing/crusting, edema/papulation, excoriation, lichenification and dryness); and c. subjective symptoms (daily pruritus and sleep loss) in the last three days/nights. A SCORAD of below 20 indicates mild AD (a few inflammatory lesions); between 20 and 40 is classified as moderate AD (intense inflammation and pruritus); and above 40 indicates severe AD (extensive, inflammatory). The maximum SCORAD score is 103.22
Descriptive analysis was performed of the sociodemographic characteristics of the family and the demographic and clinical characteristics of the children. Mean values and standard deviation were reported for continuous variables and frequencies/proportions for categorical variables. Total mean scores for each participant were computed and were treated as continuous variables. For the Brief IPQ questionnaire, each dimension was treated as a continuous variable. The distribution of total scores was examined using the Shapiro–Wilk (S-W) and Kolmogorov-Smirnov (K-S) tests. When variables appeared to be normally distributed, associations were examined using Pearson's bivariate correlation coefficient and when the assumption of normality was questioned, the Spearman correlation coefficient was used. Correlations smaller than 0.3 denote a weak, clinically unimportant correlation; between 0.3 and 0.7 moderate correlation; and larger than 0.7 denote a strong correlation. Pearson's bivariate correlation coefficient was used to explore the relationship between IDQOL, CDLQI, DFIQ and SCORAD. To compare means or distributions across two groups, the t-test was used when normality was assumed and the Mann–Whitney U-test when normality was questioned. All statistical analyses were performed using the SPSS v.23.
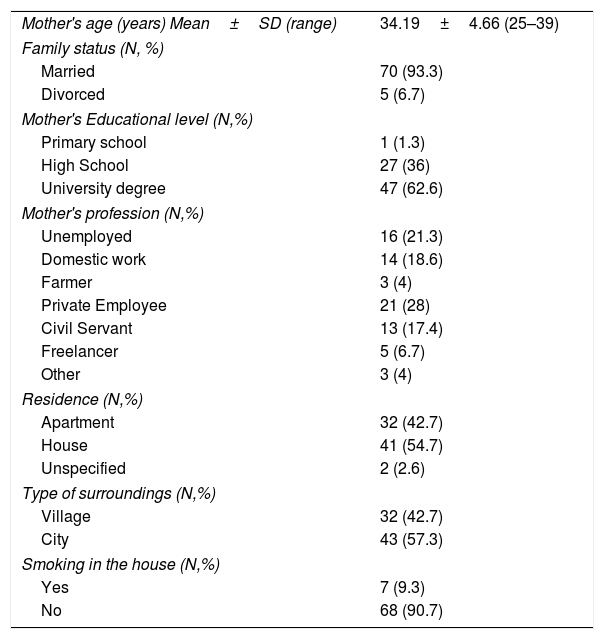
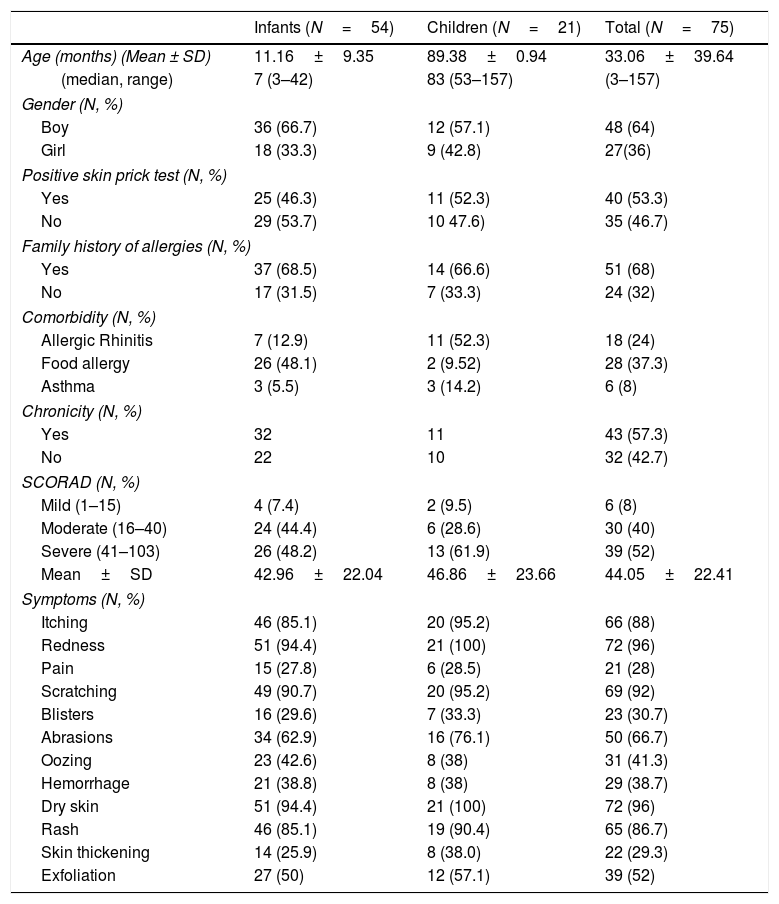
