In this review, a summary is presented of the main reports regarding the potential ocular manifestations of the new coronavirus disease (COVID-19). Scientific evidence is based on letters to the editor, clinical cases and case series, cross-sectional, and a few longitudinal studies. To date, it includes viral conjunctivitis, immune conjunctivitis, and oculomotor palsies (OCP) due to the novel coronavirus. Retinopathy is discussed, but no cases have been published yet. A viral conjunctivitis outbreak can be isolated or associated with the systemic picture, mainly pulmonary, before or after the onset of respiratory symptoms. It can be both unilateral and bilateral, follicles are typical, and duration is variable between 5 and 21 days. Immune-mediated conjunctivitis consists of eye redness, together with erythroderma and fever. It appears more frequently in children, and has been associated with a “Kawasaki-like” disease and toxic shock syndrome. OCP can present on its own, or as part of Miller-Fisher syndrome, along with ataxia, and hyporeflexia. Ophthalmologists have a considerable risk of developing COVID-19 due to close contact with the patient, exposure to tears and eye secretions, and the use of various pieces of equipment and devices susceptible to contamination.
En esta revisión resumimos las principales publicaciones que informan sobre las potenciales manifestaciones oculares de la enfermedad por el nuevo coronavirus (COVID-19). La evidencia científica se basa en cartas al editor, casos clínicos aislados y series de casos, principalmente de corte transversal. Hasta la fecha, incluimos la conjuntivitis viral, una conjuntivitis inmunomediada y parálisis oculomotoras (POM). Se discute la posibilidad de retinopatía. La conjuntivitis viral puede aparecer aislada o asociada al cuadro sistémico, principalmente pulmonar, antes o después del inicio de los síntomas respiratorios. Puede ser tanto unilateral como bilateral, es típica la presencia de folículos, y presenta una duración variable entre 5 y 20 días. La conjuntivitis inmunomediada consiste en un enrojecimiento ocular acompañada de eritrodermia y febrícula. Aparece más frecuentemente en los niños y se ha asociado a un cuadro «Kawasaki-like» y síndrome del shock tóxico. Las POM pueden presentarse de forma aislada, o formando parte de un síndrome de Miller-Fisher, junto con ataxia e hiporreflexia. Los oftalmólogos presentamos un riesgo considerable de contraer la COVID-19 debido a un contacto estrecho con el paciente, exposición a las lágrimas y a las secreciones oculares y al uso de multitud de equipos y aparatos susceptibles de contaminarse.
In December 2019, the first patients diagnosed with the disease caused by the new coronavirus (SARS-CoV-2), called COVID-19, were identified in Wuhan, China.1,2 Dr. Li Wenliang, a Chinese ophthalmologist, was one of the first physicians to warn about the effects of the virus and was suppressed and investigated by his government. Since then, multiple infections have been reported, leading to the declaration of a pandemic disease by the World Health Organization on March 11, 2020.3 At the date of writing, there are more than 14 million infected and more than 600,000 deaths worldwide.4 The main route of transmission is through the respiratory system. It can also be transmitted by fomites through human contact via hands and contaminated surfaces.5 The main clinical manifestation of the disease is pneumonia,4,5 with various described conditions such as gastroenteritis,6 disseminated intravascular coagulation (DIC),7 liver damage, renal and splenic infarction,7–9 acute respiratory distress syndrome4,5 and neurological conditions such as anosmia, ageusia, polyneuropathy, myopathy and stroke.10 This review collects the scientific evidence existing so far to summarize the ophthalmological manifestations produced by the virus and its ability to be transmitted through the eye. We prefer to use the Anglo-Saxon acronyms “COVID-19” and “SARS-CoV-2”, given the extensive diffusion of these terms in the informative literature.
