The chemical modification of allergens with glutaraldehyde improves safety while maintaining clinical efficacy, which permits the administration of higher doses of immunotherapy, reducing the risk of adverse reactions. The aim of this study is to evaluate the immunogenic capacity of a new cat dander polymer by immunizing mice and quantifying immunoglobulins in serum, in comparison with the non-modified allergen.
MethodsThe study consists of the immunization of three mice groups with the polymerized and the native extract, together with a negative control group. The immunoglobulin levels in serum have been measured by indirect ELISA. By means of the non-parametric Mann–Whitney U test, it was determined if there were significant differences in the values of specific antibodies between groups.
ResultsThe group immunized with the allergoid showed significantly higher specific IgG and IgG1 values to dander allergens and specific IgG to the major allergen Fel d 1, while there were no significant changes in IgG2a and IgE values. These results could be due to a higher immunization dose. The vaccine formulation was based on the optimal defined dose for clinical efficacy of allergen immunotherapy.
ConclusionsThis preclinical study carried out with the present assay has established that the allergoid of cat dander extract, as designed for its optimal use in allergen immunotherapy, produces a higher specific IgG than the native extract, in addition to showing significantly higher specific IgG1 levels, evidencing a greater effectiveness in immunization.
Allergy is defined as an immune-based disease IgE-mediated hypersensitivity.1 Allergic diseases are an important global health problem because of the prevalence of asthma, and allergic rhinitis is increasing worldwide.2 Specific allergen immunotherapy (AIT) is the most effective treatment against IgE-mediated allergy, and consists in the administration of increasing quantities of specific allergens in order to induce immunological tolerance.3 Despite its efficacy, difficulties continue to present with conventional immunotherapy, mainly due to the risk of anaphylaxis and the requirements for prolonged therapy with many doses of allergens.4 Allergens can be modified chemically to solve these problems; the most common procedure to chemically modify allergen extracts is through the use of formaldehyde or glutaraldehyde. Its addition produces polymerization as a result of cross-linking proteins,5 by the irreversible linking of glutaraldehyde or formaldehyde with N-terminal and/or lysine residues,5,6 resulting in structures of high molecular weight linked covalently (allergoid). This transformation process permits the maintenance of immunogenicity and reduces allergenicity of the allergoid by conformational changes, which allows for high-dose extracts in vaccines.7
Among the allergies produced by animal epithelia, cat (Felis domesticus) allergy is one of the most serious. Cat dander contains multiple allergens which can be found in places where cats do not live.8 In fact, the ever-closer contact between people and pets produces a higher prevalence of sensitization to cat dander, which is nowadays on the increase.9 The prevalence is between 10 and 15% among adults.10 The most important cat dander allergen is Fel d 1,11 a glycoprotein formed by two chains of 4 and 14kDa.12,13 The characteristics of transportability and ubiquity given by its three-dimensional structure make it the major cat allergen. In fact, more than 60% of all IgE antibodies induced by cat dander are directed to this allergen,14 and over 90% of patients sensitized to F. domesticus have specific IgE to Fel d 1.13 Another major allergen in cat dander is Fel d 4, which is a lipocalin. These molecules are small proteins produced in the liver or secretory glands, with the ability to bind small hydrophobic molecules.15
In a previous report, this group developed and characterized a new allergoid of cat dander for immunotherapy. Allergens of cat dander were polymerized with glutaraldehyde, obtaining a protein complex of molecular weight above 100kDa, and the allergenic profile was analyzed by different immunochemical methods. The presence of the most relevant allergens in the allergoid was confirmed by peptide footprint using HPLC coupled to mass spectrometry. Studying the allergenic profile by ELISA inhibition, it was shown that the IgE-binding capacity of the allergoid is reduced 18-fold as compared to the cat native extract, while the capacity for IgG-binding is maintained.16 These assays were performed following the recommendations of the European Medicines Agency: ‘Guidelines on Allergen Products: Production and Quality Issues’ (2008),17 and the product allergens monograph European Pharmacopoeia (2010, 1063, Product Allergenic-European Pharmacopeia)18; they were also based on the ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.19
A study in which the immunological dose-response of the AIT was evaluated based on the quantification of Fel d 120 concluded that 15μg per dose of Fel d 1 is the optimal maintenance dose, capable of achieving clinical and immunological efficacy with the treatment of allergen immunotherapy. However, the vast majority of AIT vaccines for the treatment of this sensitization in particular, do not currently reach this defined optimum concentration, mainly due to the fact that the increase in the concentration of the allergen may cause a significant percentage of adverse reactions.21
With the data of the high safety profile of the new allergoid of cat dander16 and the evidence showing maintenance of the binding capacity of IgG with respect to its native extract of origin, the development of a vaccine based on the new allergoid was considered. This vaccine would guarantee lower allergenicity, therefore the dose of allergens can be increased and in turn, due to the polymerization process, the antigenic determinants of the surface are not affected, obtaining enough immunogenicity to produce significant clinical effects.
