Mammalian cells release several membrane-enclosed vesicles called extracellular vesicles. Those vesicles can contain several molecules such as proteins, DNA and various RNA. Therefore, extracellular vesicles can act as a target delivery system and exert multiple biological effects. Several works demonstrated that extracellular vesicles are increased or dysregulated in patients with cirrhosis, and they can be predictive of disease progression, complications and mortality. This review aims to summarize and highlight the role of extracellular vesicles in the cirrhotic patient and how they correlate with the degree of disease and with complications, particularly with the development of portal thrombosis and hepatocellular carcinoma.
Mammalian cells release several membrane-enclosed vesicles called extracellular vesicles, which comprise exosomes, amphisomes, microvesicles (MVs) and apoptotic bodies [1,2]. Exosomes are vesicles produced from the endosomal compartment arising from intraluminal vesicles and measure 30–150 nm in diameter. The fusion of multivesicular bodies with the cell membrane induces exosome release [3]. Amphisomes are hybrid organelles generated by the fusion of endosomes with autophagosomes [4]. MVs are usually 150–1000 nm in diameter and are released by budding directly from the plasma membrane [5]. Apoptotic bodies are generated during cell apoptosis and are bigger, with a diameter of 500 nm to 2000 nm [6]. MVs can contain several molecules such as proteins, deoxyribonucleic acid (DNA) and various ribonucleic acid (RNA), including microRNA (miRNA), a small non-coding nucleic acid of 18–24 nucleotides involved in epigenetic regulation of the gene expression [7]. Extracellular vesicles can act as a target delivery system [8]. Not packed into exosomes miRNAs (non-exosomal miRNAs) can be detectable in the biological fluids in protein and lipoprotein complexes, which prevents their degradation.
Several works demonstrated that several extracellular vesicles (EVs), in particular MVs are increased or dysregulated in patients with cirrhosis, and they can be predictive of disease progression, complications and mortality [9-14].
Search strategyWe performed a systematic search of studies on microvesicles or microparticles, liver disease and cirrhosis. PubMed, EMBASE and COCHRANE were the online databases employed to search for studies. We developed a search strategy and adjusted it for each engine using different keywords: liver, cirrhosis, hepatocarcinoma, portal vein thrombosis AND (microvesicles OR microparticles).
MVs release in chronic liver diseasesMVs originating from normal cells in physiological states may exhibit substantial qualitative and quantitative changes in disease conditions (such as inflammatory or malignant conditions). Those alterations can potentially trigger or intensify inflammatory responses and fibrotic injuries [2]. Additionally, EVs may induce cells to develop a tumorigenic phenotype [2]. The mechanisms involved in the release of lipotoxic hepatocyte-derived EVs have been studied. For instance, EVs carrying the chemokine C-X-C motif chemokine ligand 10 (CXCL10) are released from lipotoxic hepatocytes through a mechanism mediated by mixed lineage kinase 3 (MLK3). Those EVs function as chemoattractants for macrophages [15].
In another study, lipotoxic hepatocyte-derived EVs were found to induce migration and tube formation in an endothelial cell line in vitro, as well as promote angiogenesis in mice. This effect is mediated by vanin-1, a surface cargo protein on these EVs [16].
Similarly, the plasma of mice with diet-induced non-alcoholic steatohepatitis (NASH) and patients with NASH contained elevated levels of hepatocyte-derived exosomes carrying mitochondrial DNA. These exosomes activated toll-like receptor (TLR) 9 signaling on myeloid cells, leading to a downstream proinflammatory response [17].
Moreover, lipotoxic endoplasmic reticulum (ER) stress in hepatocytes directly enriched EVs with ceramide, which, upon conversion to sphingosine 1-phosphate (S1P), acted as a chemoattractant for proinflammatory macrophages expressing S1P receptors [18].
EVs are also implicated in cross-talk with hepatic stellate cells (HSCs) and in mediating fibrosis progression in NASH. EVs isolated from the visceral adipose tissue of obese patients, compared to those from lean patients, caused an increase in the expression of profibrotic genes such as tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) and integrin ανβ5, and a decrease in matrix metalloproteinase (MMP) 7 and plasminogen activator inhibitor-1 (PAI-1) in a hepatocyte cell line [19]. Moreover, EVs derived from lipotoxic hepatocytes are enriched with the adhesion molecule integrin β1. This enrichment enhances the adhesion of proinflammatory monocytes to their cognate ligand, vascular cell adhesion molecule 1 (VCAM1), on liver sinusoidal endothelial cells (LSEC), promoting their homing to the liver in NASH [20].
