This is a prospective study that assessed pneumococcal antibody levels in PID patients under intravenous immunoglobulin (IVIG) treatment using different brands.
MethodsTwenty-one patients receiving regular IVIG every 28 days were invited to participate: 12 with common variable immunodeficiency, six with X-linked agammaglobulinaemia and three with hyper-IgM syndrome.
One blood sample was collected from each patient just prior to IVIG administration at a three-month time interval during one year. A questionnaire was filled in with patient's demographic data and history of infections during the study period. Streptococcus pneumoniae antibodies against six serotypes (1, 5, 6B, 9V, 14 and 19F) were assessed by ELISA both in patients’ serum (trough levels) and in IVIG samples.
ResultsMedian total IgG trough serum levels were 7.91g/L (range, 4.59–12.20). All patients had antibody levels above 0.35μg/mL to the six serotypes on all four measurements. However, only 28.6% of patients had pneumococcal antibodies for the six analysed serotypes above 1.3μg/mL on all four evaluations during the one-year period. No correlation was found between IgG trough levels and pneumococcal specific antibodies. Eighteen of the 21 patients (85.7%) had infections at some point during the 12-month follow-up, 62/64 (96.9%) clinically classified in respiratory tract infections, four of which were pneumonia.
ConclusionsPneumococcal antibodies are present in a high range of concentrations in sera from PID patients and also in IVIG preparations. Even maintaining a recommended IgG trough level, these patients can be susceptible to these bacteria and that may contribute to recurrent respiratory infections.
The main clinical features of patients with hypogammaglobinaemia are recurrent infections of the respiratory tract with Streptococcus pneumoniae being the most frequent isolated bacterium.1 Long-term administration of immunoglobulin reduces the incidence of infection. However, some patients with common variable immunodeficiency (CVID) may continue to experience upper respiratory tract infections (URTIs) that lead to permanent lung damage, and the optimal trough level for serum IgG remains under discussion.2,3
The minimum concentration of serotype-specific S. pneumoniae antibodies considered to be protective is widely debated. The World Health Organization (W.H.O.) considers the concentration of 0.35μg/mL as protective for invasive pneumococcal disease in healthy children.4 However, individuals with recurrent infections may require higher antibody levels such as 1.3μg/mL for protection.5 Moreover, antibody concentrations required for the prevention of pneumonia, otitis media or colonisation have yet to be established.4,6
Over the past two decades, administration of exogenous pooled human immunoglobulin for intravenous use has become an important therapy in clinical medicine for patients with antibody deficiencies. Passive immunisation by intravenous immunoglobulin (IVIG) has been shown to reduce infections in these patients, especially those from encapsulated bacteria such as S. pneumoniae and Haemophilus influenzae type b (Hib).7,8 Antibodies to S. pneumoniae have been quantified in IVIG samples in a few studies, without concomitant serum antibody level assessment,9,10 and some were quantified in sera only.11,12 Therefore, monitoring antibody levels in these patients is necessary.
The goal of this study was to evaluate serum antibody levels to S. pneumoniae in patients with impaired production of IgG antibodies in regular use of IVIG replacement and to correlate these levels to IVIG preparations as well as to infectious episodes in each patient during the study period.
Patients and methodsThis is a prospective study approved by the Ethics Committee of the Federal University of São Paulo that is in accordance with the World Medical Association and the Helsinki Declaration. Twenty-one patients on regular IVIG replacement therapy were evaluated: 12 with CVID, six with X-linked agammaglobulinaemia (XLA) and three with hyper-IgM syndrome (HIM). Administration of IVIG was performed at intervals of 28 days (range, 21–34 days).
At the beginning of the study, a questionnaire was filled in with the following data: date of birth, gender, diagnosis of immunodeficiency, age at diagnosis, period of treatment, dose of IVIG. During the study, episodes of infections and antibiotic administration were also recorded.
Two of the three hyper-IgM patients had absence of pneumococcal antibodies after vaccine and the third had an early diagnosis and was not immunised. Out of the 12 CVID patients, eight had absence of antibodies to polysaccharide antigens and in four of them these antibodies were not tested but IgG levels were less than 0.4g/L at diagnosis. As XLA patients do not produce antibodies, they were not tested before IVIG replacement therapy initiation.
Collection of blood and IVIG samplesAfter written informed consent and immediately before IVIG infusion, a 10mL blood sample was collected totalling 84 samples that were centrifuged at 800×g for 10min. Serum was separated and stored at −80°C until tested for IgG and pneumococcal serotypes trough levels. One five-millilitre sample of IVIG administered to each patient in the month before blood collection was also stored at 4°C until analysis. All patients were under IVIG replacement for over a year.
Measurement of antibodies to S. pneumoniae by ELISAQuantification of IgG antibodies was performed by ELISA, in accordance with the protocol formulated by the W.H.O. available at http://www.vaccine.uab.edu.
