Objective. Cystatin C is a very potent inhibitor of cysteine proteinases and, it has been clinically applied as a sensitive marker in monitoring of renal and liver functions. The aim of this study was to reveal whether cystatin C may be a useful marker for distinguishing intra-versus extrahepatic cholestasis.
Materials and methods. Serum cystatin C concentrations were determined by nephelometric immunoassay using N latex cystatin C kit in 53 patients with cholestatic disorder that included 18 patients with intrahepatic cholestasis, 17 patients with malignant extrahepatic cholestasis, 18 patients with benign extrahepatic cholestasis. Serum cystatin C concentration was also determined in 20 healthy volunteers.
Results. Mean serum cystatin C concentration was 2.82 ± 0.24 mg/l (SD) in patients with intrahepatic cholestasis, 2.05 ± 0.15 mg/l in patients with extrahepatic malignant cholestasis, 1.37 ± 0.13 mg/l in extrahepatic benign cholestatic patients and 0.93 ± 0.24 mg/l in control group. Serum cystatin C concentrations in patients with cholestatic disease were significantly higher than those in the healthy controls (p < 0.001). Moreover, mean serum cystatin C concentration in patients with intrahepatic cholestasis was higher than those in extrahepatic cholestasis groups (p < 0.001). Serum cystatin C concentrations were significantly higher in patients with malignant extrahepatic cholestasis than in patients with benign extrahepatic cholestasis (p < 0.001). There were no correlations patients among serum cystatin C concentrations and serum levels of AST, ALT, ALP, GGT, total and conjugated bilirubin.
Conclusion. Our results suggested that serum cystatin C level may be a potential biochemical marker both to point out an intrahepatic origin by excluding an extrahepatic source of cholestasis in patients with jaundice and to possibly differentiate bening and malignant extrahepatic cholestatic disorders.
Cholestatic disorders comprise intrahepatic cholestatic diseases (such as drug induced cholestasis, viral fulminant hepatitis) and extrahepatic cholestatic diseases (such as common bile duct stones, malignant tumors).1 On admission to outpatient clinics, it may be difficult to distinguish intra versus extrahepatic cholestasis on the basis of laboratory findings. Therefore it is necessary to perform clinical examination, abdominal ultrasound, endoscopic retrograde cholangiopancreatography (ERCP), percutanous transhepatic cholangiography (PTC) and magnetic resonance cholangiopancreatography (MRCP) to enable the differentiation among these diseases.2 For instance, demonstration of an elevated hepatic alkaline phosphatase (ALP) and gamma glutamyltranspeptidase (GGT) are very important tests for diagnosis of extrahepatic and intrahepatic cholestasis. However, the degree of elevation ALP and GGT do not adequately distinguish the former from the latter.1
The type I cystatins (A and B) are mainly intracellular, the type II cystatins (C, D, F, G, S, SN, and SA) are extracellular and the type III cystatins (L, H, kininogens) are intravascular proteins.3 Cystatin C, a member of the cystatin family, is a non-glycosylated 13 kDa basic protein.4 It is a very potent inhibitor of lysosomal cysteine proteinases such as cathepsin B, H, L, S.5 Serum cystatin C level is affected by sex, hyperbilirubinemia and diet. Moreover, the individual variations of serum cystatin C in healthy controls have been shown to be extremely low.6 Serum cystatin C is suggested to be a more sensitive marker of glomerular filtration rate (GFR) than creatinine in the general population.7 Cystatin C has been established in carcinoma cell lines.8,9 Previous clinical studies showed that altered serum cystatin C concentrations have been detected in patients with autoimmune disease, amyloidosis, and multiple sclerosis.10-12 Shu-Chen, et al.6 showed that serum cystatin C concentrations were significantly higher in patients with liver cirrhosis or hepatocellular carcinoma than those in the control and chronic hepatitis. Similarly, Mamiko, et al.13 reported that cystatin C may be an applicable monitoring marker in progression of liver fibrosis. Although studies have shown that serum cystatin C concentrations were significantly high in some clinical conditions, alterations in serum cystatin C concentrations have not been explored in relation to cholestatic disorders. In this study, we aimed to measure the concentrations of serum cystatin C in patients with cholestatic disorders and to investigate whether the serum cystatin C level is a useful biochemical marker in the differential diagnosis between intra and extrahepatic cholestatic diseases.