MVs and liver fibrosisThe activation of HSCs is crucial for the initiation and progression of fibrogenesis [21]. Activated HSCs release pro-fibrotic EVs that can induce the activation of other HSCs in a paracrine manner. HSCs stimulated with platelet-derived growth factor (PDGF) release fibrogenic EVs enriched with PDGF receptor α (PDGFRα) [22]. Mechanistically, when PDGF binds to PDGFRα, it promotes the phosphorylation of tyrosine 720, which recruits Src homology region 2-containing protein tyrosine phosphatase-2 (SHP2) [22]. SHP2 subsequently inhibits the degradation of PDGFRα and promotes its enrichment in EVs. In patients with cirrhosis, PDGFRα is enriched in circulating EVs compared to healthy individuals [22].
Furthermore, the release of fibrogenic EVs is increased by the activation of the mammalian target of rapamycin (mTOR) signaling and the inhibition of multivesicular body autophagic degradation [23]. As a result, the fibrogenic EVs downstream of mTOR and SHP2 induce hepatic stellate cell migration in vitro and liver fibrosis in vivo [22,23].
Another group reported that EVs released from activated HSCs contain lower levels of Twist family basic helix-loop-helix transcription factor 1 (TWIST1) and miR-214 compared to EVs from nonactivated HSCs [24]. Acting in a paracrine manner, these EVs promote the expression of cellular communication network factor 2 (CCN2) in recipient HSCs, thereby inducing their activation [24]. It would indeed be intriguing to validate the role of the TWIST1 inhibitor, harmine, on the release of pro-fibrotic EVs and its potential impact on liver fibrosis [25].
Lipotoxic EVs derived from stressed hepatocytes carry miR-128–3p and can be internalized by HSCs [16]. Subsequently, miR-128–3p downregulates PPAR-γ expression, triggering HSC activation and contributing to liver fibrosis. Furthermore, in mouse models of liver injury, elevated levels of EV-associated miR-128–3p are correlated with the presence of fibrosis [16].