The ELISA for detection of antibodies against six serotypes of S. pneumoniae (1, 5, 6B, 9V, 14 and 19F, obtained from American Type Culture Collection – ATCC, Manassas, USA) was previously calibrated at the Research Laboratory of the Division of Pediatric Infectious Diseases of the Federal University of São Paulo.
Human serum samples were mixed before analysis with C-polysaccharide (C-PS) (Statens Serum Institute, Copenhagen, Denmark) and 22F (ATCC) capsular polysaccharide (PS) to neutralise antibody binding to C-PS and other common contaminants present in the pneumococcal polysaccharide (PnPS) coating antigens. ELISA plates were coated with PnPS by adsorbing individual PnPS serotype antigens to microplates. Dilutions of absorbed human sera were then added to the ELISA plates. The serotype specific antibody bound to the ELISA plate was detected with anti-human IgG antibody conjugated with alkaline phosphatase, followed by addition of the substrate, p-nitrophenyl phosphate. The optical density of each well was measured at 405nm and 690nm using an ELISA plate reader. The antibody level of the human serum was calculated by interpolating the optical density of the sample wells to that of the standard serum (human anti-pneumococcal reference serum, lot 89-SF – Center for Biological Evaluation and Review, US Food and Drug Administration, Bethesda, MD, USA).
For serotype 14, the same method was applied, but the substrate was incubated for 45min instead of the usual 120min.
Protective antibody levels were considered to be ≥1.3μg/mL.5
Statistical analysisIntervals between IVIG administration in individuals with and without infection were compared using Mann–Whitney test. Serum antibody levels and IVIG levels were compared using Kruskal–Wallis test, with differences among groups assessed by Student–Newman–Keuls comparison. Correlation between total IgG and specific antibodies for the six serotypes was tested using Pearson's correlation coefficient. For all analyses, BioEstat 5.0 software (Instituto de Desenvolvimento Sustentável Mamirauá, Tefé, Brazil) was employed. Statistical significance was set at p<0.05.