MethodologyA search was carried out in Pubmed until July 21, 2020 in which the following publications were included in English: letters to the editor, clinical cases, bibliographic reviews and clinical studies. The search field included publication abstract and title. Different key words were combined, such as “SARS-Cov-2”, “COVID 19”, “2019-nCoV”, “coronavirus 2019”, and (term “AND” in the advanced search process) “ophthalmology”, “eye disease”, “conjunctivitis”, “ocular surface”, “glaucoma”, “orbit”, “tears”, “uveitis”, “retina”, “vasculitis”, “ophthalmoparesis”, “palsy”, “optic nerve”, “anterior ischemic optic neuropathy” (AION), “retinal venous occlusion” (RVO), “retinal artery occlusion” (RAO).
ResultsA total of 97 publications were obtained. Most were letters to the editor, isolated clinical cases, series of clinic cases, opinion articles and general recommendation guidelines. Two meta-analyses of conjunctivitis case series were found. Due to the reiteration of the content among many articles, 45 were selected for discussion in the present review.
DiscussionThe only ophthalmological complication confirmed by SARS-CoV-2, published to date, has been conjunctivitis. Case series of oculomotor palsy (OMP) and possible retinopathy have been described.
Multiple comparisons have emerged with other coronaviruses, mainly SARS-CoV-1, which produces severe acute respiratory syndrome (SARS), and with the Middle East Respiratory Syndrome Virus (MERS-CoV). Both have been found in lacrimal secretions, conjunctival and corneal cells,11 but conjunctivitis is not described in humans.12,13 Only some conjunctival congestion without excessive redness and slight increase in tear secretion, lasting a few days, has been described in isolated cases.14 The genome of SARS-CoV-2 is 75%–80% similar to that of SARS-CoV-1 and 40% similar to that of MERS,15 so the same type of eye infection would be expected. In fact, the pathogenic capacity to produce conjunctivitis by other coronaviruses is much higher, although they have little impact on clinical practice due to their low prevalence and lack of specific treatment. For example, coronavirus NL 63 (HCoV-NL63) was isolated for the first time in an infant with conjunctivitis and bronchiolitis.16 The mechanism of entry of coronaviruses into cells is via the angiotensin 2 converting enzyme (ACE-2) receptor.17 This is not only present in the alveolar epithelium, but also in the conjunctiva and the cornea.18
Unlike its predecessors, SARS-CoV-2 can cause more severe conjunctivitis, with marked cilio-conjunctival hyperemia, superficial spotted keratitis, follicles in the tarsal conjunctiva and even pseudomembranes.7,19–26 Adenopathies have not yet been reported but, being a viral infection, they may possibly be present. The incubation period of the virus ranges from 51,21 to 14 days.19 It can occur in isolation,20 as a prodrome of the respiratory infection26 and even after the beginning of systemic clinic.21 The time of evolution of the ocular clinic is also variable, with remissions described between 521 and 20 days.7,22 There is no specific treatment. Chen et al.21 used rivabirin eye drops 4 times a day for 6 days on a 30-year-old man who had already been diagnosed with COVID-19 14 days earlier. They observed a complete remission of eye symptoms on the fifth day of treatment. Shetty et al.24 comment on the possibility of using topical hydroxychloroquine (0.03%), since it has already been used in dry eye syndrome with no recorded adverse effects. Regarding the possible harmful effect of oral administration of hydroxychloroquine in patients with pneumonia, a letter to the editor was recently published in the American Journal of Ophthalmology25 in which Marmor argues that, given the short period of treatment, it is not necessary to perform ophthalmological screening. We did not find any publication that alluded to the potential ocular complications of the most commonly used drugs for COVID-19 (corticoids, antivirals, azithromycin, hydroxychloroquine, immunoglobulins, monoclonal antibodies, etc.). Such widespread use of steroids in severe patients could increase the risk of ocular hypertension and cataracts, although, as with hydroxychloroquine, this is unlikely given the short period of administration.