EVs derived from injured hepatocytes are additionally enriched with miR-192, which induces fibrogenic signaling in HSCs [26]. Furthermore, endothelial cells release EVs that are enriched with sphingosine kinase 1, promoting HSC migration. These EVs are endocytosed through a dynamin-dependent mechanism and induce phosphorylation of protein kinase B (AKT) in recipient HSCs, subsequently promoting cell migration [27].
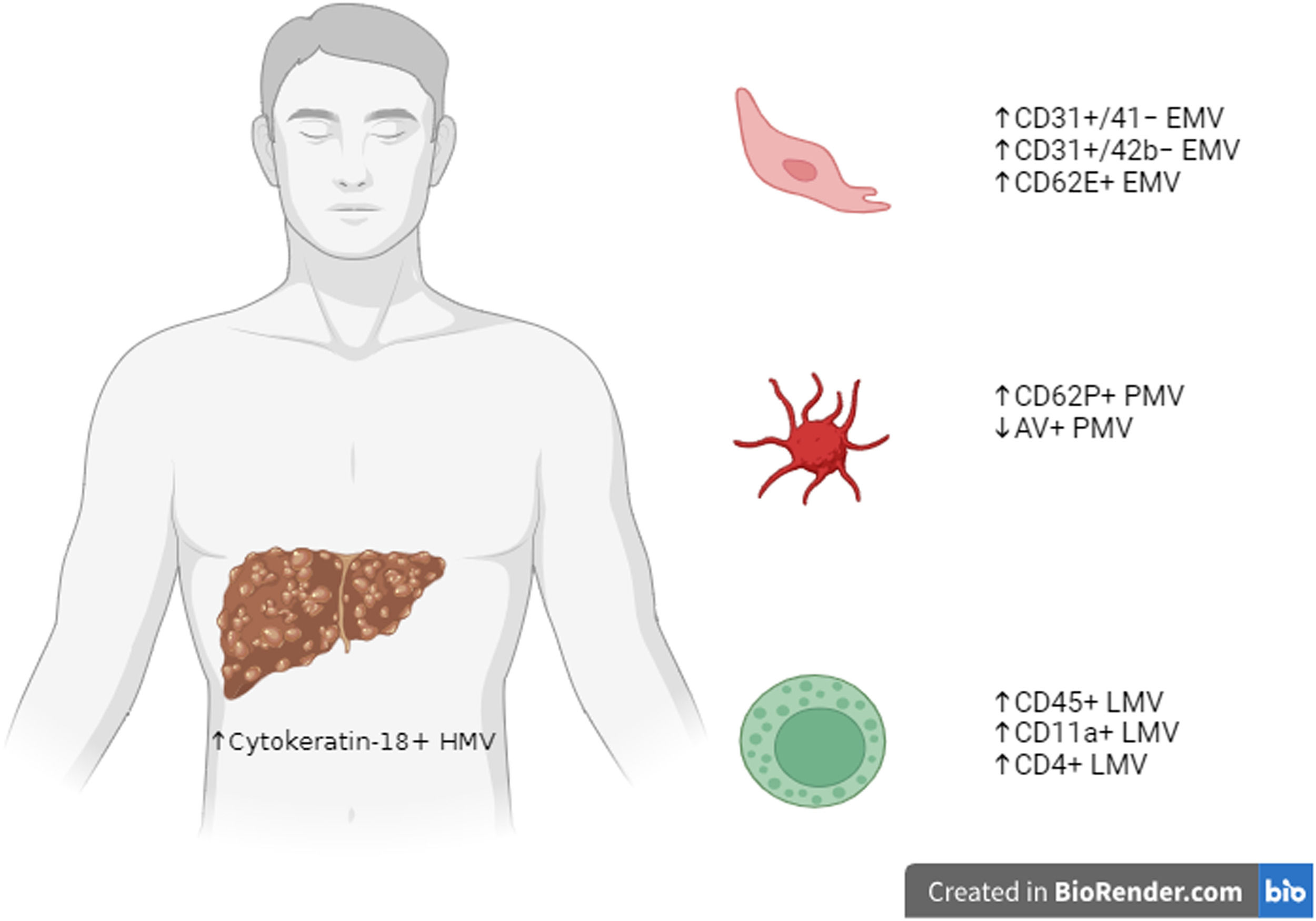
MVs in cirrhotic patientsHepatocyte-derived MVs are characterized by cytokeratin-18 expression, and they are significantly increased in connection with the degree of severity of liver function measured for instance, through the Child-Pugh score [9,10] (Fig. 1).
Summary figure on the main subtypes of MVs studied in cirrhotic patients, divided according to their origin from HMV, PMV, LMV or endothelial cells EMV. Created in BioRender.com. microvesicles, MVs; hepatocytes-derived MV, HMV; platelet-derived MV, PMV; leukocyte-derived MV, LMV; endothelial cell-derived MV, EMV.
Hepatocyte-derived MV levels are also related to the model for end-stage liver disease (MELD) score and its components [10]. Levels of hepatocyte extracellular vesicles directly correlate also with hepatic necro-inflammatory activity, thus suggesting that hepatocyte injury could be the driver of extracellular vesicle release [10].
Moreover, chronic liver disease can induce hemodinamic changes including systemic vasodilation, increased cardiac output and increased intrahepatic vascular resistance. Indeed, cirrhotic patients are characterized by higher levels of endothelial-derived MVs (CD31+/41−, CD31+/42b− or CD62e) than healthy subjects. Some data demonstrated a correlation between cirrhosis severity (Child-Pugh or MELD score) and plasma levels of larger leuko-endothelial (CD31+/41−) extracellular vesicles [9], but this finding was subsequently not confirmed by other works by using the same markers (CD31+/41−) [10], and no link was observed with different markers (CD31+/42b−) [13].
Different subtypes of leukocyte-derived extracellular vesicles (CD45+, CD11a+ and CD4+) have been evaluated and found to be more abundant than in healthy individuals [9,10,14]. Otherwise, a direct correlation with cirrhosis severity was not observed.