Materials and MethodsSubjectsThe study population comprised 53 patients with cholestatic diseases and 20 healthy controls. Cholestatic diseases included intrahepatic cholestasis (n:18), malignant extrahepatic cholestasis (n:17) and benign extrahepatic cholestasis (n:18). All cases were diagnosed by laboratory findings, abdominal ultrasound, ERCP, MRCP and liver biopsy in some patients. The diagnosis of intrahepatic cholestasis was confirmed by liver biopsy in 18 patients. In extrahepatic cholestatic patients, the diagnosis was supported by clinical findings, biochemical and imaging studies. Patients with abnormal kidney functions were excluded.
All patients and controls gave informed consent to participate in the study. The study protocol was approved by the ethics committee of Gaziantep University. Blood samples obtained from all participants were centrifuged and stored at-80 C for further studies.
Measurement of serum cystatin C concentrations:The N Latex Cystatin C kit (Dade Behring Diagnostics, Marburg, Germany) was used in this study. The assay is a fully automated particle enhanced nephelometric immunoassay for measuring serum cystatin C. The assay is carried out using a fixe time method. The antigen-antibody complexes scatter a beam of incident light at 840 nm and the intensity of the light is proportional to the concentration of cystatin C in the sample. The assay utilizes a stored six-point calibration curve generated from multiple dilutions of a human calibrator. The accepted reference range for serum cystatin C is from 0.5-1.3 mg/l.6
Laboratory testsSerum transaminases, albumin, alkaline phosphatase, serum bilirubin level and prothrombin time were measured in all subjects by standard laboratory methods.
StatisticsAll results are expressed as mean±SD. Non-parametric data were compared by Mann-Whitney and Kruskall-Wallis tests. A p-value of < 0.05 was considered to be statiscally significant. The Spearman’s rank test and Pearson test were used to assess correlation between parameters examined. A p-value of < 0.05 was considered to be statistically significant. The data was analysed to calculate sensitivity and specificity by creating various cut-off points for each disease group with ROC curve analysis.
All analyses were two tailed and conducted using a computer based statistics software (SPSS for Windows 8.0, 1997, SPSS Inc. Chicago, USA).
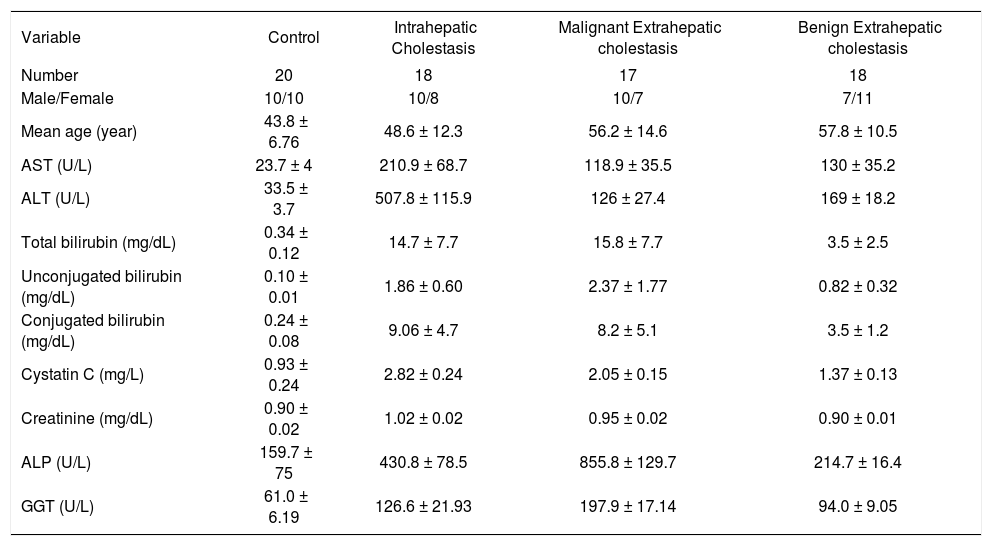
ResultStudy subjectsPatients with cholestatic diseases were recruited from the department of Gastroenterology, Gaziantep University Hospital. They were categorized into three groups. Groups were including intrahepatic cholestasis (Group IHC; n=18), malignant extrahepatic cholestasis (Group EHMC; n= 17), and benign extrahepatic cholestasis (Group EHBC; n:18). In addition, 20 persons without cholestatic diseases were included to be controls. The main clinical and laboratory characteristics of the 53 patients with cholestatic diseases and the control group are summarized in table 1. In addition; etiological classification of patients are shown in table 2.
