To quantify the long-term impact (24 months) on the visual results and activity of neovascular lesions of COVID-19 confinement in patients with nAMD in our population.
MethodsA retrospective observational study of patients with nAMD who attended consultation or were treated during the 3 months before confinement was carried out.
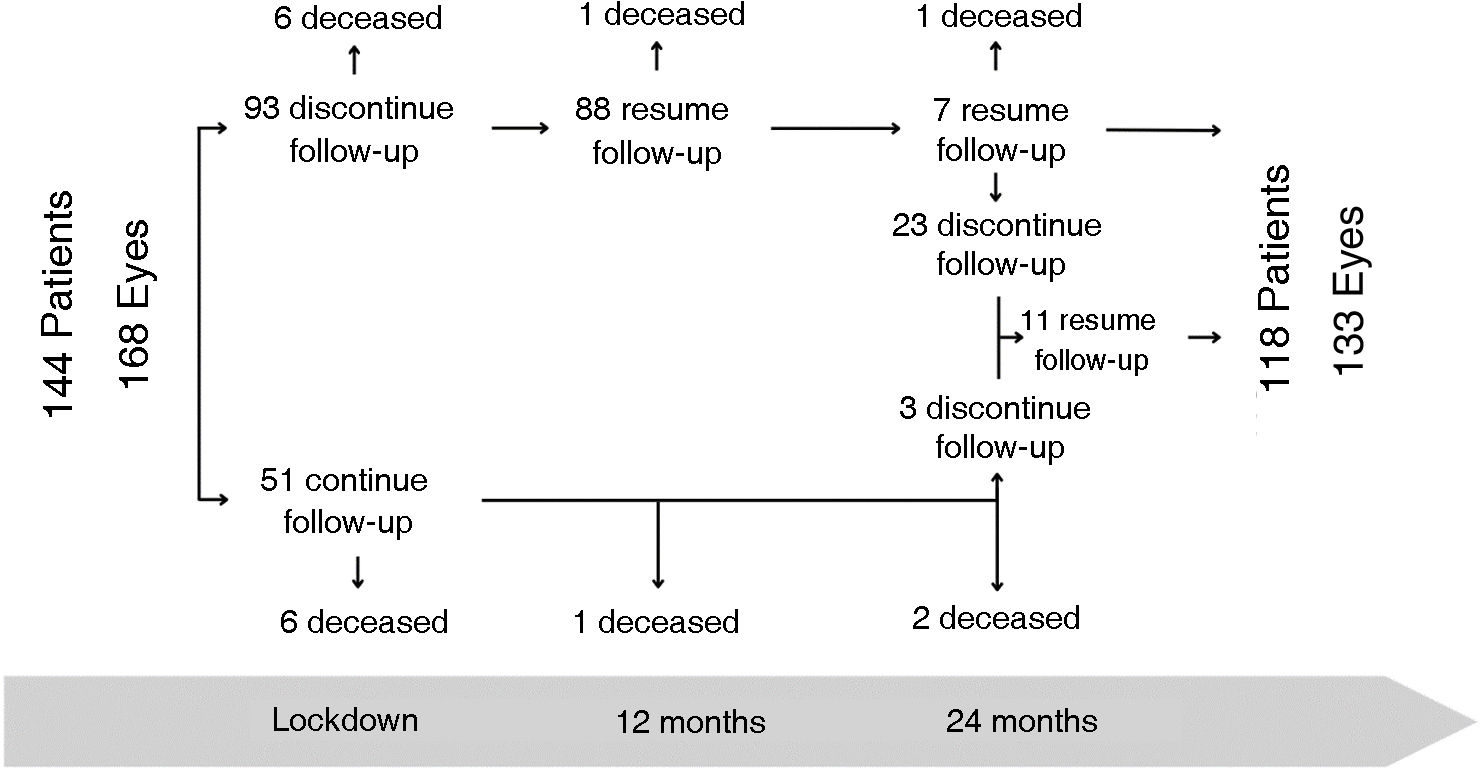
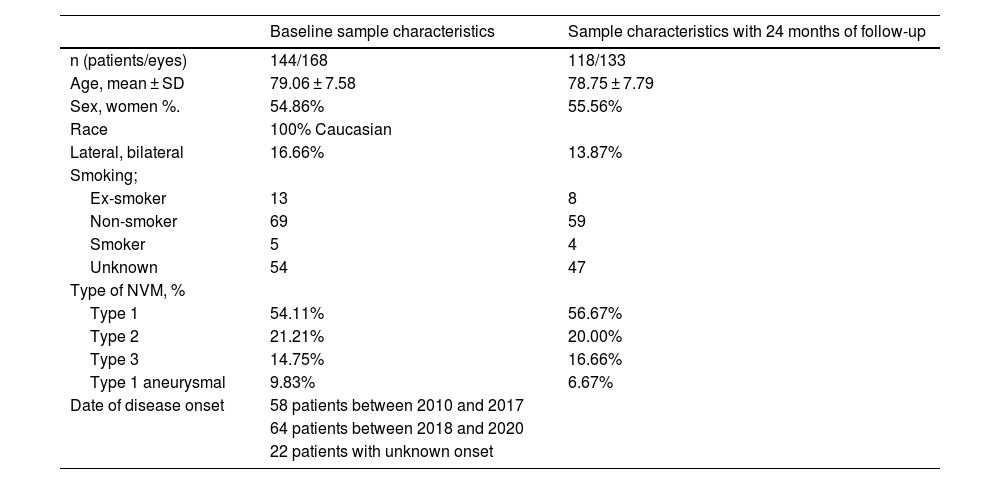
Results144 patients (168 eyes) with nAMD were included, 51 of them (35.42%) came during confinement, and at 24 months the final cohort was 118 patients (133 eyes).