There are controversial data on platelet-derived MVs (PMVs) in cirrhosis. Some studies demonstrated a significant increase in platelet-derived MVs in patients with cirrhosis, compared with healthy subjects, by using CD62P+ marker [14,28,29]. A recent study by Weil et al. showed that PMVs were 2.5-fold higher in healthy volunteers compared with cirrhotic patients [30]. Moreover, circulating annexin V (AV)+ PMV levels were lower in cirrhotic patients and inversely correlated with MELD scores [30]. Other studies found no significant difference using the CD41+ marker [9,10,31,32], and CD41+ extracellular vesicle levels did not change with cirrhosis severity [9,10,31]. Those different findings may be due to different experimental settings, as well as several factors influencing PMV release, such as acute infections as demonstrated by a recent study by Campello et al. [33].
MVs & cirrhosis complicationsIn a work by Payance et al., hepatocyte cytokeratin-18+ extracellular vesicles were weakly related to hepatic venous pressure gradient (HVPG), but they could not discriminate patients with an HVPG ≥ 10 mmHg [10]. Clear data about the role of MV subpopulation in reliably estimating portal hypertension are still lacking. Some data demonstrated the ability of MVs to predict the future occurrence of cirrhosis complications such as portal vein thrombosis, ascites, hepatic encephalopathy, hepato-pulmonary, and hepato-renal syndromes [9,13,14,34]. Plasma levels of hepatocyte-derived MVs were shown to predict 6-month mortality, independently of Child-Pugh and MELD scores [9]. Other subpopulations of plasma vesicles, such as pan-leukocyte (CD11+) and endothelial (CD144+, CD62E+), were instead found not to be useful in predicting mortality [9]. However, leuko-endothelial (CD31+/41−) extracellular vesicles have been shown to predict 6-month mortality independently of Child-Pugh score in other works [9,10].
A recent study evaluated MVs in ascites in 163 patients with cirrhosis [11]. Low total extracellular vesicle levels in ascites predicted 30-day mortality independently of MELD score and antibiotic treatment. High percentages of neutrophil-derived (CD66b+) or lymphocyte-derived (CD3) extracellular vesicles in ascites were more commonly observed in patients with poor outcomes [11].
A recent work by Campello et al. demonstrated that patients with cirrhosis and infection had significantly higher tissue factor (TF)+ EVs, P-Selectin+ EVs (activated platelet-derived), CD14+ EVs (monocyte/macrophages derived) and CD14+ TF+ EVs versus those with cirrhosis without infection [33]. Otherwise, no difference was found in total EVs [33]. After infection resolution, levels of those EVs returned to those without infection [33]. Patients with infections were characterized by a significant relation to decreased P-Selectin+ EVs and bleeding complications (HR 8.0 [95 %CI 1.3–48.1]), whereas high levels of leukocyte-derived EVs (CD45+) and CD14+ EVs were significantly correlated with thrombotic complications (HR 16.4 [95 %CI 1.7–160] and 10.9 [95 %CI 1.13–106], respectively) [33].A very intriguing concept is to detect specific MV changes or subtypes to facilitate early hepatocellular carcinoma (HCC) diagnosis in patients with chronic liver diseases. Levels of all MVs, as well as some subpopulations, were found to be increased in the plasma and sera of HCC patients, and their levels normalize after liver transplantation or hepatectomy, thus arguing a direct link with cancer development [12,35]. In a work by Julich-Haertel et al., AV+ EpCAM+ CD147+ tumor-associated MVs were elevated in HCC and cholangiocarcinoma (CCA) [36]. Evaluation of AV+ epithelial cell adhesion molecule (EpCAM)+ asialoglycoprotein receptor (ASGPR)1+ CD133+ tumor-associated MVs allowed the distinction of liver malignancies (HCC or CCA) and cirrhosis from tumor-free individuals (both patients with cirrhosis and no liver neoplasia both control subjects) and from patients carrying other non-liver cancers [36]. Furthermore, AV+ EpCAM+ ASGPR1+ tumor-associated MVs were increased in liver cancer-bearing patients compared to patients with cirrhosis that lacked any detectable liver malignancy [36]. Receiver operating characteristic (ROC) analysis demonstrated a good diagnostic accuracy (>78 %) for AV+ EpCAM+ ASGPR1+ cancer-associated MVs towards HCC diagnosis [36].
In the work of Wang et al. [12], blood MVs were significantly higher in HCC patients in comparison with subjects with liver cirrhosis; MVs levels were related to the HCC tumor size, pathological classification and TNM stage [12]. Whereas, they did not correlate with liver enzymes, alpha-fetoprotein (AFP) levels, alcohol drinking or smoking habits [12]. ROC analysis showed better performance of MVs than of AFP for early HCC detection [12].