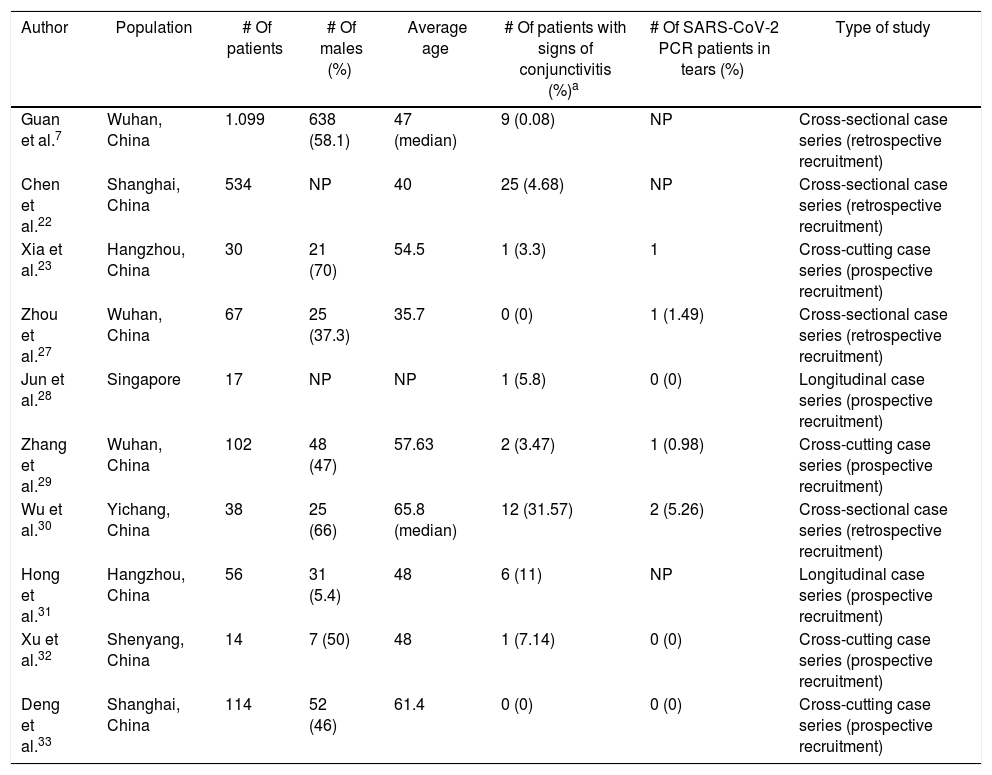
The first reference to conjunctivitis from SARS-CoV-2 was found in a letter to the editor published in The Lancet,26 describing the picture of unilateral eye redness in a senior pulmonologist (dressed in his protective gear and an N95 mask, but without goggles), days after visiting a hospital in Wuhan. He later developed pneumonia. Since then, numerous clinical cases and case series have been published, mostly by Chinese authors7,22,23,27–33 (Table 1), all of them reporting low prevalence of ocular infection. In the longest published series, only 9 (0.8%) of 1099 patients developed ocular congestion.7 In a study of 534 patients, Chen et al.22 observed conjunctivitis in only 25 (4.68%). Xia et al.23 studied a cohort of 30 patients with COVID-19 and found the virus in only one of the eyes of one patient without complications. In a retrospective series of 67 patients, Zhou et al.27 did not record any conjunctivitis. However, one patient presented a positive conjunctival exudate. Likewise, in another study in Singapore with 17 confirmed COVID-19 patients, none were found to have SARS-CoV-2 in the conjunctiva in repeated takes, even though one developed unilateral eye redness.28 Zhang et al.29 studied 102 patients with COVID-19, of whom only 2 had conjunctivitis and, again, only one was found to have the virus on the surface of the eye. Wu et al.30 described up to 31% of ocular complications (12 patients with ocular congestion), in a series of 38 COVID-19 patients. However, they only obtained positive PCR in 2 eye samples. In this study, a positive correlation was found between the severity of the systemic and ocular infection. The authors conclude that conjunctivitis could be a factor of bad prognosis of systemic disease when it appears in intermediate phases. Hong et al.31 studied 56 subjects before and after the development of COVID-19 and found that 6 patients (11%) had ocular redness before the onset of respiratory symptoms. Scores on the OSDI (Ocular Surface Disease Index) and SEEQ (Salisbury Eye Evaluation Questionnaire) tests, which assess both dry eye symptoms, were significantly worse after passing COVID-19, even in patients without conjunctivitis.