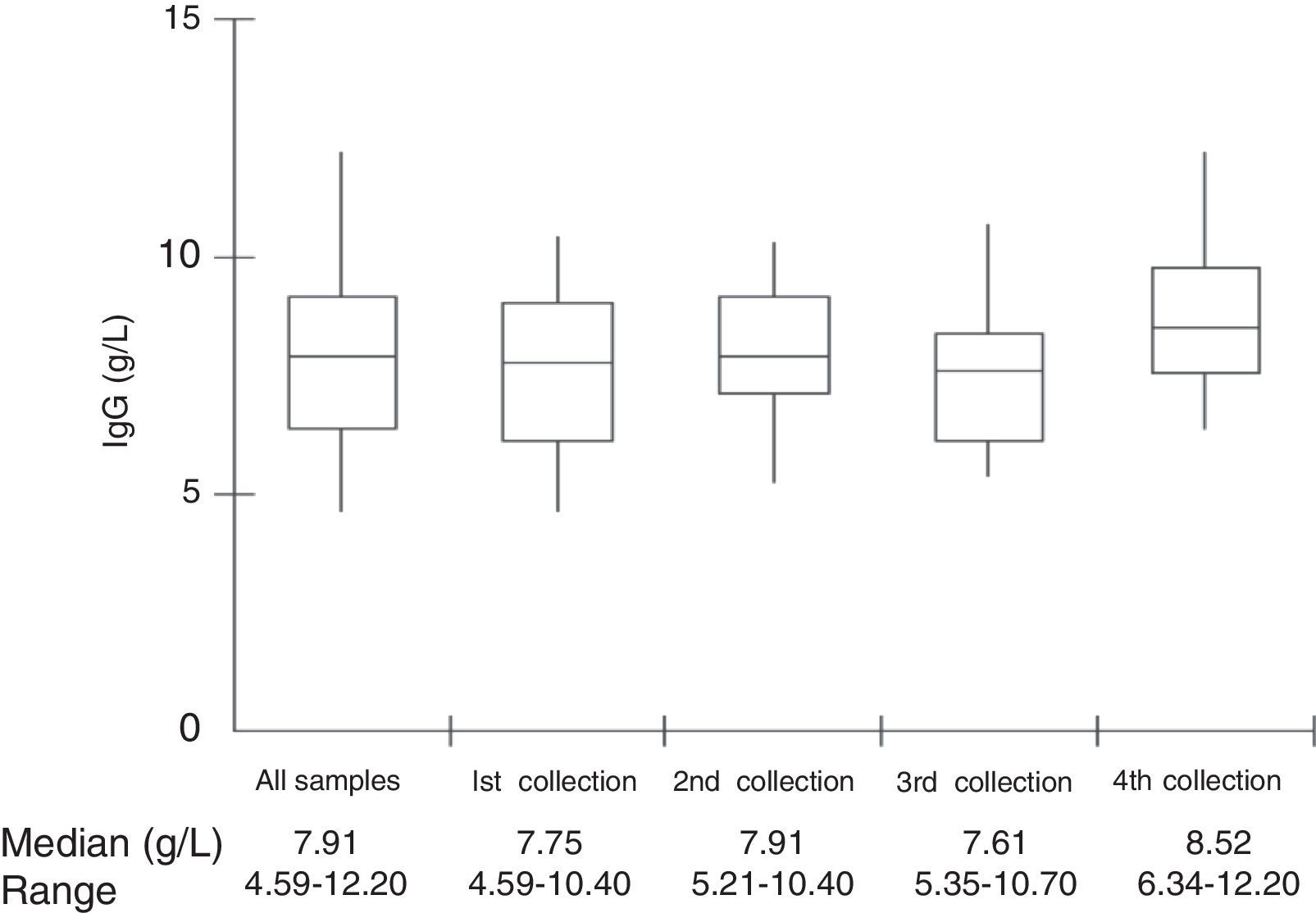
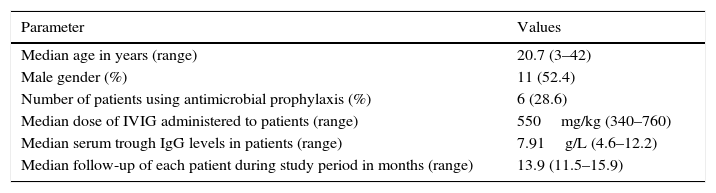
ResultsThe median age of patients was 20.7 years and the median age at diagnosis was 8.0 years. The median IgG at PID diagnosis was 1.49g/L (range, 0.03–5.95g/L). Demographic characteristics of study patients are shown in Table 1.
Demographic characteristics of study patients.
| Parameter | Values |
|---|---|
| Median age in years (range) | 20.7 (3–42) |
| Male gender (%) | 11 (52.4) |
| Number of patients using antimicrobial prophylaxis (%) | 6 (28.6) |
| Median dose of IVIG administered to patients (range) | 550mg/kg (340–760) |
| Median serum trough IgG levels in patients (range) | 7.91g/L (4.6–12.2) |
| Median follow-up of each patient during study period in months (range) | 13.9 (11.5–15.9) |
Over the course of the study, the IVIG dose (median, 522mg/kg/month) remained unchanged for all patients. Most patients received more than one IVIG commercial preparation during the study, but never in the same infusion, because they depended on preparations provided by the Brazilian government. Thirty-eight lots of six different commercial IVIG preparations (Flebogamma®, Octagam®, Tegeline®, Immunoglobulin®, Endobulin® and Vigam®) were evaluated.
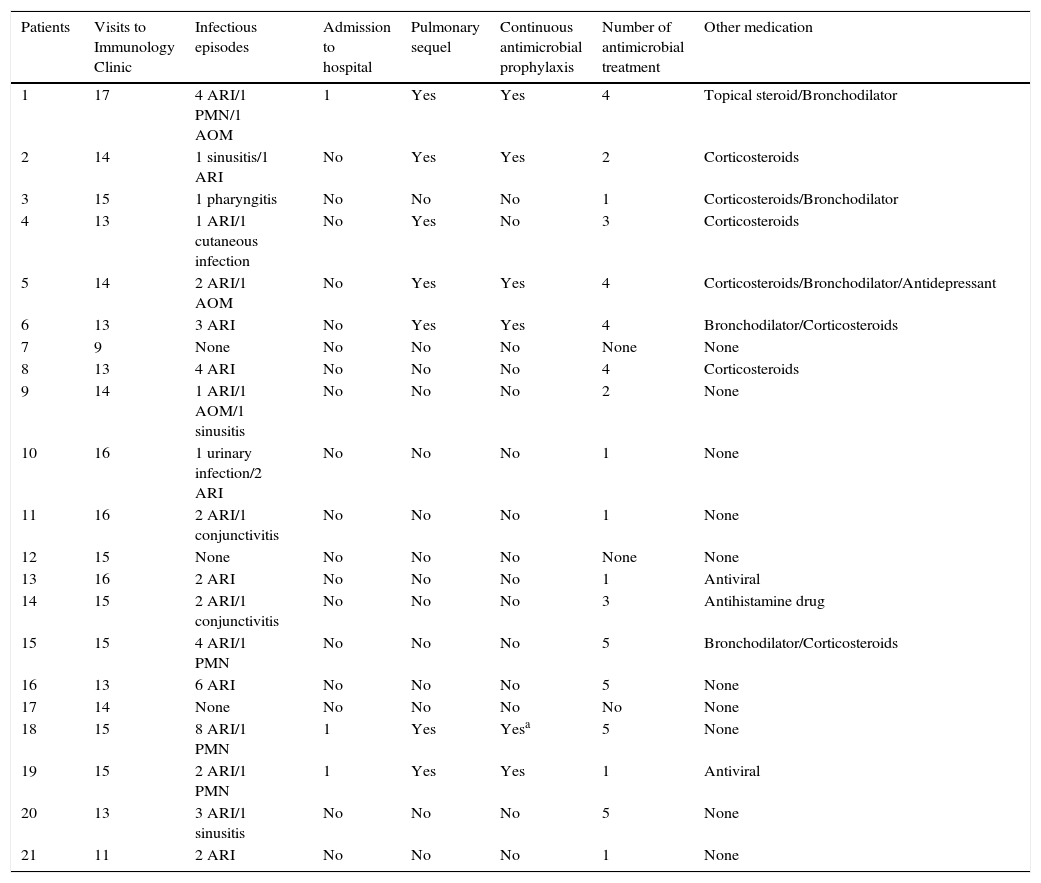
Out of the 21 patients, 18 (85.7%) had some infection within the study period, with a median of three infections per patient. Sixty-two out of 64 infectious episodes (96.9%) were clinically classified as respiratory tract infections – such as sinusitis, otitis, pharyngitis, and pneumonia – or conjunctivitis (Table 2). Any clinical manifestation of cough with secretion or change in the current secretion without a definitive diagnosis was considered as acute respiratory infection (ARI) and antibiotics were recommended. There were 4/64 (6.2%) episodes of pneumonia, but the pathogen was not identified, and 3/4 of these (75%) required hospitalisation (Table 2). The four episodes of pneumonia occurred during the study period, but not at the time of blood sample collection. Thus, an assessment of serum antibody levels close to those pneumonia intervals was not possible.