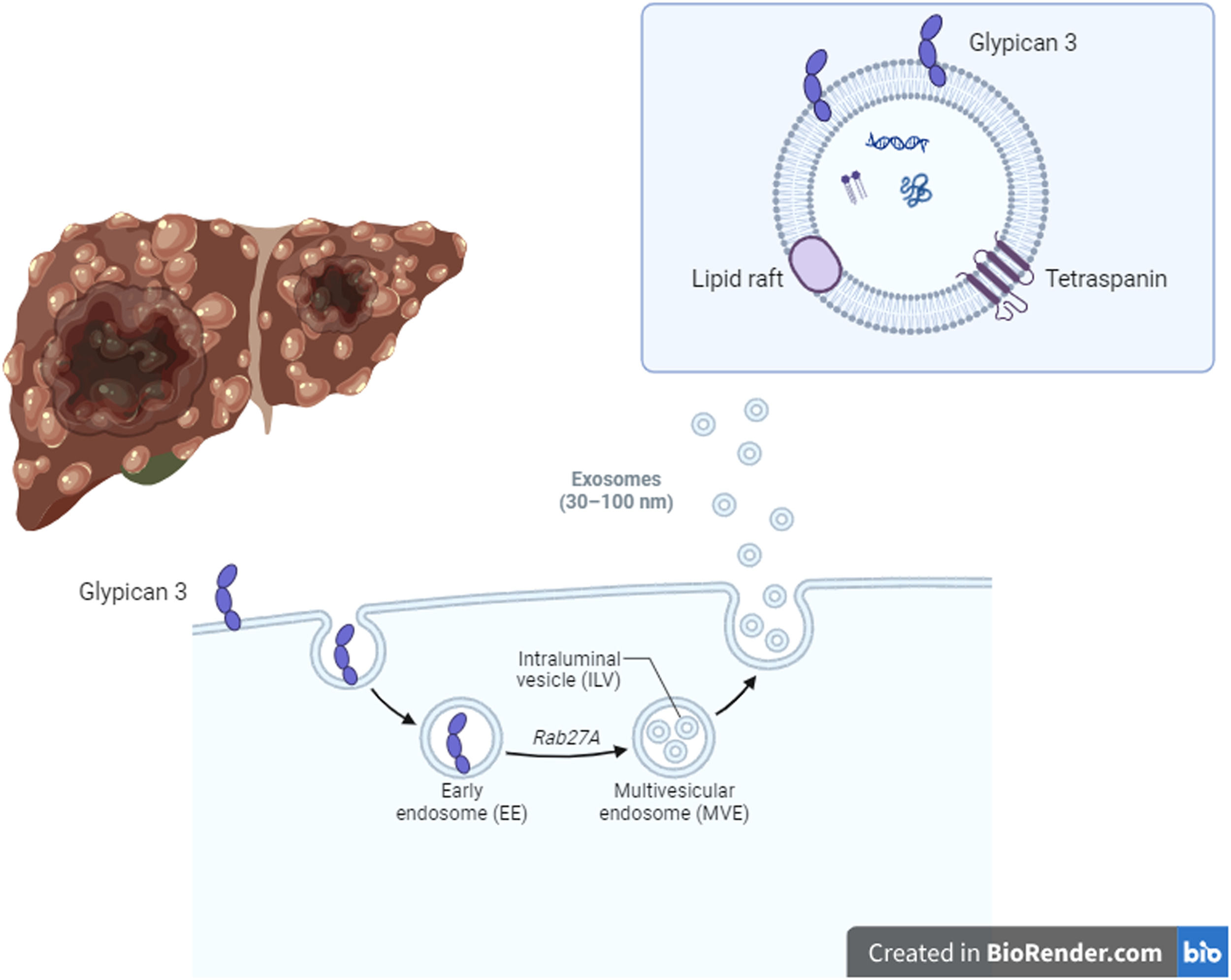
HCC development in liver cirrhosis is associated with impaired autophagy leading to increased production of HCC-specific biomarker glypican-3 (GPC3) [37] (Fig. 2). GPC3 is preferentially released through exosomes [37]. A recent work by Koksal et al. confirmed that the rate of GPC3-positive exosomes was higher in patients with HCC (12.4 %) compared to exosomes isolated from non-cirrhotic and healthy controls (3.7 % and 1.3 %, respectively, p < 0.001) [37].