Main clinical studies evaluating conjunctivitis in COVID patients.
| Author | Population | # Of patients | # Of males (%) | Average age | # Of patients with signs of conjunctivitis (%)a | # Of SARS-CoV-2 PCR patients in tears (%) | Type of study |
|---|---|---|---|---|---|---|---|
| Guan et al.7 | Wuhan, China | 1.099 | 638 (58.1) | 47 (median) | 9 (0.08) | NP | Cross-sectional case series (retrospective recruitment) |
| Chen et al.22 | Shanghai, China | 534 | NP | 40 | 25 (4.68) | NP | Cross-sectional case series (retrospective recruitment) |
| Xia et al.23 | Hangzhou, China | 30 | 21 (70) | 54.5 | 1 (3.3) | 1 | Cross-cutting case series (prospective recruitment) |
| Zhou et al.27 | Wuhan, China | 67 | 25 (37.3) | 35.7 | 0 (0) | 1 (1.49) | Cross-sectional case series (retrospective recruitment) |
| Jun et al.28 | Singapore | 17 | NP | NP | 1 (5.8) | 0 (0) | Longitudinal case series (prospective recruitment) |
| Zhang et al.29 | Wuhan, China | 102 | 48 (47) | 57.63 | 2 (3.47) | 1 (0.98) | Cross-cutting case series (prospective recruitment) |
| Wu et al.30 | Yichang, China | 38 | 25 (66) | 65.8 (median) | 12 (31.57) | 2 (5.26) | Cross-sectional case series (retrospective recruitment) |
| Hong et al.31 | Hangzhou, China | 56 | 31 (5.4) | 48 | 6 (11) | NP | Longitudinal case series (prospective recruitment) |
| Xu et al.32 | Shenyang, China | 14 | 7 (50) | 48 | 1 (7.14) | 0 (0) | Cross-cutting case series (prospective recruitment) |
| Deng et al.33 | Shanghai, China | 114 | 52 (46) | 61.4 | 0 (0) | 0 (0) | Cross-cutting case series (prospective recruitment) |
NP: not published.
Ulhaq and Soraya34 conducted a meta-analysis in which they included most of the present studies. They concluded that the aggregate prevalence of conjunctivitis in COVID-19 patients was very low [5.5% (42/735 subjects); 95% CI (1.6%–9.4%)], with lacrimal exudate CRP being barely sensitive [0.6%; 95% CI (0.1–5.1)], although very specific [100%; 95% CI (0–100)] compared to nasopharyngeal exudate. Another meta-analysis that included 3 of the above mentioned, concluded that conjunctivitis may be associated with a more severe form of disease.35
Transmission of the virus through the eye surface is feasible since it is an exposed microenvironment connected to the airway through the nasolacrimal duct. In addition, the lymphatic drainage of the ocular mucosa is the same as that of the nostril and nasopharynx.36,37 However, as we have seen, few patients develop conjunctivitis. Viral replication is probably more limited in the conjunctiva due to a greater “turn over” than the cells of the respiratory epithelium.12 Low PCR sensitivity in the tear may be due to several factors: (1) a short window period because the virus quickly “washes” into the nasal cavity; (2) a lower viral load and therefore less ability to obtain a representative sample, unlike the nasopharyngeal exudate; (3) sampling at different times of the day; (4) use of different diagnostic methods with different sensitivities and specificities. Furthermore, there is no unanimous definition of “conjunctivitis”. While some series include patients with minimal eye redness, others report is a clear infectious condition. In any case, there is evident risk of infection for the ophthalmologist due to close contact with patients, exposure to tears and ocular secretions and the use of a large array of equipment and devices susceptible to contamination.