Laboratory Data for Control and Experimental Groups.
| Variable | Control | Intrahepatic Cholestasis | Malignant Extrahepatic cholestasis | Benign Extrahepatic cholestasis |
|---|---|---|---|---|
| Number | 20 | 18 | 17 | 18 |
| Male/Female | 10/10 | 10/8 | 10/7 | 7/11 |
| Mean age (year) | 43.8 ± 6.76 | 48.6 ± 12.3 | 56.2 ± 14.6 | 57.8 ± 10.5 |
| AST (U/L) | 23.7 ± 4 | 210.9 ± 68.7 | 118.9 ± 35.5 | 130 ± 35.2 |
| ALT (U/L) | 33.5 ± 3.7 | 507.8 ± 115.9 | 126 ± 27.4 | 169 ± 18.2 |
| Total bilirubin (mg/dL) | 0.34 ± 0.12 | 14.7 ± 7.7 | 15.8 ± 7.7 | 3.5 ± 2.5 |
| Unconjugated bilirubin (mg/dL) | 0.10 ± 0.01 | 1.86 ± 0.60 | 2.37 ± 1.77 | 0.82 ± 0.32 |
| Conjugated bilirubin (mg/dL) | 0.24 ± 0.08 | 9.06 ± 4.7 | 8.2 ± 5.1 | 3.5 ± 1.2 |
| Cystatin C (mg/L) | 0.93 ± 0.24 | 2.82 ± 0.24 | 2.05 ± 0.15 | 1.37 ± 0.13 |
| Creatinine (mg/dL) | 0.90 ± 0.02 | 1.02 ± 0.02 | 0.95 ± 0.02 | 0.90 ± 0.01 |
| ALP (U/L) | 159.7 ± 75 | 430.8 ± 78.5 | 855.8 ± 129.7 | 214.7 ± 16.4 |
| GGT (U/L) | 61.0 ± 6.19 | 126.6 ± 21.93 | 197.9 ± 17.14 | 94.0 ± 9.05 |
Numbers and Types of Hepatic Abnormalities Studied.
| • Extrahepatic Malignant Cholestasis (n:17) |
| Pancreatic carcinoma (n: 6)Periampullary carcinoma (n: 3)Cholangiocarcinoma (n: 8) |
| • Extrahepatic Benign Cholestasis (n: 18) |
| Choledocholithiasis (n: 16)Benign Sitricture (post-cholecystectomy (n: 2) |
| • Intrahepatic Cholestasis |
| Fulminant hepatitis (n: 5)Toxic hepatitis (n: 6)Primary Biliary cirrhosis (n: 2)Liver cirrhosis (n: 5) |
As shown in table 1 and figure 1, the mean cystatin C concentration was 0.93 ± 0.24 mg/l in healthy controls, 2.82 ± 0.24 mg/l in patients with intrahepatic cholestasis and 2.05 ± 0.15 mg/l in patients with malignant extrahepatic cholestasis and 1.37 ± 0.13 mg/l in patients with benign extrahepatic cholestasis. Concentrations in the patients with cholestatic disease were all significantly higher than concentrations in the healthy controls, (p < 0.001). Furthermore, mean serum cystatin C concentration of patients in intrahepatic cholestasis groups was remarkably higher than that in extrahepatic cholestasis groups (p < 0.001) and serum cystatin C concentrations were significantly higher in patients with malignant extrahepatic cholestasis than in patients with benign extrahepatic cholestasis (p < 0.001).