The formulation of the new vaccine was based on the concentration of the major allergen Fel d 1 and a preclinical study in mice was designed to evaluate the immune system activation capacity of the new allergoid by sequential measures of specific IgG produced against allergens from cat dander.
MethodsPreclinical studyThe aim of this study was to evaluate the immunogenic capacity of the new vaccine, based on its capacity to induce allergen-specific IgG. This preclinical study called “Determination of immunogenic capacity of new products for immunotherapy treatment against F. domesticus allergy” was accepted by the Spanish Ethical Committee for Animal Experimentation (CEEA), and the project was classified as Type II (Project number A13170501) by the Animal Health Service of the General Directorate of Agriculture, Livestock and Fisheries of the Region of Murcia, in accordance with the current legislation.22 The assay was carried out entirely in the establishment of the University of Murcia (UMU), recognized as a user and breeding activity center (N°REGA ES300305440012).
Vaccine designTwo different extracts were used for the production of the vaccine: native extract and polymerized cat dander extract. The polymerized extract was developed by modification with glutaraldehyde and characterized in protein and allergenic profile.16 Vaccines were produced under Probeltepharma protocols: phenol and NaCl were used as preservatives, water as solvent and aluminum hydroxide as adjuvant. Extract dosage was determined by the Fel d 1 concentration in each extract:
- -
Beltavac® DEPOT PLUS, vaccine obtained from the native cat dander extract, with a Fel d 1 concentration of 5.25μg/mL.
- -
Beltavac® POLYMERIZED, vaccine obtained from the polymerized cat dander extract, with a Fel d 1 concentration of 30μg/mL.
Twenty-four female BALB/c mice (Envigo, Netherlands) were used in this study. They were distributed in three groups of eight mice each, in accordance with the treatment they would receive:
- -
Group 1: Beltavac® DEPOT PLUS of Alternaria alternata (negative control).
- -
Group 2: Beltavac® DEPOT PLUS cat dander native.
- -
Group 3: Beltavac® POLYMERIZED cat dander.
The immunization process consisted of four subcutaneous injections: one immunization and three boosters, each fifteen days. Finally, after 52 days, the blood of each mouse was extracted by cardiac puncture, subsequently sacrificing the individuals in the euthanasia chamber (with progressive liberation of CO2).
All inoculations were administered subcutaneously in a volume per injection corresponding to 1/10 of the recommended human maintenance dose.23 Thus, the Beltavac® DEPOT PLUS immunized groups received inoculations of 100μL, and the Beltavac® POLYMERIZED immunized group was inoculated with 50μL. The serum was obtained by centrifugation (2500rpm for 20min), and was conserved at −80°C.
In vitro evaluation of the immune responseSpecific IgG, IgG1, IgG2a and IgE concentrations against cat dander and specific IgG concentrations against allergen Fel d 1 were determined by indirect ELISA in each individual sample. In this case, the serum obtained from each immunized mouse was used as the primary antibody, and the secondary antibodies used were anti-mouse IgG, anti-mouse IgG2a (Sigma-Aldrich, St. Louis, MO, USA), and anti-mouse IgG1, anti-mouse IgE (Thermo Fisher Scientific, Waltham, Mass., USA). All antibodies were conjugated with horseradish peroxidase.