ResultsCharacteristics of the participantsThe sample consisted of 75 children with AD, specifically 54 infants, in the age range 3–42 months, and 21 older children, in the age range 4.4–13.0 years, and their mothers. The demographic characteristics of the study population are shown in Table 1. The majority of mothers were married (93.3%), university graduates (62.6%), employees (78.7%) and city residents (57.3%). The clinical characteristics of the study children are shown in Table 2. The majority of the children were boys (64%), and most had a family history of allergies (68%). A positive skin prick test, according to the European Academy of Allergy and Clinical Immunology (EAACI) (i.e., wheal diameter ≥3mm), was recorded in 53.3% of the children.23 The most common symptoms were redness, scratching and dry skin. Regarding disease severity, the total mean score of SCORAD was 42.96±22.04 for the infants and 46.86±23.66 for the children. Additionally, no significant difference in the distribution of SCORAD scores was found between boys and girls or between the two age groups.
Sociodemographic characteristics of the mothers of children with atopic dermatitis (N=75).
| Mother's age (years) Mean±SD (range) | 34.19±4.66 (25–39) |
| Family status (N, %) | |
| Married | 70 (93.3) |
| Divorced | 5 (6.7) |
| Mother's Educational level (N,%) | |
| Primary school | 1 (1.3) |
| High School | 27 (36) |
| University degree | 47 (62.6) |
| Mother's profession (N,%) | |
| Unemployed | 16 (21.3) |
| Domestic work | 14 (18.6) |
| Farmer | 3 (4) |
| Private Employee | 21 (28) |
| Civil Servant | 13 (17.4) |
| Freelancer | 5 (6.7) |
| Other | 3 (4) |
| Residence (N,%) | |
| Apartment | 32 (42.7) |
| House | 41 (54.7) |
| Unspecified | 2 (2.6) |
| Type of surroundings (N,%) | |
| Village | 32 (42.7) |
| City | 43 (57.3) |
| Smoking in the house (N,%) | |
| Yes | 7 (9.3) |
| No | 68 (90.7) |
SD: Standard Deviation.
Demographic and clinical characteristics of the children with atopic dermatitis.