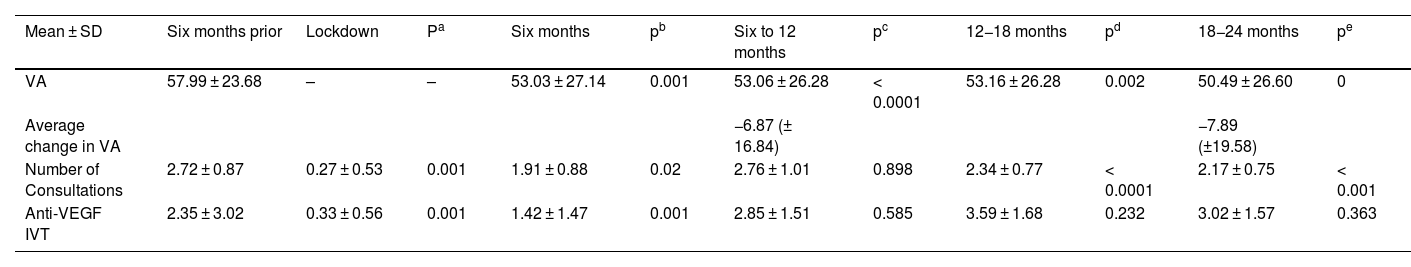
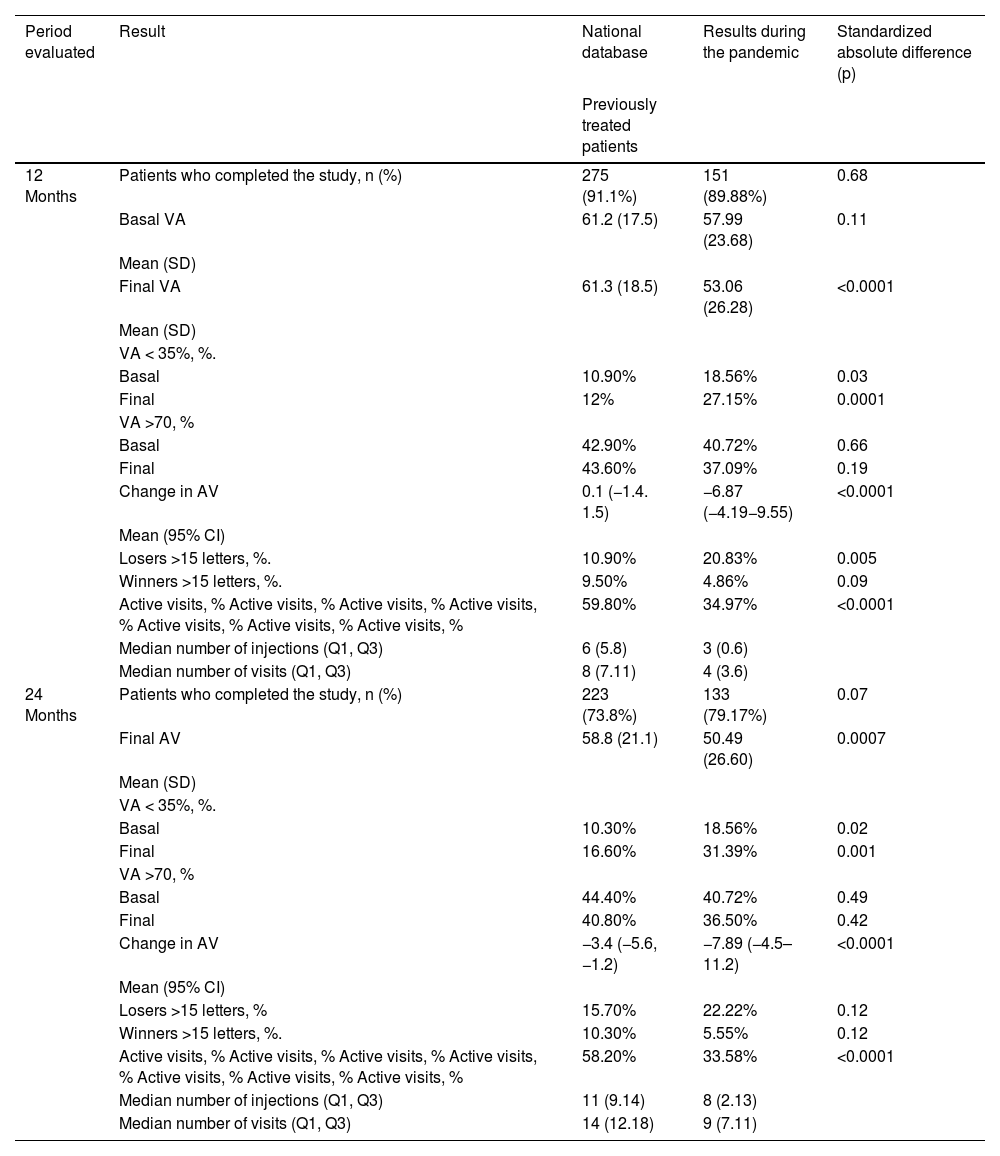
The previous VA of 57.99 ± 23.68 letters decreased, clinically relevant and statistically significant, by an average of 6.87 (±16.84) and 7.89 (±19.58) at 12- and 24-months follow-up. This change differs significantly from the two-year vision change observed in the national database of pretreated patients.
