Children who experience bronchial asthma attacks also experience hypoxic conditions, especially after severe attacks or respiratory failure. Hypoxic conditions during severe asthma attacks may induce neural damage such as axonal damage and neurodegeneration. However, to our knowledge, no studies have been published in this regard. The tau protein plays an important role in the assembly of tubulin monomers into microtubules to form the neuronal microtubule network, maintain microtubule structure and stability, and establish links between microtubules and other cytoskeletal filaments.1 The tau protein is mainly produced in the central nervous system (CNS). Brain injuries introduce the tau protein into the cerebrospinal fluid (CSF) and blood; therefore, tau protein levels are markers of axonal damage and neurodegeneration. In this study, we compared the serum tau protein levels in children who experienced severe asthma attacks with those in the control subjects.
The parents of the patients enrolled in this study provided informed consent. Serum samples were obtained from 18 patients admitted to the Department of Pediatrics, Yamaguchi University Hospital, between March 2009 and August 2010 (age range, 7 months to 14 years; mean age, 5.2 years; median age, 5 years; male–female, 13:5; mean SpO2 on admission, 89.9%) (Table 1). We diagnosed bronchial asthma attacks according to the Japanese Pediatric Guidelines for the Treatment and Management of Asthma 2008 (JPGL 2008).2,3 We also determined asthma attack severity using the JPGL 2008 criteria.2 All of the serum samples were obtained from children who experienced severe asthma attacks at the time of their admission to our hospital. All the patients were given corticosteroids, and 14 required continuous isoproterenol inhalation. In addition, two patients required mechanical ventilation; however, none of these patients displayed neurological sequelae. The control group included 22 healthy children (age range, 1–13 years; mean age, 4.9 years; median age, 5 years; male–female, 12:10). All of the samples were stored at −80°C after collection, and we measured all of the tau protein levels at the same time without freeze thawing.
Summary of children with severe asthma attack and control subjects.
| Severe asthma attack (n=18) | Control (n=22) | |
| Age | 5.2 (7 months to 14 years) | 4.9 (1–13 years) |
| Gender (M:F) | 13:5 | 12:10 |
| SpO2 on admission (%) | 89.9 (81–94) | – |
| Treatment | Corticosteroid: 18Continuous isoproterenol inhalation: 14Mechanical ventilation: 2 | – |
The serum tau protein levels were measured according to the manufacturer's instructions, using an enzyme-linked immunosorbent assay kit (Immunoassay Kit Human Tau [Total], Invitrogen Corporation, Camarillo, CA, USA) which had a detection limit of 12pg/mL.
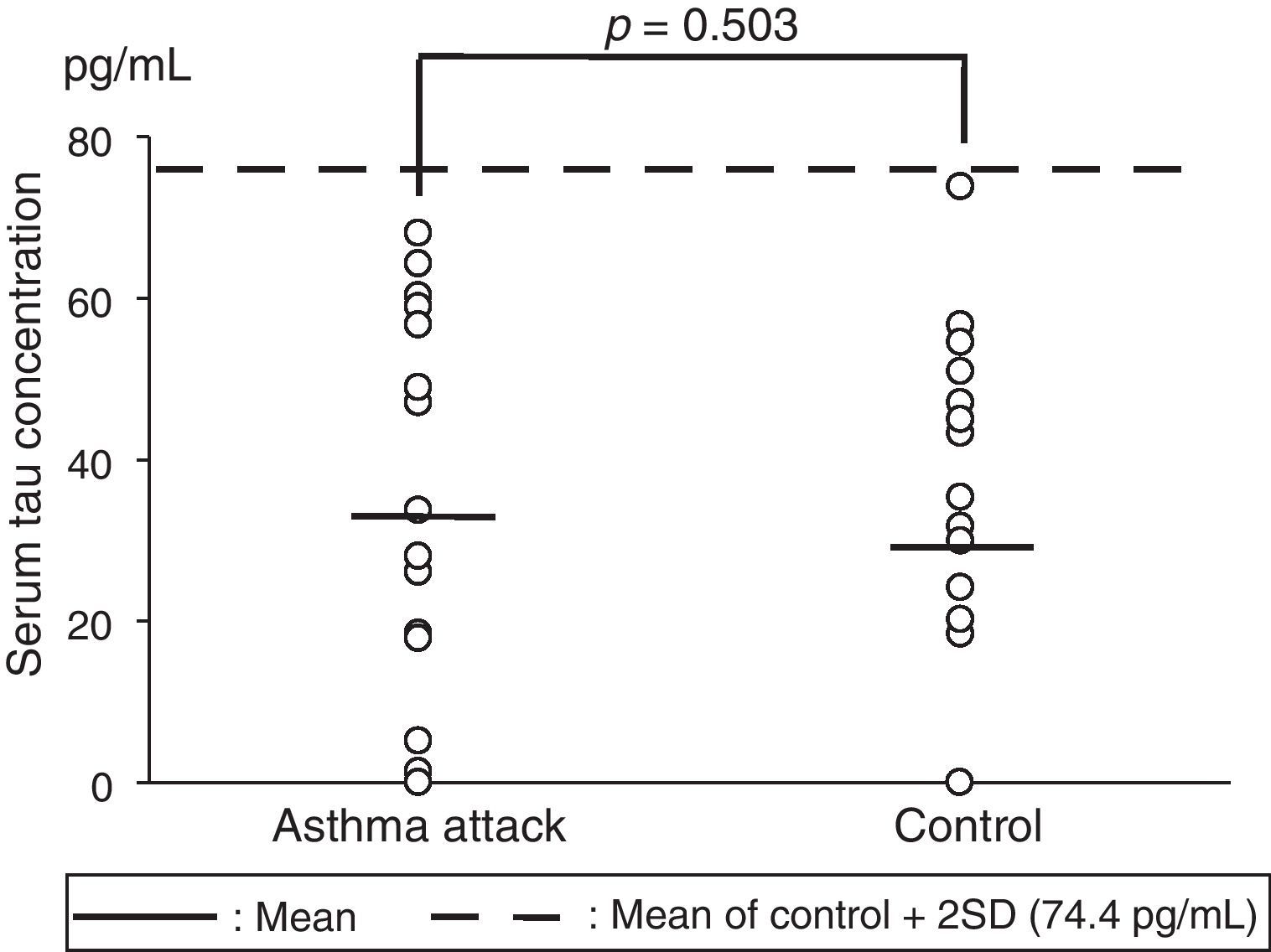
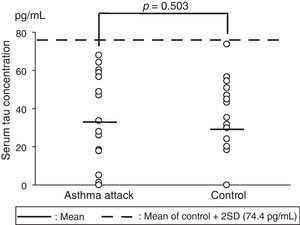
The serum tau protein levels (mean±standard deviation [SD]) were 33.4±24.5pg/mL in the children who experienced severe asthma attacks and 29.2±22.6pg/mL in the control group (Fig. 1). No significant differences were observed in the serum tau protein levels for the two groups on analysis using the Mann–Whitney U test (p=0.503). We established a mean value of the control group+2 SD as the reference range, and all the samples in both groups fell within the range. In addition, no correlations were observed between the serum tau protein levels and SpO2 levels in the asthma attack group (data not shown).