These patients are a focus of infection mainly due to contaminated hands after touching the face or rubbing the eyes, as occurs in adenoviral conjunctivitis, since there is no “expulsive mechanism” such as coughing, sneezing or speech, as occurs in the airway. Even though the virus has been isolated in tear secretions from patients without conjunctivitis,27 the risk of transmission through the eye in these subjects seems negligible since the viral load would be much lower. Even so, in these moments of uncertainty in which we do not fully know the behavior of the infection and being aware of the initial mistakes in the management of the outbreak, it seems advisable to remain cautious and consider the risk, however minimal it may seem. Therefore, in addition to repeatedly recommended safety measures (such as the use of masks, frequent hand washing, slit lamp screens, use of topical single-dose medication, etc.), the vast majority of authors advise the use of protective glasses in clinical practice.24
Children suffer from COVID-19 in milder forms.38 This seems to be mainly due to a more effective immune response. Another possibility is the presence of an increased number of respiratory viruses in the nasopharyngeal mucosa of children which could limit SARS-CoV-2 replication and growth through direct interactions or competition among them38 or decreased expression of ACE-2 in epithelial cells.39 The prevalence of conjunctivitis in infected children is low (1%–5%).37 However, recently the Spanish Society of Pediatrics issued a statement warning about case reports of children with COVID-19 who exhibited high fever, erythrodermia and conjunctival injection.40,41 This picture overlaps with Kawasaki disease, a rare mucocutaneous disease that is relatively unknown to ophthalmologists, consisting of small and medium vessel vasculitis that produces fever, adenopathies, cutaneous and palmoplantar erythema, conjunctivitis, limb edema and, less frequently, coronary aneurysms.41 While most got better in a few days, some (0.5%–5%) evolved unfavorably to toxic shock.37,41 Most of these cases occurred in older children, some already in their teens. Even so, the case of a 6-month-old baby with generalized erythema and conjunctivitis was also described, who recovered completely with intravenous immunoglobulins (IG) in 48 h.42 In this case, far from appearing to be a direct infection by the virus (absence of follicles, adenopathies, ocular secretions), the conjunctivitis seemed clearly immune-mediated. An abdominal condition with diarrhea and vomiting has also been described, with acceptable general condition, which in a few hours evolved into a shock with tachycardia and hypotension.40
Verdoni et al.43 conducted a comparative study in Bergamo (one of the Italian epidemic hotspots) between two groups: (1) 19 children with debut of “Kawasaki-like” disease before the beginning of the SARS-CoV-2 outbreak; (2) 10 children with debut of the disease after the beginning of the outbreak. They observed that the second group had a higher mean age (7.5 years vs. 3 years), most had AC antibodies to the virus (8 out of 10), and exhibited a more severe form of the disease, including more cardiac involvement (6 out of 10 vs. 2 out of 19), toxic shock syndrome (5 out of 10 vs. 0 out of 19), and macrophage activation syndrome (5 out of 10 vs. 0 out of 19). SARS-CoV-2 may involve a strong host stimulus capable of triggering a disproportionate immune response. The predictors of such a response are still unknown.