Correlations between cystatin C concentrations and other laboratory findings:
ALT and AST concentrations were significantly higher in patients with intrahepatic cholestasis than in patients with extrahepatic cholestasis as were levels of cystatin C (p < 0.001). However, there is no statistically any significant difference between serum aminotransferase levels in patients with malignant and benign extrahepatic cholestasis (p > 0.05). On the other hand, GGT and ALP concentrations were significantly higher in patients with extrahepatic malignant cholestasis than in patients with intrahepatic cholestasis (p < 0.001, Figure 2). But no correlations were observed between serum cystatin C concentrations and AST, ALT, ALP, GGT, total and conjugated bilirubin levels. The data was analysed to calculate sensitivity and specificity by creating various cut-off points for each disease group (ROC curve analysis) levels. IHC between control group; Cystain C:1.8 mg/l (cut-off points) %100 sensitivity and %100 specificity AUC (area under the curve): 1.00 (CI 1.00-1.00). M.EHC and control group; Cystain C: 1.55 mg/l (%100 sensitivity and %100 specificity), AUC:1.00 (CI 1.00-1.00). B.EHC with control group; Cystain C: 1.15 mg/l (%94 sensitivity and %75 specificity), AUC:0.950 (CI 0.889-1.011). IHC with M.EHC group; Cystain C: 2.35 mg/l (%94 sensitivity and %100 specificity), AUC:0.997(CI 0.986-1.007). IHC with B.EHC group; Cystain C: 2.95 mg/l (%100 sensitivity and %100 specificity), AUC:1.00 (CI 1.00-1.00). M.EHC with B.EHC group; Cystain C: 1.70 mg/l (%100 sensitivity and %100 specificity), AUC:1.00 (CI 1.00-1.00).
DiscussionCholestatic diseases include intrahepatic and extrahepatic cholestatic diseases, whereas extrahepatic cholestasis comprises benign (choledocolitiasis) and malignant cholestasis (cholangiocarcinoma, pancreas carcinoma and periampullary carcinoma). Causes of intrahepatic cholestasis are multifactorial: metabolic (such as drug induced, TPN (total parenteral nutrition) induced and cystic fibrosis), immunologic; (primary biliary cirrhosis and sarcoidosis), infectious; (viral hepatitis), enigmatic; (Benign recurrent intrahepatic cholestasis.)14
Aminotransferase levels are usually elevated in any hepatic disorder in which there is evidence of hepatocyte inflammation or necrosis and especially ALT elevation is often the first biochemical abnormality detected in a patient with viral, drug induced and alcoholic hepatitis. On the other hand, while serum levels of ALP is often raised in extrahepatic malignant cholestasis, it is usually normal or moderately raised in intrahepatic cholestasis. But there are no differences in the levels of hepatic alkaline phosphatase and ALT in patients with intrahepatic or extrahepatic cholestasis. Likewise the previous studies were reported that serum cystatin C concentrations were increased in patients with chronic liver disease. In particular, patients with liver cirrhosis had mean serum cystatin C levels higher than that of the control subjects.13 It has been suggested that an imbalance between cathepsins and cystatin C contributes to liver fibrosis. Several previous studies showed that metalloproteinases and tissue inhibitors of metalloproteinases were played important roles in the regulation of liver fibrosis.15-16 Furthermore, lysosomal cathepsins are also enzymes capable of degrading interstitial collagens. An increased of activity of cathepsin was observed in liver fibrosis.17-18
Intrahepatic cholestasis, especially due to fulminant hepatitis and toxic hepatitis, leads to hepatocyte injury and inflammation, resulting in destruction of hepatocyte. After destruction of hepatocyte, lysosomal cathepsin levels increases in serum. Increased activities of cathepsins have been observed in tumour tissues. Moreover cystatin C, specific inhibitors of cathepsins, is also increased in several tumour tissues and cancer cell lines.8,9 Alterations in the balance between cystatin C and cysteine proteinases have been associated with malignant tumour progression.19 The previous clinical study has shown that serum cystatin C concentration was significantly higher in the hepatocellular carcinoma patients than in the control or chronic hepatitis patients. In the present study; serum cystatin C concentrations in the patients with malignant extrahepatic cholestasis were remarkably higher than those in the control group and benign extrahepatic cholestasis group, whereas serum cystatin C concentrations in the intrahepatic cholestasis patients were significantly higher than in the control and other extrahepatic cholestatic patients. Serum levels of the ALP were detected significantly higher in extrahepatic malignant cholestasis group than intrahepatic cholestasis group. In intrahepatic cholestasis group, serum ALT levels were higher than in patients with extrahepatic cholestasis groups and control. However, we did not observe positive correlation between cystatin C concentrations and serum ALT levels in cholestatic patients.
ConclusionOur results suggested that serum cystatin C level may be a potential biochemical marker both to point out an intrahepatic origin by excluding an extrahepatic source of cholestasis in patients with jaundice and to differentiate bening and malign extrahepatic cholestatic. Although our study size is very small, the results may be useful for planning larger studies and it can be useful as an hypothesis-generating paper for future studies. But further studies with larger number of patients are needed to make more comments.