These assays were carried out in 96-well multiwell MaxiSorp™ immunoplate polystyrene plates (Thermo Fisher Scientific), in which the native antigen of cat dander or the allergen Fel d 1 (NA-FD1-1) (Indoor Biotech, VA, USA) were incubated. The concentrations were 25μg/mL and 10μg/mL respectively, in a 50mM sodium carbonate-bicarbonate buffer (pH 9.6). The non-specific binding sites were blocked by the incubation with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS) with 0.05% Tween (PBS-T). After another cycle of washing, each well was incubated overnight with diluted sera 1:6400 of PBS-T+BSA (except in the case of IgG2a, in which the dilution was 1:600). In addition, a pool of group 2 sera was used to establish specific antibody values as arbitrary units. The plate was washed three times and incubated with the corresponding secondary antibody at the manufacturer's recommended dilution. SIGMAFAST™ OPD peroxidase substrate (Sigma–Aldrich) was used for reading at 450nm in a spectrophotometer Multiskan™ (Thermo Fisher Scientific), in accordance with the manufacturer's instructions.
With the optical density (OD) obtained from the pool of sera of group 2, arbitrary units per ml (AU/ml) were established. It was determined that for IgG and IgE, the OD mean of the pool of group 2 corresponded to 100AU/ml of specific antibody. In the case of IgG1 and IgG2a, it was established that the mean OD corresponded to 60AU/ml and 20AU/ml, respectively. This is because the percentage of IgG1 in the global IgG antibodies was 60% approximately, and approximately 20% in the case of IgG2a.
Statistical analysisData processing was performed with SPSS 15.0 statistical software for Windows (IBM SPSS statistic, Chicago, IL, USA) to obtain the data of the mean, standard deviation and median of each group.
In addition, comparison of the specific antibody-data of each group was performed to establish significant differences using the non-parametric Mann–Whitney U test with a confidence level of 95%. In this comparison, a value of p<0.05 value was considered a significant difference.
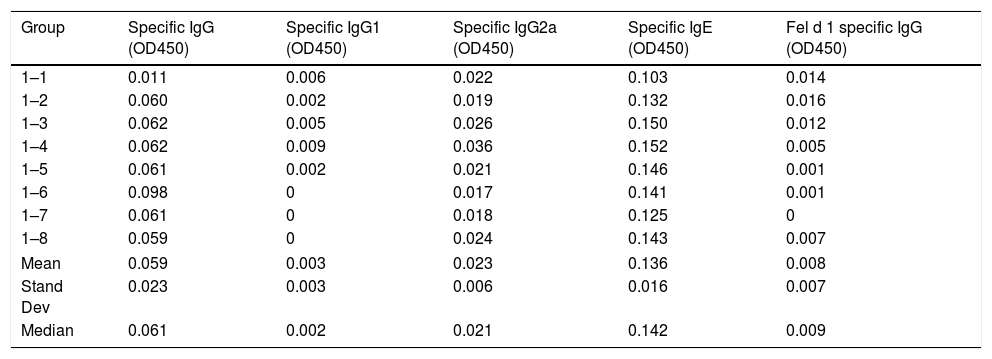
ResultsThe values of specific antibodies in mice serum from groups 2 and 3 are significantly higher than the values from group 1, which was immunized with an allergen without cross-reactivity with cats. Therefore, the values from group 1 are baseline values and so subcutaneous injection with cat dander specific products is found to generate a humoral specific response. Tables 1–3 show the values of OD450 subtracted from blank controls (without antigen) obtained from different concentrations of specific antibodies produced to cat dander proteins and to Fel d 1 in mice of each of the groups included in this study.