Retinopathy has been questioned by SARS-CoV-2. The already known picture of DIC and the vascular involvement of different organs such as the lung, kidneys or brain,7,10 could suggest some type of retinal vasculopathy. There is evidence of a greater concentration of ACE-2 in the pigment epithelium of the retina and neurosensorial retina than in the conjunctival and corneal cells.44 Furthermore, in experimental studies with animal models of coronavirus infection, retinal alterations such as vasculitis and degenerations have been observed.45 Recently, a series of 12 cases of COVID has been published in The Lancet (9 confirmed by nasopharyngeal PCR, 2 by serology; 6 males, 6 females; age: 25–69 years) with retinal lesions, detected incidentally in a systematic ophthalmological study on infected patients.46 All exhibited fever, asthenia and dyspnea, and 11 of the 12 patients presented anosmia. None had ophthalmological clinic. In the 12 patients, optical coherence tomography (OCT) showed hyperreflective foci in the ganglion cell layer and internal plexiform in both eyes, mainly at the level of the papillomacular bundle. Angio-OCT and quantitative analysis of the thickness of the ganglion cell layer were normal. Four patients presented cotton-like exudates and microhemorrhages in the temporal vascular arches. Only one case report has been published relating central retinal artery occlusion (CRAO) to COVID-1947 in a 60-year-old man with high blood pressure, dyslipidemia, stable coronary disease and chronic obstructive pulmonary disease. On the twelfth day of hospital admission for COVID-19 (confirmed by nasopharyngeal PCR), said patient presented sudden and painless decrease in vision in his right eye. The authors relate the patient's state of hypercoagulability, produced by the inflammation attributable to SARS-CoV-2, with the ophthalmological complication, being consistent with other vascular complications described in the literature such as stroke or pulmonary embolism.7,10 Paradoxically, no published cases of retinal vein occlusion (CRVO) were found in thie review. It is likely that during the coming months we will see some publication on the subject. A recent letter to the editor has also described 2 clinical cases of acute maculopathy with paracentral scotoma in 2 patients confirmed with COVID-1948: a 37-year old woman with normal ophthalmological