Serum tau protein concentrations in children who experienced severe asthma attacks versus those in the control subjects. The solid horizontal lines show the mean values, whereas the broken horizontal line shows the mean+2 SD value for the control (74.4pg/mL).
SD: standard deviation.
Tau protein is reported to be localised in the neuronal axons of the CNS.4 Many studies have shown elevated CSF tau protein levels in patients with Creutzfeldt-Jakob disease (CJD), stroke, or Alzheimer disease.5–7 In addition, some studies have already reported that serum tau protein levels are elevated in patients with acute neurological disorders such as strokes and CJD and are below the detection limit in neurologically healthy individuals.7–9 We speculate that the hypoxic conditions induced by asthma attacks may cause not only axonal damage but also failure of the blood–brain barrier and that tau protein may appear in the blood and the CSF after axonal damage. The detailed mechanism of how tau protein leaks into the blood remains unclear; however, the serum tau protein levels reflect the CSF levels in patients with CJD.7 We conclude that serum tau protein levels may be a biomarker of brain damage or axonal damage in addition to CSF tau protein levels.
In this study, the serum tau protein levels were not elevated in the children who experienced severe asthma attacks, a finding similar to that seen in the control subjects. All the patients recovered without neurological symptoms after severe asthma attack-induced hypoxic conditions. We treated all of these patients with corticosteroids, continuous isoproterenol inhalation, and mechanical ventilation according to the JPGL criteria. It is most important that the appropriate treatment relieves them from the hypoxic condition as soon as possible. These results suggest that use of the appropriate treatment of severe asthma attacks according to the JPGL criteria can prevent brain damage, axonal damage, neurodegeneration, and destruction of the blood-brain barrier in hypoxic conditions.
This study has a limitation in that there were only two patients with respiratory failure who needed mechanical ventilation. We must, therefore, investigate the serum tau protein levels in more patients with respiratory failure. We also need to examine serum tau levels in patients with neurological sequelae, as none of the patients in the current study displayed such symptoms.
The results of the present study suggest that the hypoxic conditions induced by severe asthma attacks do not induce axonal damage and neurodegeneration in children and that the use of appropriate treatments for asthma attacks in accordance with the JPGL criteria can help prevent neuronal damage.
Ethical disclosuresPatient's data protectionConfidentiality of data. We have followed the protocols of our work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. We have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflicts of interestNone of the authors have conflicts of interest to disclose.