Serum-specific antibody values (as OD450 subtracted from blank controls) of group 1 mice immunized with Alternaria alternata. The data of mean, standard deviation and median of the antibody values are included.
| Group | Specific IgG (OD450) | Specific IgG1 (OD450) | Specific IgG2a (OD450) | Specific IgE (OD450) | Fel d 1 specific IgG (OD450) |
|---|---|---|---|---|---|
| 1–1 | 0.011 | 0.006 | 0.022 | 0.103 | 0.014 |
| 1–2 | 0.060 | 0.002 | 0.019 | 0.132 | 0.016 |
| 1–3 | 0.062 | 0.005 | 0.026 | 0.150 | 0.012 |
| 1–4 | 0.062 | 0.009 | 0.036 | 0.152 | 0.005 |
| 1–5 | 0.061 | 0.002 | 0.021 | 0.146 | 0.001 |
| 1–6 | 0.098 | 0 | 0.017 | 0.141 | 0.001 |
| 1–7 | 0.061 | 0 | 0.018 | 0.125 | 0 |
| 1–8 | 0.059 | 0 | 0.024 | 0.143 | 0.007 |
| Mean | 0.059 | 0.003 | 0.023 | 0.136 | 0.008 |
| Stand Dev | 0.023 | 0.003 | 0.006 | 0.016 | 0.007 |
| Median | 0.061 | 0.002 | 0.021 | 0.142 | 0.009 |
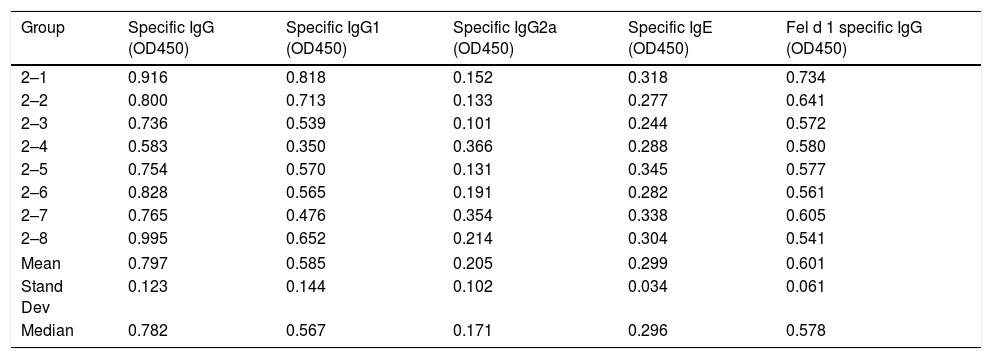
Serum-specific antibody values (as OD450 subtracted from blank controls) of group 2 mice immunized with cat dander native. The data of mean, standard deviation and median of the antibody values are included.
| Group | Specific IgG (OD450) | Specific IgG1 (OD450) | Specific IgG2a (OD450) | Specific IgE (OD450) | Fel d 1 specific IgG (OD450) |