examination in which a hyper-reflective focal area in the plexiform layers with volume loss of the internal nuclear layer was seen in OCT (acute paracentral maculopathy), and a 32-year old man, also with normal examination, with the following findings in OCT: hyperreflective area in the external plexiform layer with interruption of the band of union of the internal and external segments of the photoreceptors (acute macular neuroretinopathy).
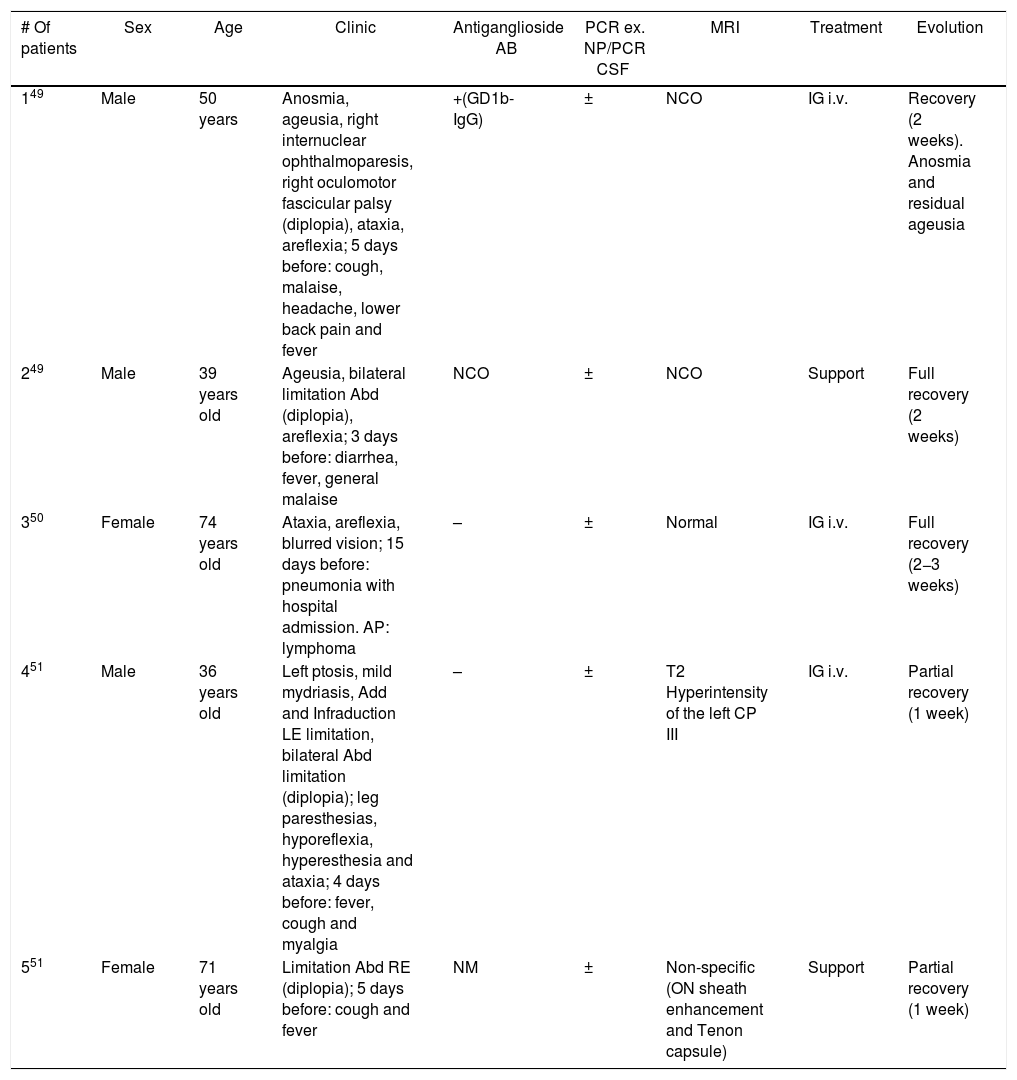
Three articles have been published describing 5 cases of patients with COVID-19 and OMP (Table 2).49–51 The most repeated pattern is limited abduction [paralysis of the VI cranial nerve (CP)], followed by incomplete involvement of the III CP. The distribution by sex was 3 males and 2 females. The age range was variable (36–74 years). In 4 of the 5 patients, the picture was accompanied by ataxia and hyporeflexia, and they were diagnosed with Miller-Fisher syndrome (ophthalmological variant of Guillain-Barré syndrome). This condition is typical after an infection of the upper respiratory and/or gastrointestinal tract (herpes virus and bacteria such as Haemophilus influenzae, Campylobacter jejuni, Chlamydia, Salmonella, and mycoplasma).49 All of them exhibited diplopia and/or blurred vision days after presenting systemic COVID-19 symptoms. The presence of antiganglioside antibodies (ACG), especially anti-GD1b, strongly supports the diagnosis. It was found in one of said 5 patients. The authors question the pathogenic mechanism by which SARS-CoV-2 induces ophthalmoplegia (direct neuropathic vs. aberrant immune reaction). In fact, some neurotropism has been described in several coronaviruses.52 Gutiérrez-Ortiz et al.49 conclude that it is probably due to an autoimmune meta-infectious reaction due to several factors: the absence of virus detection in the cerebrospinal fluid in any patient, the effect of the already known pro-inflammatory “cytochemical storm” of SARS-CoV-2,53 the satisfactory treatment with IG in several patients and the presence of ACG in the first one. The prothrombotic state that generates the infection can also be a causal mechanism, through myroembolisms in the oculomotor nerves. Only one case of facial palsy associated with COVID-19 has been described, although without lagophthalmos or other ocular complications.54
COVID patients with OMP.