| Infants (N=54) | Children (N=21) | Total (N=75) | |
|---|---|---|---|
| Age (months) (Mean ± SD) | 11.16±9.35 | 89.38±0.94 | 33.06±39.64 |
| (median, range) | 7 (3–42) | 83 (53–157) | (3–157) |
| Gender (N, %) | |||
| Boy | 36 (66.7) | 12 (57.1) | 48 (64) |
| Girl | 18 (33.3) | 9 (42.8) | 27(36) |
| Positive skin prick test (N, %) | |||
| Yes | 25 (46.3) | 11 (52.3) | 40 (53.3) |
| No | 29 (53.7) | 10 47.6) | 35 (46.7) |
| Family history of allergies (N, %) | |||
| Yes | 37 (68.5) | 14 (66.6) | 51 (68) |
| No | 17 (31.5) | 7 (33.3) | 24 (32) |
| Comorbidity (N, %) | |||
| Allergic Rhinitis | 7 (12.9) | 11 (52.3) | 18 (24) |
| Food allergy | 26 (48.1) | 2 (9.52) | 28 (37.3) |
| Asthma | 3 (5.5) | 3 (14.2) | 6 (8) |
| Chronicity (N, %) | |||
| Yes | 32 | 11 | 43 (57.3) |
| No | 22 | 10 | 32 (42.7) |
| SCORAD (N, %) | |||
| Mild (1–15) | 4 (7.4) | 2 (9.5) | 6 (8) |
| Moderate (16–40) | 24 (44.4) | 6 (28.6) | 30 (40) |
| Severe (41–103) | 26 (48.2) | 13 (61.9) | 39 (52) |
| Mean±SD | 42.96±22.04 | 46.86±23.66 | 44.05±22.41 |
| Symptoms (N, %) | |||
| Itching | 46 (85.1) | 20 (95.2) | 66 (88) |
| Redness | 51 (94.4) | 21 (100) | 72 (96) |
| Pain | 15 (27.8) | 6 (28.5) | 21 (28) |
| Scratching | 49 (90.7) | 20 (95.2) | 69 (92) |
| Blisters | 16 (29.6) | 7 (33.3) | 23 (30.7) |
| Abrasions | 34 (62.9) | 16 (76.1) | 50 (66.7) |
| Oozing | 23 (42.6) | 8 (38) | 31 (41.3) |
| Hemorrhage | 21 (38.8) | 8 (38) | 29 (38.7) |
| Dry skin | 51 (94.4) | 21 (100) | 72 (96) |
| Rash | 46 (85.1) | 19 (90.4) | 65 (86.7) |
| Skin thickening | 14 (25.9) | 8 (38.0) | 22 (29.3) |
| Exfoliation | 27 (50) | 12 (57.1) | 39 (52) |
SD: Standard Deviation, SCORAD: Severity Scoring of Atopic Dermatitis.
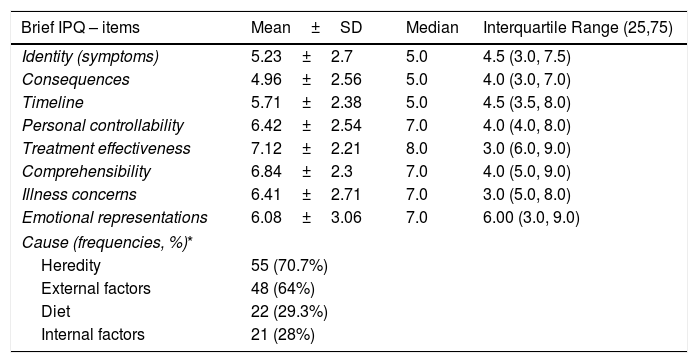
Table 3 presents the mean scores on the dimensions of the Brief IPQ. Perceptions about the identity of the disease (5.23±2.70, median 5) and about the possibility of serious consequences were low (4.96±2.56, median 5), perceptions of worry and emotional effects were slightly elevated (6.41±2.71, median 7 and 6.08±3.06, median 7, respectively), while the strongest perception was that of controllability of the disease through treatment (7.12±2.21, median 8). Regarding the mothers’ beliefs about the causes of AD, inheritance (70.7%) and other external factors (64%) were included as the perceived main causes, while 28% of the mothers ranked internal factors, such as stress and personality traits, among the most important causal factors.