|---|---|---|---|---|---|
| 2–1 | 0.916 | 0.818 | 0.152 | 0.318 | 0.734 |
| 2–2 | 0.800 | 0.713 | 0.133 | 0.277 | 0.641 |
| 2–3 | 0.736 | 0.539 | 0.101 | 0.244 | 0.572 |
| 2–4 | 0.583 | 0.350 | 0.366 | 0.288 | 0.580 |
| 2–5 | 0.754 | 0.570 | 0.131 | 0.345 | 0.577 |
| 2–6 | 0.828 | 0.565 | 0.191 | 0.282 | 0.561 |
| 2–7 | 0.765 | 0.476 | 0.354 | 0.338 | 0.605 |
| 2–8 | 0.995 | 0.652 | 0.214 | 0.304 | 0.541 |
| Mean | 0.797 | 0.585 | 0.205 | 0.299 | 0.601 |
| Stand Dev | 0.123 | 0.144 | 0.102 | 0.034 | 0.061 |
| Median | 0.782 | 0.567 | 0.171 | 0.296 | 0.578 |
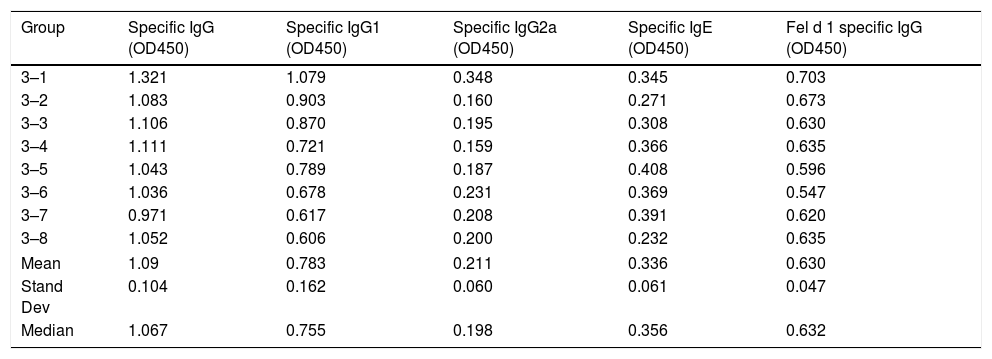
Serum-specific antibody values (as OD450 subtracted from blank controls) of group 3 mice immunized with polymerized cat dander. The data of mean, standard deviation and median of the antibody values are included.
| Group | Specific IgG (OD450) | Specific IgG1 (OD450) | Specific IgG2a (OD450) | Specific IgE (OD450) | Fel d 1 specific IgG (OD450) |
|---|---|---|---|---|---|
| 3–1 | 1.321 | 1.079 | 0.348 | 0.345 | 0.703 |
| 3–2 | 1.083 | 0.903 | 0.160 | 0.271 | 0.673 |
| 3–3 | 1.106 | 0.870 | 0.195 | 0.308 | 0.630 |
| 3–4 | 1.111 | 0.721 | 0.159 | 0.366 | 0.635 |
| 3–5 | 1.043 | 0.789 | 0.187 | 0.408 | 0.596 |
| 3–6 | 1.036 | 0.678 | 0.231 | 0.369 | 0.547 |