The median number of injections and consultations is lower in our group at 12 months, compared to the pre-pandemic national database, and tends to equalize at 24 months.
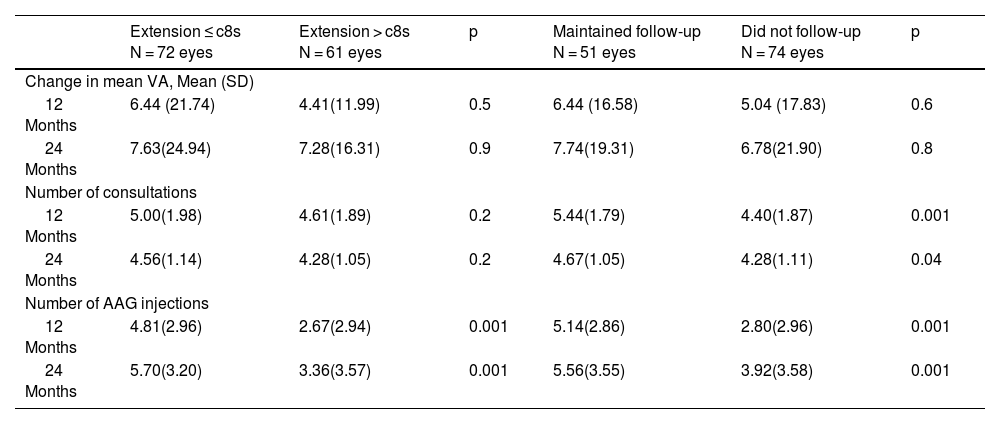
We did not find differences in vision when we compared patients who attended consultations during confinement or in treatment intervals greater than 8 weeks (Tq8w).
ConclusionsThe VA of patients with nAMD decreased significantly after confinement, probably due to the lower number of antiangiogenic injections and consultations during the first year, and did not recover during the second year despite the increase in the number of injections and visits close to those reported before confinement.
El objetivo de este trabajo es cuantificar el impacto a largo plazo (24 meses) en los resultados visuales y la actividad de las lesiones del confinamiento por COVID-19 en pacientes con DMAEn en nuestra población.
MétodosEstudio observacional retrospectivo de pacientes con DMAEn que acudieron a consulta o fueron tratados durante los 3 meses previos al confinamiento.
ResultadosSe incluyeron 144 pacientes (168 ojos) con DMAEn, 51 de ellos (35,42%) acudieron durante el confinamiento, a los 24 meses la cohorte final es de 118 pacientes (133 ojos).
La AV previa de 57,99 ± 23,68 letras, disminuyó una media de 6,87 (±16,84) y 7,89 (±19,58) letras a los 12 y 24 meses de seguimiento (p < 0,001). Este cambio difiere significativamente al cambio de visión a dos años observado en la base de datos nacional de pacientes pretratados.
La mediana de inyecciones y consultas es menor en nuestro grupo a los 12 meses, comparado con la base de datos nacional prepandemia y tiende a igualarse a los 24.
No encontramos diferencias en la visión cuando comparamos los pacientes que acudieron a las consultas durante el confinamiento ni en los intervalos de tratamiento superiores a 8 semanas (c8s).
ConclusionesLa AV de los pacientes con DMAEn disminuyó significativamente tras el confinamiento, probablemente debido al menor número de inyecciones de antiangiogénicos y consultas durante el primer año, y no se recuperó durante el segundo año pese al aumento del número de inyecciones y visitas en cifras cercanas a las reportadas antes del confinamiento.