Scores of the mothers of children with AD on the Brief Illness Perception Questionnaire (Brief IPQ).
| Brief IPQ – items | Mean±SD | Median | Interquartile Range (25,75) |
|---|---|---|---|
| Identity (symptoms) | 5.23±2.7 | 5.0 | 4.5 (3.0, 7.5) |
| Consequences | 4.96±2.56 | 5.0 | 4.0 (3.0, 7.0) |
| Timeline | 5.71±2.38 | 5.0 | 4.5 (3.5, 8.0) |
| Personal controllability | 6.42±2.54 | 7.0 | 4.0 (4.0, 8.0) |
| Treatment effectiveness | 7.12±2.21 | 8.0 | 3.0 (6.0, 9.0) |
| Comprehensibility | 6.84±2.3 | 7.0 | 4.0 (5.0, 9.0) |
| Illness concerns | 6.41±2.71 | 7.0 | 3.0 (5.0, 8.0) |
| Emotional representations | 6.08±3.06 | 7.0 | 6.00 (3.0, 9.0) |
| Cause (frequencies, %)* | |||
| Heredity | 55 (70.7%) | ||
| External factors | 48 (64%) | ||
| Diet | 22 (29.3%) | ||
| Internal factors | 21 (28%) | ||
For the infants, the total mean score of IDQOL was 6.67±5.30 and the highest scores were observed in “total timedisturbed” (0.93±1.11) and in “mood” (0.80±0.91). For the older children, the total mean score on CDLQI was 7.86±7.19, and the three subscales of CDLQI on which the highest scores were reported were “symptoms andfeelings” (1.30±0.92), “problem withsleep” (0.90±0.99) and “problems caused by thetreatment” (0.86±0.79).
Regarding the effects of the child's AD on the QoL of the family, the total mean score on DFIQ was 9.42±7.03, corresponding to a moderate effect. The AD had the highest impact on “family'sexpenditure” (1.19±0.99), including costs related to treatment or clothes, on “helping with treatment on main caregiver'slife” (1.08±0.98) and on “housework” (1.03±1.05) (e.g., washing and cleaning).
Quality of life associationsNo statistically significant correlation was demonstrated between the demographic or clinical characteristics of the sample and the IDQOL score. Regarding CDLQI, the only correlation observed was between the total CDLQI score and SCORAD (r=0.448, p<0.05).
Concerning the QoL of the family, associations were demonstrated between the scores on DFIQ and certain dimensions of the Brief IPQ. Specifically, the total mean score on DFIQ was correlated with illness identity (high symptom burden) (r=0.615, p=0.000), belief about the consequences of the illness on various domains of the child's life (r=0.542, p=0.000), concerns about the illness (r=0.421, p=0.000), and emotional representations, which include negative reactions such as fear, anger and distress (r=0.512, p=0.000) (Table 4).
Correlations between DFIQ and IDQOL, CDLQI, SCORAD and Brief-IPQ.
| DFIQ | |
|---|---|
| IDQOL | .662*** |
| CDLQI | .832 *** |
| Brief-IPQ | |
| Identity (symptoms) | .615 *** |
| Consequences | .542*** |
| Timeline | NS |
| Personal Controllability | NS |
| Treatment effectiveness | NS |
| Illness Concerns | .421*** |
| Comprehensibility | NS |
| Emotional representations | .512*** |
| Cause | NS |
| SCORAD | .255* |
IDQL: Infant's Dermatitis Quality of Life Index, CDLQI: Children Dermatology Life Quality Index, DFIQ: Dermatitis Family Impact Questionnaire, Brief – IPQ: Brief – Illness Perception Questionnaire.
Values are the Spearman's correlation coefficients.