Medical records of patients in the study period (AOM, acute otitis media; ARI, acute respiratory infection; PNM, pneumonia).
| Patients | Visits to Immunology Clinic | Infectious episodes | Admission to hospital | Pulmonary sequel | Continuous antimicrobial prophylaxis | Number of antimicrobial treatment | Other medication |
|---|---|---|---|---|---|---|---|
| 1 | 17 | 4 ARI/1 PMN/1 AOM | 1 | Yes | Yes | 4 | Topical steroid/Bronchodilator |
| 2 | 14 | 1 sinusitis/1 ARI | No | Yes | Yes | 2 | Corticosteroids |
| 3 | 15 | 1 pharyngitis | No | No | No | 1 | Corticosteroids/Bronchodilator |
| 4 | 13 | 1 ARI/1 cutaneous infection | No | Yes | No | 3 | Corticosteroids |
| 5 | 14 | 2 ARI/1 AOM | No | Yes | Yes | 4 | Corticosteroids/Bronchodilator/Antidepressant |
| 6 | 13 | 3 ARI | No | Yes | Yes | 4 | Bronchodilator/Corticosteroids |
| 7 | 9 | None | No | No | No | None | None |
| 8 | 13 | 4 ARI | No | No | No | 4 | Corticosteroids |
| 9 | 14 | 1 ARI/1 AOM/1 sinusitis | No | No | No | 2 | None |
| 10 | 16 | 1 urinary infection/2 ARI | No | No | No | 1 | None |
| 11 | 16 | 2 ARI/1 conjunctivitis | No | No | No | 1 | None |
| 12 | 15 | None | No | No | No | None | None |
| 13 | 16 | 2 ARI | No | No | No | 1 | Antiviral |
| 14 | 15 | 2 ARI/1 conjunctivitis | No | No | No | 3 | Antihistamine drug |
| 15 | 15 | 4 ARI/1 PMN | No | No | No | 5 | Bronchodilator/Corticosteroids |
| 16 | 13 | 6 ARI | No | No | No | 5 | None |
| 17 | 14 | None | No | No | No | No | None |
| 18 | 15 | 8 ARI/1 PMN | 1 | Yes | Yesa | 5 | None |
| 19 | 15 | 2 ARI/1 PMN | 1 | Yes | Yes | 1 | Antiviral |
| 20 | 13 | 3 ARI/1 sinusitis | No | No | No | 5 | None |
| 21 | 11 | 2 ARI | No | No | No | 1 | None |
Seven out of 21 patients (33.3%) had pulmonary sequel and six (85.7%) were on antimicrobial prophylaxis with amoxicillin or azithromycin or ciprofloxacin or trimethoprim-sulfamethoxazole during the study period (Table 2).
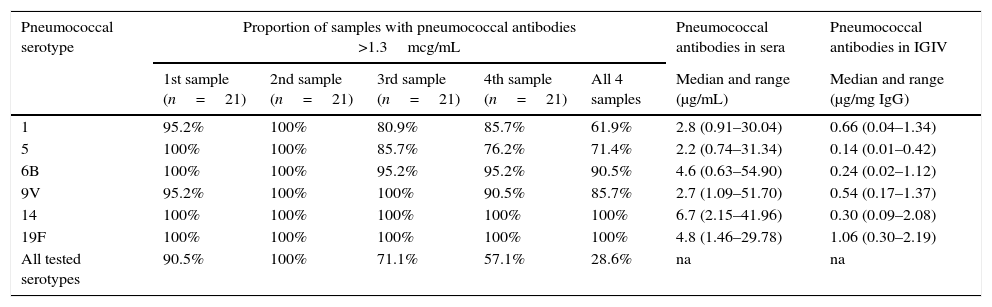
Although the median concentration of all tested pneumococcal serotypes was above 1.3μg/mL in serum, the variation between minimum and maximum levels was very high, reaching more than 80 fold for some serotypes (Table 3). Antibody levels below 1.3μg/mL were observed in 8/84 (9.5%) blood samples for serotype 1, 8/84 (9.5%) blood samples for serotype 5, 2/84 (2.4%) blood samples for serotype 6B and 3/84 (3.6%) blood samples for serotype 9V. Antibodies to serotypes 14 and 19F were above 1.3μg/mL in all evaluations. Antibody levels to serotype 14 were significantly higher than antibodies to the other five serotypes (Table 3).