| # Of patients | Sex | Age | Clinic | Antiganglioside AB | PCR ex. NP/PCR CSF | MRI | Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|
| 149 | Male | 50 years | Anosmia, ageusia, right internuclear ophthalmoparesis, right oculomotor fascicular palsy (diplopia), ataxia, areflexia; 5 days before: cough, malaise, headache, lower back pain and fever | +(GD1b-IgG) | ± | NCO | IG i.v. | Recovery (2 weeks). Anosmia and residual ageusia |
| 249 | Male | 39 years old | Ageusia, bilateral limitation Abd (diplopia), areflexia; 3 days before: diarrhea, fever, general malaise | NCO | ± | NCO | Support | Full recovery (2 weeks) |
| 350 | Female | 74 years old | Ataxia, areflexia, blurred vision; 15 days before: pneumonia with hospital admission. AP: lymphoma | – | ± | Normal | IG i.v. | Full recovery (2−3 weeks) |
| 451 | Male | 36 years old | Left ptosis, mild mydriasis, Add and Infraduction LE limitation, bilateral Abd limitation (diplopia); leg paresthesias, hyporeflexia, hyperesthesia and ataxia; 4 days before: fever, cough and myalgia | – | ± | T2 Hyperintensity of the left CP III | IG i.v. | Partial recovery (1 week) |
| 551 | Female | 71 years old | Limitation Abd RE (diplopia); 5 days before: cough and fever | NM | ± | Non-specific (ON sheath enhancement and Tenon capsule) | Support | Partial recovery (1 week) |
Abd: abduction; Add: adduction; PH: personal history; ex.: exudate; IG: immunoglobulins; IV: intravenous line; CSF: cerebrospinal fluid; NP: nasopharyngeal; NM: not mentioned; ON: optic nerve; NCO: not carried out; RE: right eye; LE: left eye; CP: cranial pair; PCR: polymerase chain reaction; OMP: oculomotor paralysis; MRI: magnetic resonance imaging.
Expert committees have commented on the possibility of an increased risk of ischemic optic neuropathy due to the prone position during hospital admission and ICU stay of patients with COVID-19. This position improves arterial oxygenation to unventilated dorsal areas of the lung but, due to the redistribution of the flow, it could favor ischemic phenomena. Even so, it seems to be a fairly safe, effective technique with a low rate of complications.55
The main limitation of this review is the quality of referenced articles. As mentioned, most are clinical case series and therefore have low scientific evidence. It is difficult to establish the causality of the virus in all the mentioned ophthalmological manifestations, especially in the presence of prothrombotic or autoimmune phenomena. In addition, the very important alteration of clinical epidemiology could have generated false associations. The exceptional situation we have experienced has led to the publication of works without double review (peer review) to generate information quickly. This also invariably causes confusion. And it is often more difficult to get out of the confusion than to get out of the error.
ConclusionsIt can be concluded that SARS-CoV-2 conjunctivitis is rare in relation to the magnitude and prevalence of other manifestations caused by the virus. It could be related to a more serious form of disease. The ophthalmic transmission route is possible, but not very relevant in comparison with the aereal route and by contact with contaminated hands and surfaces. Pneumonia acquired by direct ophthalmic transmission is unlikely. The main impact of suffering from isolated conjunctivitis is the epidemiological control of the disease, rather than the ocular repercussions. Even so, we endorse the general recommendations of the rest of the authors, advising the use of protective glasses (in addition to protective equipment and mask), given the already evidenced possibility of contracting the infection through the ocular route. Children and adolescents may exhibit immune-mediated conjunctivitis accompanied by erythrodermia and fever, which usually evolves well but in some it may even worsen with toxic shock. The factors that determine this worsening are still to be elucidated. Close monitoring of these patients is therefore essential. The OMP by COVID also seems to be due to an aberrant immune response and generally has a good prognosis. Only a single case report has been described of CRVO attributable to SARS-CoV-2. We did not find any case reports or studies that referred to vascular pathology of the optic nerve. Further and higher quality studies will result in greater understanding of the management and prevention of ocular complications from SARS-CoV-2.
Conflict of interestNo conflict of interests was declared by the authors.
Please cite this article as: Pérez-Bartolomé F, Sánchez-Quirós J. Manifestaciones oftalmológicas del SARS-CoV-2: Revisión de la literatura. Arch Soc Esp Oftalmol. 2021;96:32–40.