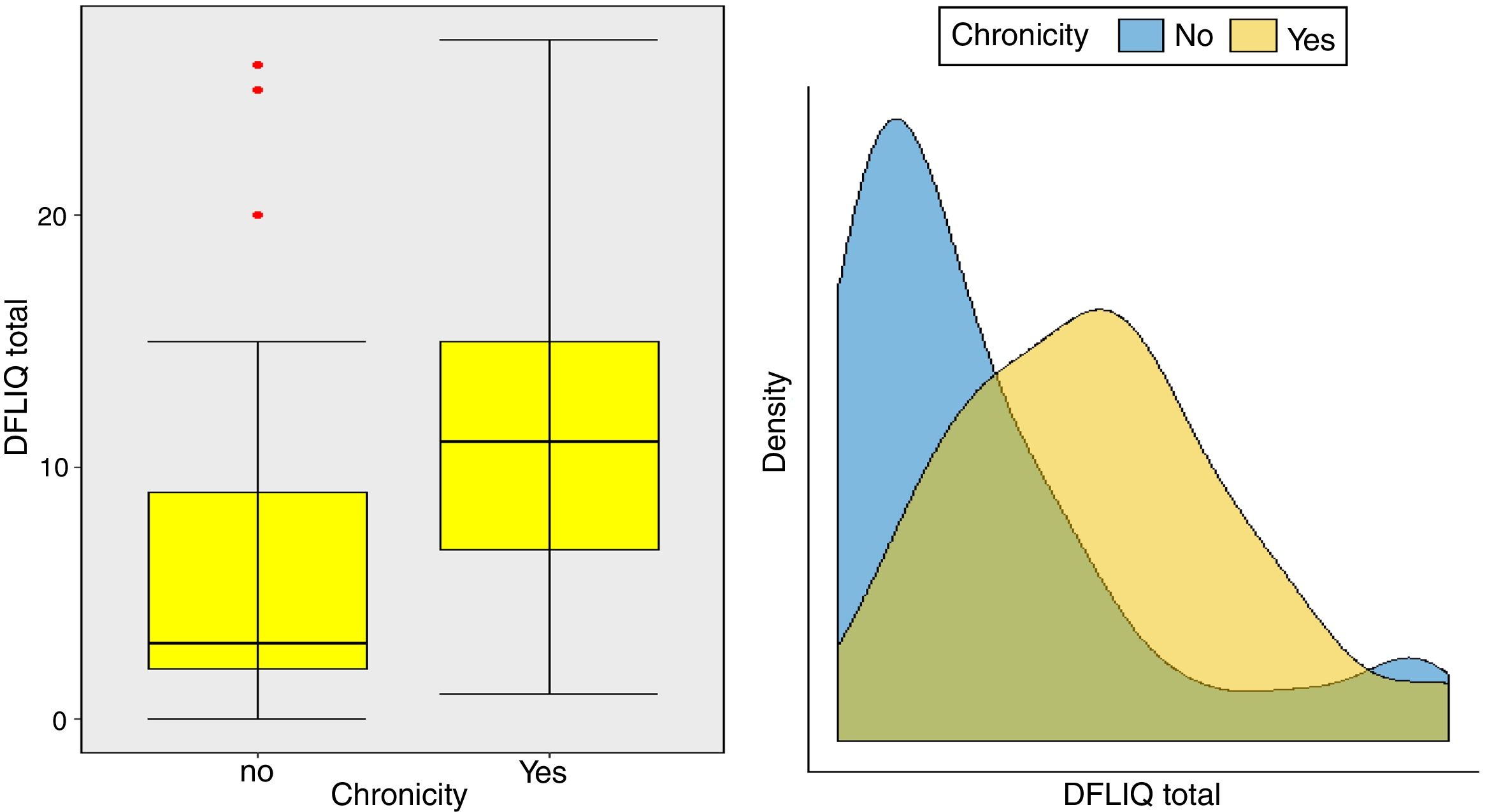
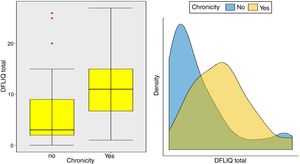
In addition, statistically significant correlations were observed between IDQOL and DFIQ (r=0.662, p=0.000) and between CDLQI and DFIQ (r=0.832, p=0.000). Correlation between SCORAD and DFIQ, although statistically significant, was weak (r=0.255, p=0.035) (Table 4). The association of the total score on DFIQ (DFIQtotal) with chronicity was investigated, using the non-parametric Mann–Whitney U statistic because the normality assumption was not met (S-W: pno<0.001, pyes=0.226, K-M: pno<0.001, pyes=0.2) (Fig. 1). Regarding chronicity, the results indicated that the QoL of families with a child suffering from AD for a longer period is more negatively affected (p<0.001).