Prospective immunological evaluation of patients on regular use of IGIV.
| Pneumococcal serotype | Proportion of samples with pneumococcal antibodies >1.3mcg/mL | Pneumococcal antibodies in sera | Pneumococcal antibodies in IGIV | ||||
|---|---|---|---|---|---|---|---|
| 1st sample (n=21) | 2nd sample (n=21) | 3rd sample (n=21) | 4th sample (n=21) | All 4 samples | Median and range (μg/mL) | Median and range (μg/mg IgG) | |
| 1 | 95.2% | 100% | 80.9% | 85.7% | 61.9% | 2.8 (0.91–30.04) | 0.66 (0.04–1.34) |
| 5 | 100% | 100% | 85.7% | 76.2% | 71.4% | 2.2 (0.74–31.34) | 0.14 (0.01–0.42) |
| 6B | 100% | 100% | 95.2% | 95.2% | 90.5% | 4.6 (0.63–54.90) | 0.24 (0.02–1.12) |
| 9V | 95.2% | 100% | 100% | 90.5% | 85.7% | 2.7 (1.09–51.70) | 0.54 (0.17–1.37) |
| 14 | 100% | 100% | 100% | 100% | 100% | 6.7 (2.15–41.96) | 0.30 (0.09–2.08) |
| 19F | 100% | 100% | 100% | 100% | 100% | 4.8 (1.46–29.78) | 1.06 (0.30–2.19) |
| All tested serotypes | 90.5% | 100% | 71.1% | 57.1% | 28.6% | na | na |
na: not applicable.
Considering the four blood samples, only six out of 21 patients (28.6%) maintained antibodies above 1.3μg/mL for the six pneumococcal serotypes during the one-year period (Table 3). Median IgG trough levels (676 versus 698mg/dL) and median number of infections during the study (2.5 versus 3.0) were similar in the groups of patients with and without serum samples with pneumococcal levels above 1.3μg/mL in all samples.
Patients who received IVIG doses greater than 500mg/kg (n=14) were compared with those who received 500mg/kg of IVIG or less (n=7): antibody levels for the six serotypes analysed were higher in the group that received lower doses of IVIG (Mann–Whitney, p≤0.01 for all serotypes). Median pneumococcal levels for patients on IVIG doses >500mg/kg and ≤500mg/kg were the following, respectively. For serotype 1: 2.28 and 3.97; 5: 2.00 and 3.10; 6B: 3.92 and 5.76; 9V: 2.54 and 4.56; 14: 6.18 and 8.09; 19F: 4.33 and 7.20.
Patients with XLA had higher pneumococcal antibody levels for serotypes 1, 6B and 14 (Mann–Whitney, serotype 1: p=0.04; 6B: p=0.04; 14: p=0.02) compared to the other patients, without difference in the dose of IVIG between them (Mann–Whitney, p=0.38). By contrast, antibody levels for the other pneumococcal serotypes (5, 9V and 19F) were similar to those observed in the other patients (Mann–Whitney, serotype 5: p=0.18; 9V: p=0.08; 19F: p=0.12).
The most frequent IVIG administered to patients was Octagam® (Octapharma Pharmazeutika Produktionsges, Viena, Austria) (42/84; 50.0%). The other IVIG brands used were: 23/84 (24.4%) from Flebogamma 5%® (Grifols, S.A, Barcelona, Spain); 8/84 (9.5%) from Tegeline® (LFB Biomedicaments, Lille, France); 5/84 (5.9%) from Vigam® (BPL Bio Products Laboratory, Hertfordshire, United Kingdom); 4/84 (4.8%) from Endobulin® (Baxter, Vienna, Austria) and 2/84 (2.4%) from Blausiegel® (Korea Green Cross Corporation, Korea).