| 3–7 | 0.971 | 0.617 | 0.208 | 0.391 | 0.620 |
| 3–8 | 1.052 | 0.606 | 0.200 | 0.232 | 0.635 |
| Mean | 1.09 | 0.783 | 0.211 | 0.336 | 0.630 |
| Stand Dev | 0.104 | 0.162 | 0.060 | 0.061 | 0.047 |
| Median | 1.067 | 0.755 | 0.198 | 0.356 | 0.632 |
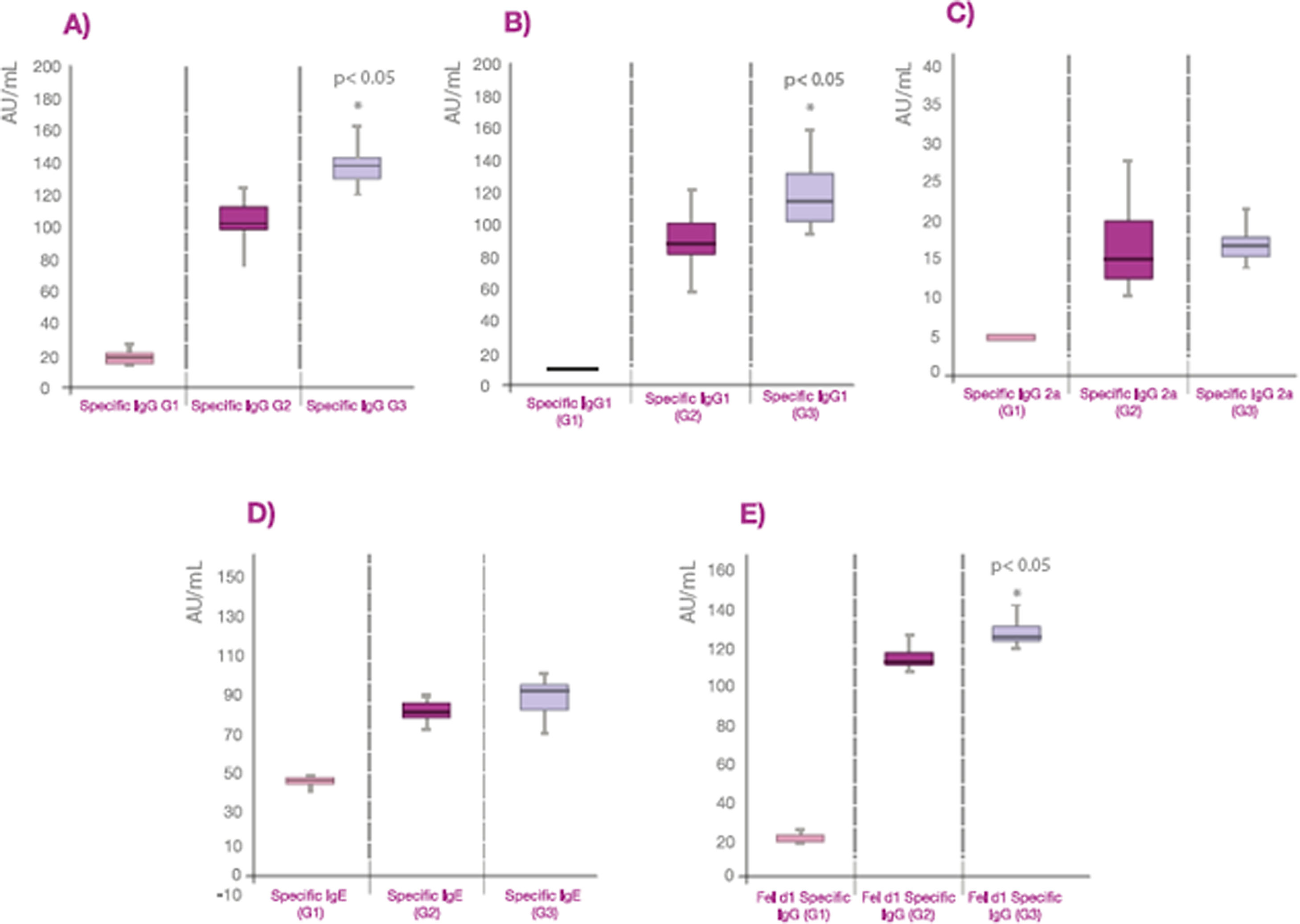
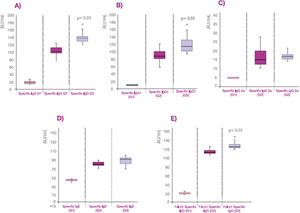
The values of specific antibodies expressed as AU/ml are also represented in box plot graphs showing the median and the quartile values (Fig. 1). The values of specific IgG to cat dander allergens and Fel d 1 from group 3 are greater than group 2 (p<0.05) probably due to the higher dose of allergens in Beltavac® POLYMERIZED versus Beltavac® DEPOT PLUS (Fig. 1A, 1B, 1E). Regarding IgG1, values from group 3 are also higher than group 2 (p<0.05), whereas there are no differences between the values of specific IgG2a and IgE in both groups (Fig. 1C and D).
Graphical representation of the specific antibody values in box diagrams, showing the data distribution of each group. A: representation of the specific IgG in AU/ml. B: representation of the specific IgG1 in AU/ml. C: representation of the specific IgG2a in AU/ml. D: representation of the specific IgE in AU/ml. E: representation of the specific IgG against Fel d 1 antigen in AU/ml.
