Severe asthma is a compelling challenge in clinical practice. Adolescence represents a relevant aspect of this issue. We report a series of adolescents with severe asthma and evaluated before and after a one-year standardized guideline-oriented treatment. We explored the relevance of symptom perception, including nasal and bronchial complaints, assessed by visual analog scale (VAS) and the perception of asthma control measured by the asthma control test (ACT). The current study demonstrated that adolescents perceived a significant improvement in their symptoms (p < 0.0001) and asthma control (p < 0.001) after adequate treatment. In conclusion, the management of severe asthma in adolescents should be carefully addressed to also consider the patient’s perception.
Asthma is characterized by chronic inflammation of airways and respiratory symptoms, including wheeze, breathlessness, chest tightness, and cough.1,2 Asthma prevalence is about 7% in adolescents worldwide.3 The economic burden of asthma is notable also considering school absences and emergency department admissions.4,5 The current asthma definition consists of a clinical umbrella syndrome.6 Consequently, classifying asthmatic patients is clinically relevant to correctly manage them.7 In this regard, severe asthma represents a challenge in clinical practice, as it leads to an increase in morbidity and mortality.8 Moreover, the adolescence represents a further issue as it is associated with emotional, psychological, and social aspects that may significantly affect adherence to the therapy and correct lifestyle.9,10
International guidelines have been provided to optimize management and treatment.7 In this regard, asthma control is the main target of the treatment strategy. Symptoms perception represents a particular issue that deserves adequate attention, mainly concerning the perception of asthma control. In this regard, a standardized questionnaire, namely the asthma control test (ACT) is widely used in clinical practice.11
Therefore, we tested the hypothesis that the perception of symptoms and asthma control could be clinically relevant in the management of adolescents with severe asthma. The current longitudinal study evaluated adolescents suffering from severe asthma before and after a one-year treatment.
Materials and methodsThe current longitudinal study included 40 adolescents (22 males and 18 females; mean age 14.18 + 1.97 years) suffering from severe asthma before and after a 12-month treatment. The study was conducted in a real-life setting, such as a tertiary level asthma clinic.
The patients were consecutively enrolled in the study.
Inclusion criteria were: adolescent age (12–17 years) and documented asthma diagnosis based on typical symptoms history consistent with reversibility to bronchodilators and/or bronchial hyperresponsiveness to methacholine (MCH). Exclusion criteria were a history of lung disease other than asthma, severe comorbidity that could affect the interpretation of the results. Patients discontinued the use of short-acting and long-acting bronchodilators, respectively for 4 and 12 h before lung function measurement.
The visit entailed a careful history, current inhaled corticosteroids dosage, clinical examination, perception of breathing and nasal patency assessed by the visual analog scale (VAS), lung function testing, body mass index (BMI), asthma control test (ACT) questionnaires, asthma control level and asthma severity grade according to the Global Initiative for Asthma (GINA) guidelines.7 The Hospital Review Board approved the study procedure and written informed consent was obtained from all parents.
Patients were treated consistently with the GINA guidelines and were evaluated at baseline, and after 6- and 12-months follow-up.
Spirometry was performed using a computer-assisted spirometer (Pulmolab 435-spiro 235, Morgan, England– predictive values ECCS 1993), with an optoelectronic whirl flow meter. This spirometer fulfils the ATS/ERS standards according to guidelines.12,13 It was performed as stated by the European Respiratory Society.12
ACT questionnaire consisted of five questions with five possible responses, exploring the patient’s perception of his/her asthma control.11 The result could range between 0 and 25, where 25 was the optimal asthma control.
The VAS consisted of one ruler asking for breathlessness and nasal symptoms. Patients indicated their actual perception of breathlessness or nasal patency by marking a VAS. In this study, the VAS was a 10-cm vertical line on which 0 implied breathlessness or nasal obstruction, while 10 corresponded to no breathlessness or completely open nose. Initially, patients were instructed to place a mark on the line indicating their perception at that moment. With a movable marker, the adolescent could mark any point on the 10-cm segment which best described his/her perception. No interval marker was visible on the line.
Data were reported as mean with standard deviation (SD) or median with inter-quartile range (lower and upper values) or as absolute and relative (percentages) numbers. The Wilcoxon signed-rank test, the one-way ANOVA, or the non-parametric Kruskal Wallis test when appropriate, were performed. Statistica software 9.0 (StatSoft Corp., Tulsa, OK, USA) was used.
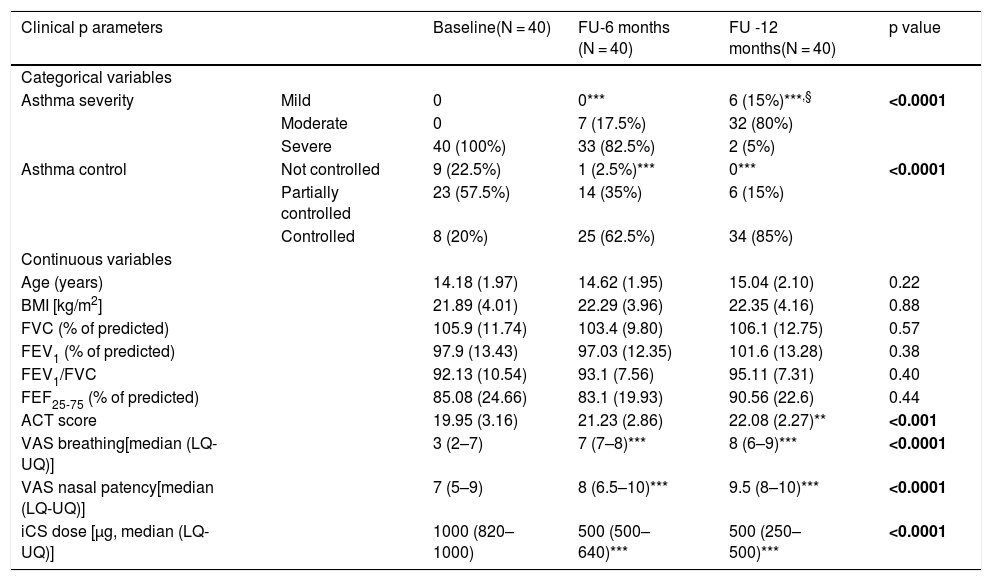
ResultsTable 1 shows the most relevant findings at baseline, after six, and 12 months.