DiscussionThe present study assessed the QoL of the children with AD and their families and the impact of the mothers’ illness perceptions about AD on the family QoL, a subject that has not been adequately studied. The results showed that AD has a moderate impact on the QoL of infants and children, and on the family unit. Specifically, from the IDQOL scores it was observed that mothers report that AD affects their infants’ mood (irritability, crying, etc.) and the time that they are disturbed. Mothers are involved in both practical and emotional terms in the management of AD and are able to identify and, possibly overestimate, even minor changes in the mood of their children.24 Similarly, from the CDLQI dimensions with the highest scores, it was shown that AD affects mainly the emotional state of the children and their sleep. Studies in large samples indicate that mood changes and sleep disturbances are important issues for children with AD affecting their physical, emotional and social wellbeing.25–27 Our findings indicated that AD has a moderate impact on the QoL of infants and children with mean total scores corresponding to those of similar studies.28,29 Additionally, an international multicenter study highlighted that despite some national peculiarities, parents in different countries assessed the QoL of their children and the family QoL in similar ways.30
Regarding the illness perceptions of the mothers and their impact on the family QoL, certain dimensions of the Brief IPQ were correlated with family QoL. Specifically, lower family QoL was significantly associated with stronger beliefs about symptom burden, fear of serious consequences on the child's life, higher levels of worry and strong emotional representations, which correspond to the experience of psychological distress symptoms. It appears that the high demands of caring for children suffering from AD increase parents’ anxiety, depression, frustration and fatigue, while, at the same time, they become highly overprotective.5,6 It is reported that parents are concerned about the factors that trigger the aggravation of symptoms of AD, but also about the safety of the long-term use of topical corticosteroids. Because of this fear, they often seek alternative therapies, with dubious effectiveness and at an extra cost.31 Moreover, taking into consideration that emotional factors are involved in the evolution of the AD, parents’ high levels of anxiety and their consequent overprotection behaviors are likely to impact on the child's physiological stress response system and the exacerbation of AD symptoms.
With regard to the main causes of AD according to the mothers’ belief, the mothers in this study considered inheritance (70.7%) and external factors (64%) to be the major factors. Viewing inheritance to be the main cause of AD could lead to parental guilt, negative self-concept and increased depression or anxiety. Conversely, attributing the cause to external factors (e.g., the urban environment) places the responsibility for their child's condition beyond their own influence. According to the literature, parents’ beliefs vary, attributing AD mainly to internal-naturalistic causes (e.g., body-malfunctioning),12 or placing environmental factors in the first causative place and genetics last.32 Causal attributions interact through a complex process that results in the adoption by the parents of specific health behavior and care activities, with varying impact on coping and adjustment. All the subjective aforementioned perceptions are formed during the period of illness and guide the decision-making process, the symptoms management, and the QoL.33
Concerning the impact of AD on the family QoL (DFIQ), AD was reported to affect the family's expenditure, but also to increase the mothers’ time spent on childcare and additional housework, findings that are in line with previous studies.24,34 With respect to the associations between children's QoL and family's QoL, correlations were demonstrated between the total mean scores on DFIQ and IDQOL and CDLQI. It is well documented that the more affected the QoL of children was, the more impaired the QoL of the family was, in particular, in the social domain, leisure and daily expenditure.24,35
Examining the impact of the chronicity of AD on the family's QoL – an association that has not been investigated adequately up to the present – it was found that the QoL of families with a child who suffers from AD for a long period of time is negatively affected. Regarding the association between the severity of the AD and the family QoL, a positive but weak correlation was observed in this study, possibly because of the way the symptoms and the treatment were perceived and managed by the parents. Several studies indicate a strong association between the severity of the AD and its impact on the family QoL. Additionally, it is reported that high levels of parental anxiety were accompanied by inadequate symptom management, overprotection, frustration and aggressiveness toward children's behaviors such as scratching.6,24,36