Vaccine immunogenicity can be defined as its capacity to induce a specific immune response. Allergen immunotherapy can redirect the T cells response of atopic subjects: from IgE to IgG4 (IgG1 in mice). Although the data obtained in murine model assays should be evaluated with caution, certain strains such as Balb/c permit good immunological characterization of the allergy vaccines developed.24,25
A study was performed to test the new product, evaluating its immunogenic capacity to induce specific antibodies against the allergenic extract and the major allergen Fel d 1. The values of specific antibodies in sera from groups 2 and 3 mice were significantly higher than the value from the control group mice, immunized with fungi allergens, without cross-reactivity with cat allergens.
Regarding the new allergoid developed, the results show that the group immunized with the allergoid has higher specific IgG levels to dander allergens and Fel d 1 than the native extract. This may be because the dose of allergen inoculated with the polymer is higher: extract with 1.5μg Fel d 1 per dose instead of the extract containing 0.525μg Fel d 1 in the group immunized with native extract. In order to verify this assertion a study using different dosing regimens should be carried out in mice. The decision to reconstitute the allergoid extract in order to obtain a vaccine with this dose of Fel d 1 is due to a study showing the effectivity of treatment with cat extract containing 15μg Fel d 1 per dose.20 Higher doses may be inoculated due to the safety profile of the allergoid, because it maintains the immunogenic capacity, and therefore, at higher doses, the specific IgG synthesis response is greater. This corresponds to the characteristics of the polymerized products, which maintain their immunogenic capacity, and reduce their allergenicity as compared to the native extract. Moreover, in vitro analysis of allergoid IgE-binding capacity showed an 18-fold reduction compared to the native extract, so it allows higher doses of allergen without compromising safety.16 In vivo analysis through skin response could confirm the reduction of IgE binding capacity of the new allergoid from cat dander.
Specific IgG1 levels were also evaluated due to their homology with IgG4 proteins in humans,26 whose production is stimulated by specific immunotherapy and it acts as a specific IgE blocking antibody.27 The results show elevated levels of specific IgG1 in the immunized group with polymerized dander, while, in the case of IgG2a, levels are similar in both groups. This is because the production of IgG2a is via lymphocytes Th1, whereas IgG1 is via lymphocytes Th2 which is dependent on IL-4.28 The adjuvant used in vaccines, aluminum hydroxide, also stimulates the Th2 pathway. From these results, we conclude that specific production of IgG1 and IgE is stimulated by the new allergoid. However, the difference is significantly greater in the case of IgG1 since the higher inoculation dose favors the production of IgG1 versus IgE.29
Some authors have reservations about the maintenance of immunogenicity in allergoids, as several studies in animal models show a lower capacity of synthesis of specific IgG compared to native extracts.30,31 Since the products compared come from different manufacturers, and due to the heterogeneity existing in immunotherapy products, this does not seem to be a very suitable design to reach this conclusion. However, it is appropriate to compare an allergoid with the native extract of origin, as in the case of an animal model study performed with an allergoid of Betula alba, where it was also shown that the allergoid stimulates the formation of specific IgG that recognizes new epitopes in allergens of B. alba.32 The preclinical study carried out in this assay has established that the designed cat dander allergoid is optimal in allergen immunotherapy as it produces a higher specific IgG than the native extract. In addition, as it offers significantly higher specific IgG1 levels, it evidences greater immunization effectiveness.
Development and fundingThis study was funded by Laboratorios Probelte Pharma S.L.U., Spain.
Authors’ contributionsMP coordinated and supervised all the assays developed in the study. JPS developed the allergoid. JPS and AC made the biochemical and immunological characterizations of the extracts and manufactured the new vaccines. JPS and MP designed and developed the preclinical study. JPS and MP performed the statistical analysis. EB and JPS wrote the manuscript. JPS, EB, AC and MP approve of and agree to the publishing of this manuscript.
Conflict of interestThe authors declare no competing financial interests. All authors are employees of Probeltepharma S.L.U.
The authors would like to thank the staff of the research support service of the University of Murcia (UMU), for their support in the maintenance of the animals during the preclinical study.
This document has been revised by Ann Hannigan-Breen, BA (UCD); HDipEd; Member of CIOL (Chartered Institute of Linguists) UK.