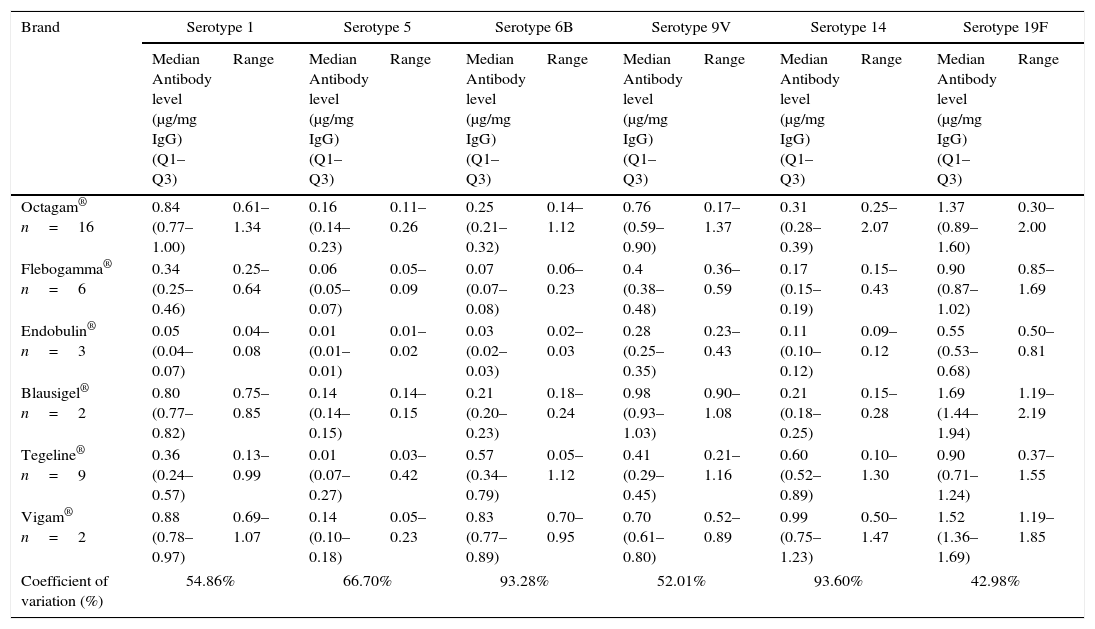
All IVIG samples contained antibodies to each of the six different pneumococcal serotypes studied. However, a considerable variation in antibody levels to different serotypes was noted among the 38 lots of IVIG, reaching over 90% for serotypes 6B and 14 (Table 4). In one of the brands of IVIG, more than one lot had very low antibody levels. The pneumococcal serotype with the highest levels of antibodies in IVIG preparations was serotype 19F, and that was significantly different from the other serotypes (Table 4).
Pneumococcal antibodies in different lots of commercially available preparations of intravenous immunoglobulin used in study.
| Brand | Serotype 1 | Serotype 5 | Serotype 6B | Serotype 9V | Serotype 14 | Serotype 19F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Antibody level (μg/mg IgG) (Q1–Q3) | Range | Median Antibody level (μg/mg IgG) (Q1–Q3) | Range | Median Antibody level (μg/mg IgG) (Q1–Q3) | Range | Median Antibody level (μg/mg IgG) (Q1–Q3) | Range | Median Antibody level (μg/mg IgG) (Q1–Q3) | Range | Median Antibody level (μg/mg IgG) (Q1–Q3) | Range | |
| Octagam® n=16 | 0.84 (0.77–1.00) | 0.61–1.34 | 0.16 (0.14–0.23) | 0.11–0.26 | 0.25 (0.21–0.32) | 0.14–1.12 | 0.76 (0.59–0.90) | 0.17–1.37 | 0.31 (0.28–0.39) | 0.25–2.07 | 1.37 (0.89–1.60) | 0.30–2.00 |
| Flebogamma® n=6 | 0.34 (0.25–0.46) | 0.25–0.64 | 0.06 (0.05–0.07) | 0.05–0.09 | 0.07 (0.07–0.08) | 0.06–0.23 | 0.4 (0.38–0.48) | 0.36–0.59 | 0.17 (0.15–0.19) | 0.15–0.43 | 0.90 (0.87–1.02) | 0.85–1.69 |
| Endobulin® n=3 | 0.05 (0.04–0.07) | 0.04–0.08 | 0.01 (0.01–0.01) | 0.01–0.02 | 0.03 (0.02–0.03) | 0.02–0.03 | 0.28 (0.25–0.35) | 0.23–0.43 | 0.11 (0.10–0.12) | 0.09–0.12 | 0.55 (0.53–0.68) | 0.50–0.81 |