Certain clinical implications emerge from the findings of this study. It is evident that recognition and understanding of the factors that affect the QoL of families with a child suffering from AD is important for clinicians, to help to reduce the impact of AD on both the children and their families. In addition to assessing the severity and chronicity of AD, physicians, therefore, should also focus on the parents’ thoughts and emotional manifestations. By introducing parental illness perceptions as a key topic of discussion, the parent-doctor relationship can be significantly improved. In cases where the parental illness perceptions generate anxiety and ineffective coping strategies, the doctor can cooperate with the parents to help them to deal with the disease, and refer them to mental health professionals for psychological interventions when necessary.37
Parents need information and education about symptom management skills, and should be included in the decision making and therapeutic planning process. This policy can reduce dysfunctional parenting practices and promote the wellbeing of both the children with AD and their families.38 Better understanding of the disease may lead to positive effects on the QoL of the family and enhancement of compliance with recommended management practices.39
AD in a child generates emotional, behavioral and interpersonal challenges within the family. Consultation and cognitive-behavioral interventions can be applied in a variety of clinical settings to help modify the parents’ dysfunctional beliefs and reduce their anxiety, which will also improve clinical outcomes and the QoL of the family.40
Limitations and suggestionsCautious interpretation of the results is needed, as the present study has some limitations. First, it was based on a rather small convenience sample and the results cannot be generalized. Given the population receiving services from the specific outpatient clinic and the incidence of AD in infants and children, it was possible to recruit only 75 participants during the study period. Second, the study took place at an outpatient pediatric allergy clinic of a single tertiary center, which also limits the generalization of the findings. On the other hand, a strength of the study was the use of the Brief IPQ, which, as far as we know, has not been used in a similar population. Finally, as the present study was cross-sectional, it would be of further interest – in a longitudinal study – to examine the parents’ illness perceptions over a long-term disease course and to explore their association with the family QoL and the possible changes in parental illness perceptions following an intervention program.
ConclusionsIn conclusion, AD appeared to have a moderate impact on the QoL of both the affected children and their families. The family QoL is associated with illness perceptions about AD of the mothers, and both the severity and the chronicity of the disease. Specifically, strong beliefs about the illness identity, the consequences of the AD, and mothers’ intense concerns and emotional representations all have a negative impact on the QoL of the family. Pediatricians and allergists should address this aspect of childhood AD. The management of AD in infants and children should include multidisciplinary intervention, with the provision of psychoeducational programs for parents, to provide them with systematic and accurate information on the characteristics of AD, along with emotional support, to reduce the negative impact on family QoL.
Authors’ contributionVassiliki Siafaka: Conceived and designed the research, collected data, contributed data tools and wrote the manuscript.
Aikaterini Zioga: Reviewed the literature, collected the data and wrote the manuscript.
Theodoros Evrenoglou: Contributed analysis tolls, performed the analysis.
Dimitrios Mavridis: Contributed analysis tolls, performed the analysis.
Sophia Tsabouri: Conceived and designed the research, collected data, contributed data tools, wrote the manuscript and was in charge of overall supervision.
DeclarationsAll authors discussed the results, provided critical feedback and contributed to the final manuscript
There is not prior presentation of study data as an abstract or poster.
Ethical approvalAll procedures performed were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (1964) and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the University Hospital of Ioannina (Greece).
Consent to participateA written informed consent was obtained from the adult participants.
Conflict of interestNo funding was received for this work from any organization. There is no conflict of interest.