Hepatocellular carcinoma (HCC) development in liver cirrhosis is associated with impaired autophagy, thus inducing an increase of an HCC-specific biomarker called glypican-3 (GPC3). GPC3 is preferentially released through exosomes. The rate of GPC3-positive exosomes was higher in patients with HCC compared to exosomes isolated from non-cirrhotic and healthy controls. Therefore, GPC3+ exosomes could be promising biomarkers of HCC development. Created in BioRender.com.
Levels of all MVs decrease/normalize after liver transplantation or hepatectomy, thus arguing a direct link with cancer development and presence [12,35]. MV levels, in particular AV+ EpCAM+ ASGPR1+ tumor-associated MVs, decreased at 7 days after curative R0 tumor resection suggesting close correlations with tumour presence [36]. Low levels of AV+ PMVs were related to significantly lower transplant-free survival in cirrhotic patients independently of MELD score and platelet counts [30]. Indeed, patients with higher AV+ PMV levels displayed a relevant 5- to 8-fold higher risk of 6-month death or liver transplant on multivariate analyses adjusted for age, ascites, esophageal varices, encephalopathy, clinical decompensation, total platelet counts, MELD score, and/or Child-Pugh C stage [30].
Role of miRNA in extracellular vesiclesSeveral non-coding RNAs are contained in MVs, and their expression is modified in patients with HCC. A recent meta-analysis showed that a pooled analysis of 30 small extracellular vesicle miRNA reached a sensitivity of 80 % and a specificity of 81 % for HCC diagnosis [38]. In particular, miRNA-21 is overexpressed in circulating MVs of patients with HCC compared with subjects with chronic hepatitis B virus infection without cirrhosis and healthy controls [39,40]. Several studies found serum miRNA-21+ MVs to be independently associated with overall survival and disease progression in patients with HCC [41,42]. However, it is unclear whether HCC or cirrhosis alone was responsible for the higher expression of miRNA-21.
Moreover, miRNA-122 expression in circulating serum MVs was demonstrated to be differently expressed in early HCC and cirrhosis without HCC [43,44]. A study by Suehiro et al. demonstrated that miRNA-122 expression decreased after transarterial chemoembolization [45]. The evaluation of two different serum long non-coding RNAs (lncRNAs) named ENSG00000258332.1 and LINC00635 in MVs discriminated patients with HCC from patients with chronic hepatitis B [46]. The combination of the 2 RNAs and AFP reached a sensitivity of 84 % and specificity of 88 %, better than AFP alone [46].
The levels of some non-exosomal miRNAs (let-7a, miRNA-21–5p, −22–3p, −103a, −122–5p, −221–3p and 222–3p) were strongly associated with liver cancer in plasma when normalized to non-exosomal miRNA-16–5p [47]. Furthermore, levels of exosomal miRNAs showed a stronger association with primary liver cancer than non-exosomal miRNAs when they were measured in saliva [47].
High pre-operative levels of miRNA155+ MVs were predictive of shorter relapse-free survival in patients with HCC treated with first-line curative hepatectomy, although no differences were demonstrated in 3- and 5-year overall survival [48]. In a work by Liu et al., low serum miRNA-125+ MV levels in patients with HCC treated with surgical resection were related to a high risk of recurrence [49]. RNA expression in circulating MVs can also be predictive of overall survival. Higher levels of RAB11A RNA in small Mvs were related to lower relapse-free survival among 60 patients with early HCC [50].
ConclusionsIn conclusion, several subpopulations of EVs seem to be promising biomarkers to ascertain cirrhosis severity or to predict the risk of mortality in such condition. Although there are findings regarding the risk of portal thrombosis and portal hypertension, clear data about the prediction of more specific complications of cirrhosis are still lacking. The evaluation of specific molecules contained in the vesicles such as RNAs could be predictors of outcome in patients with liver disease and of HCC risk. Currently, the high costs for their dosage, the need for stains and specific materials, and the experience of the operators are relevant limits to their use in clinical practice. The disagreement between different scientific evidence represents a further relevant concern for the application of the method in clinical practice. Future studies to be conducted in larger patient populations and with dedicated study designs may clarify the possible predictive role of EVs, especially because of a clinical application in daily practice.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsAB: conceptualization, writing−review & editing; FP: writing−review & editing, supervision.