Clinical data in 40 adolescents with severe asthma at baseline, and 6- and 12-month follow-up (FU).
| Clinical p arameters | Baseline(N = 40) | FU-6 months (N = 40) | FU -12 months(N = 40) | p value | |
|---|---|---|---|---|---|
| Categorical variables | |||||
| Asthma severity | Mild | 0 | 0*** | 6 (15%)***,§ | <0.0001 |
| Moderate | 0 | 7 (17.5%) | 32 (80%) | ||
| Severe | 40 (100%) | 33 (82.5%) | 2 (5%) | ||
| Asthma control | Not controlled | 9 (22.5%) | 1 (2.5%)*** | 0*** | <0.0001 |
| Partially controlled | 23 (57.5%) | 14 (35%) | 6 (15%) | ||
| Controlled | 8 (20%) | 25 (62.5%) | 34 (85%) | ||
| Continuous variables | |||||
| Age (years) | 14.18 (1.97) | 14.62 (1.95) | 15.04 (2.10) | 0.22 | |
| BMI [kg/m2] | 21.89 (4.01) | 22.29 (3.96) | 22.35 (4.16) | 0.88 | |
| FVC (% of predicted) | 105.9 (11.74) | 103.4 (9.80) | 106.1 (12.75) | 0.57 | |
| FEV1 (% of predicted) | 97.9 (13.43) | 97.03 (12.35) | 101.6 (13.28) | 0.38 | |
| FEV1/FVC | 92.13 (10.54) | 93.1 (7.56) | 95.11 (7.31) | 0.40 | |
| FEF25-75 (% of predicted) | 85.08 (24.66) | 83.1 (19.93) | 90.56 (22.6) | 0.44 | |
| ACT score | 19.95 (3.16) | 21.23 (2.86) | 22.08 (2.27)** | <0.001 | |
| VAS breathing[median (LQ-UQ)] | 3 (2–7) | 7 (7–8)*** | 8 (6–9)*** | <0.0001 | |
| VAS nasal patency[median (LQ-UQ)] | 7 (5–9) | 8 (6.5–10)*** | 9.5 (8–10)*** | <0.0001 | |
| iCS dose [μg, median (LQ-UQ)] | 1000 (820–1000) | 500 (500–640)*** | 500 (250–500)*** | <0.0001 |
Numerical data are expressed as mean (SD), unless otherwise specified.
At baseline, all adolescents had severe asthma, nine adolescents (22.5%) had not controlled asthma, 23 partially controlled (57.5%), and eight controlled (20%). At the six-month follow-up visit, 33 adolescents (82.5%) had still severe asthma and seven (17.5%) moderate asthma; one (2.5%) had not controlled asthma, 14 (35%) partially controlled, and 25 (62.5%) controlled. At the 12-month follow-up visit, two adolescents (5%) still had severe asthma, 32 (80%) moderate, and six (15%) mild asthma; nobody had uncontrolled asthma, six (15%) partially controlled, and 34 (85%) controlled. There were significant differences in the changes over time for both the asthma classifications (p < 0.0001 for both).
BMI and lung function did not change over time (at any visit).
VAS breathing and VAS for nasal patency significantly increased over time (p < 0.0001 for both).
ACT scores significantly increased over time (p < 0.001).
Inhaled corticosteroid dose significantly diminished after the treatment (p < 0.0001).
DiscussionSevere asthma is a demanding challenge in daily practice and adolescence represents a further complicating issue. The current study provided some information that may be fruitful in common practice as the outcomes may mirror what occurs in the day-by-day medical visits.
Treatment based on guidelines indication was able to improve asthma severity in almost all adolescents, but two. Consistently, the asthma control level significantly improved. It must be noted that asthma severity and asthma control do not correlate: 20% of adolescents had controlled asthma at baseline. This apparent inconsistency is well known since the definition of severe asthma includes controlled asthma even though obtained with high-dose anti-inflammatory drugs.
Lung function did not significantly change during the study, so it seems to be a poor marker to measure asthma severity and control or to predict the asthma trend over time. On the contrary, perception of asthma control assessed by ACT and perception of breathing and nasal patency measured by VAS significantly changed during the follow-up. Moreover, these outcomes do comply with the findings provided by a recent study investigating the management of pediatric asthma.10 That study concluded that patient education and better monitoring are crucial issues for the self-management to tailor treatment for asthmatic children over time. Therefore, the severe adolescent asthma treatment strategy should adequately consider the subjective assessment of control by ACT and respiratory symptoms by VAS.
The current study has some limitations, including the lack of biomarkers measurement and the relatively low number of participants. However, it must be noted that severe asthma accounts for about 5% of the asthmatic population, so about one thousand adolescents were screened to be included in this study.
In conclusion, the management of severe asthma in adolescents should be carefully addressed to consider the patient’s perception of symptom severity and asthma control.
Conflict of interestAll authors declare that there is no conflict of interest.
Financial disclosureAll authors declare that there is no funding.
Ethical disclosuresProtection of human subjects and animals in research
The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protection
Confidentiality of data
The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent
The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.