| Blausigel® n=2 | 0.80 (0.77–0.82) | 0.75–0.85 | 0.14 (0.14–0.15) | 0.14–0.15 | 0.21 (0.20–0.23) | 0.18–0.24 | 0.98 (0.93–1.03) | 0.90–1.08 | 0.21 (0.18–0.25) | 0.15–0.28 | 1.69 (1.44–1.94) | 1.19–2.19 |
| Tegeline® n=9 | 0.36 (0.24–0.57) | 0.13–0.99 | 0.01 (0.07–0.27) | 0.03–0.42 | 0.57 (0.34–0.79) | 0.05–1.12 | 0.41 (0.29–0.45) | 0.21–1.16 | 0.60 (0.52–0.89) | 0.10–1.30 | 0.90 (0.71–1.24) | 0.37–1.55 |
| Vigam® n=2 | 0.88 (0.78–0.97) | 0.69–1.07 | 0.14 (0.10–0.18) | 0.05–0.23 | 0.83 (0.77–0.89) | 0.70–0.95 | 0.70 (0.61–0.80) | 0.52–0.89 | 0.99 (0.75–1.23) | 0.50–1.47 | 1.52 (1.36–1.69) | 1.19–1.85 |
| Coefficient of variation (%) | 54.86% | 66.70% | 93.28% | 52.01% | 93.60% | 42.98% | ||||||
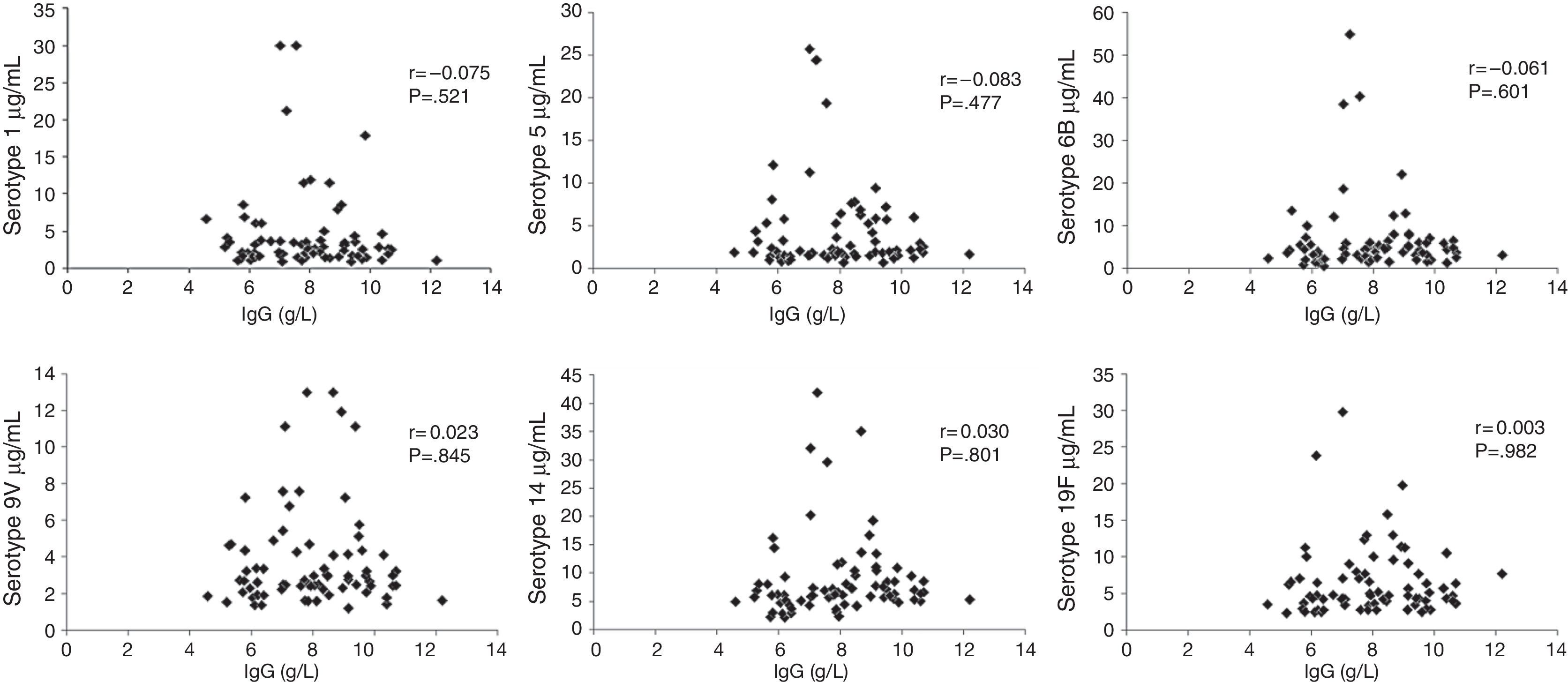
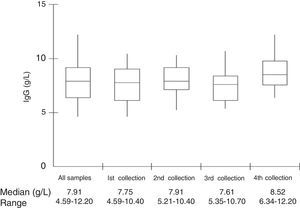
Median total IgG serum levels were 7.91g/L (Table 1 and Fig. 1). No correlation was found between IgG trough levels and pneumococcal specific antibodies for the six serotypes (Pearson's correlation coefficient, p>0.05) (Fig. 2).
Replacement therapy with IVIG is the main treatment for PID patients who do not produce antibodies, since it leads to a reduction in infection rates and prevents the most serious infections.2,13 Hence it is crucial that intravenous immunoglobulin preparations have adequate levels of antibodies against extracellular bacteria such as S. pneumoniae.10,14
However, the multiplicity of serotypes, the differences in methods used to analyse the specific antibodies to S. pneumoniae, together with the criteria used for the interpretation of these antibodies5 are factors that make it difficult to analyse results from such studies.
We chose three known PID diseases in which low IgG levels and lack of antibody production are present. Moreover, we believe that age is not a limitation of the study as antibody production by these patients is not age-dependent.
During the 296 clinical visits of the study period, the vast majority of patients presented respiratory infections and antibiotics were prescribed in 52 situations. Respiratory infections are the most frequent infections in these patients even those treated with IVIG.15,16 Some patients with ARI might have had viral infections.17 However, as viral episodes of infection may lead to secondary bacterial infection in patients with lung disease,18 antibiotics were recommended in all cases.
The recommended time interval for IVIG is 3–4 weeks and that was kept in the vast majority of our patients (median, 28 days; range, 21–34 days). The interval between infusions ranged from 26 to 50 days in another study that showed a common pattern in these patients.19 The recommended dose for each patient was maintained during the study in accordance with other reference centres of PID20 and patients with any lung disease received higher doses compared with patients without pulmonary sequels.
Antibodies to pneumococcal serotypes 1, 5, 6B and 9V were present in levels below 1.3μg/mL in many patients in our study. Serotypes 14 and 19F are also important causes of pneumococcal invasive disease in our country,21 but fortunately all patients presented protective antibody levels to these serotypes in all analysed samples. Altogether, the six pneumococcal serotypes analysed in this study represent 20.6% of S. pneumoniae isolated in adults and children over five years in Brazil in 2011.21
Pneumococcal antibody levels were not lower in patients who received doses equal to or lower than 500mg/kg or in those diagnosed with XLA. Patients who are receiving lower IVIG doses are those who present mild disease without lung sequel and this could be a reason for a lower intake of IVIG. However, other factors might interfere with IgG catabolism. As reviewed by Bonilla,22 the IgG catabolism is mediated by FcRn and higher cumulative IgG doses over time tend to be associated with a shorter half-life of IgG in serum.
Different from previous studies, the behaviour of pneumococcal antibody levels was shown in a prospective way. In general, it is assumed that patients on regular IVIG treatment are provided with high antibody levels against varied pathogens and are protected. However, our results showed that the maintenance of antibodies above 1.3μg/mL during one year for all six serotypes was only observed in a minority of individuals (28.6%).
Another very important issue was the opportunity this study provided to evaluate different brands of IVIG in the same group of patients. The analyses also show that there is a high variation of antibody concentrations in the samples studied. IVIG purification methods are manufacturer specific and unique with respect to specific conditions during fractionation and the combination of methods used to decrease aggregate formation, to remove proteins associated with adverse events, and to provide viral clearance.23
The administration of IVIG results in a peak concentration of total IgG that decreases over time before the next infusion. The concentration of IgG in the serum immediately before the next infusion of IVIG is seen by most immunologists as an important guide to therapy24 and a significant reduction in pneumonia incidence is associated with higher trough IgG levels in patients with antibody deficiency.25 However, no correlation between total IgG levels and specific antibody levels were noted in our results, suggesting that high or adequate trough IgG levels might not always ensure protective antibody levels to different pathogens. Different results were observed when only one IVIG brand was used.19
It was also not possible to correlate the occurrence of respiratory infections with pneumococcal antibody levels. Likewise, a previous attempt to correlate pneumococcal antibody levels with acute exacerbation in patients with chronic pulmonary diseases was not successful.26 For PID patients these correlations are more difficult to establish: even in patients with recommended IgG levels (IgG >7.0g/L), suboptimal pneumococcal antibodies were observed.27 Also, even when protective antibody levels are present in serum, patients with severe impaired antibody production are not fully protected from presenting some form of disease.28
There is a wide variation in specific antibody levels in different IVIG brands and lots to different antigens.10,14,29 The variation in this study of pneumococcal antibodies in IVIG analysed was above 30% for several serotypes in the different brands and lots, a little bit higher when compared to the results from Lejtenyi and Mazer. However, these authors analysed only one brand of IVIG.10 Interestingly, the higher antibody concentrations found for serotype 19F in IVIG preparations were not found when the patients’ serum samples were analysed: in these samples, antibodies to serotype 14 were usually higher than the other serotypes. This might indicate that assessing specific antibodies only in IVIG preparations cannot be sufficient to predict antibody concentrations in the patients’ serum.
Therefore, despite the proven efficacy of IVIG, it would be interesting to monitor the causative pathogens of infections affecting these patients and the level of specific antibodies. This investigation could generate important data on the behaviour of specific antibodies in these patients.
ConclusionPneumococcal antibodies are present in a high range of concentrations in sera from PID patients and also in IVIG preparations. Even maintaining a recommended IgG trough level, these patients can be susceptible to these bacteria and that may contribute to recurrent respiratory infections.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Financial supportCAPES, a Brazilian Funding Agency.
Conflict of interestThe authors declare no conflict of interest.