4 pacientes del grupo A presentaron dolor leve en la zona de punción y 15 personas del grupo B tuvieron cefalea (8 de los casos abandonaron el tratamiento debido a ello). Conclusión. El tratamiento con la NTG no es una clara alternativa a las infiltraciones en las TMR, dado que no es un tratamiento más efectivo y, por el contrario, hay un mayor número de pacientes con efectos adversos que deben abandonar el tratamiento.
Introduction
«Painful shoulder», which can significantly limit the patient's quality of life, is a frequent complaint in primary health care with an estimated yearly incidence of 11.2/1000 patients1. The most frequent cause of this syndrome is rotator cuff tendinitis (RCT), although it can also arise from other disorders such as muscular injury, bursitis, or, less often, from joint problems.
Treatment of this disorder is usually provided by the primary care physician, and the choice of treatment depends on the practitioner's preference, based mainly on acquired knowledge and personal experience. No clearly established criteria for choosing the most suitable treatment are available; despite the many published studies on this topic, there is little scientific evidence to support one option over another2.
The three potential pharmacological treatments consist of transdermal nitroglycerin, infiltration with corticosteroids and local anesthesia, and nonsteroid antiinflammatory drugs. Transdermal nitroglycerin has been shown to be effective as an antiinflammatory and analgesic for different indications such as thrombophlebitis3, dysmenhorrea4 and RCT5,6. Advocates of this approach explain these effects on the basis of the fact that nitroglycerin is transformed into nitric oxide in the vascular smooth muscle, and thus imitates the action of endogenous nitric oxide (produced in the endothelium) on the peripheral nervous system, and on the modulation of the inflammatory process5. On the other hand, infiltration of corticosteroids with local anesthesia has been found more effective than oral nonsteroid antiinflammatory drugs for RCT7,8.
Given the high prevalence of RCT in our setting and the contraindications, the potential adverse effects of some treatments (i.e., oral antiinflammatory drugs) and the invasive nature of other treatments (i.e., local infiltration), we believed it worthwhile to investigate the efficacy of transdermal nitroglycerin for the treatment of pain in RCT. If this treatment were found to be at least as effective as conventional treatments, it might offer advantages (ease of application, low cost, nonaggressive nature) that would make nitroglycerin an attractive alternative.
The aim of this study was to compare the effects of transdermal nitroglycerin and local infiltration of corticosteroids in controlling the pain of RCT in patients who had not responded to oral nonsteroid antiinflammatory drugs.
Material and methods
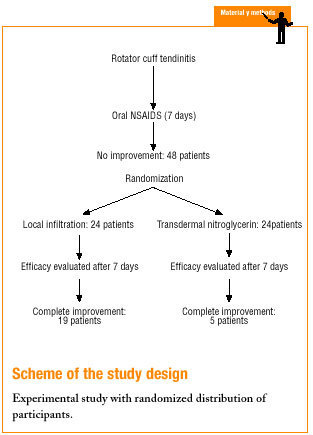
This experimental study, in which patients were assigned randomly to receive one treatment or the other, was done at the primary health care center of a semirural basic health care area in the Garraf region, Barcelona province, Spain. The health care center serves a population of approximately 12000 inhabitants, most of whom have received a moderate to low level of formal schooling. About 60% of the population are of legal working age, and the main types of employment are construction work (men) and cleaning work (women).
The participants were selected during a visit to the health center at which they were seen by members of one of three different primary care teams. The inclusion criteria were: a) clinical diagnosis of RCT, defined as pain on moving the arm through a 60120o arc, positive impingement test and positive Jove (supraspinatus), Gerbe (subscapularis) or Pate (infraspinatus and teres minor) test10; b) symptoms present for less than 6 weeks at the time of diagnosis; and c) no response to previous treatment for one week with oral nonsteroid antiinflammatories. Patients were excluded if they had adhesive capsulitis (limited or painful passive mobility of the shoulder), biceps tendinitis (positive palm up test or Yergason test), allergy or intolerance of any of the drugs used in the study, or if they were receiving treatment with nitroglycerin patches for heart disease.
Sample size was calculated at 24 patients per group according to a comparison of proportions test for a unilateral hypothesis. Alpha error was set at 0.05, and beta error at 0.20. Keeping in mind that the efficacy of infiltration is 7090%, we assumed that a difference of 30% or less would be clinically relevant.
The main variable in the study was pain, measured with a visual analog scale (VAS) at the time of inclusion in the study and after 7 days of treatment. Other variables studied were age, sex and adverse effects of both treatments.
From May 1999 to April 2000 all patients who came to the center and fulfilled the inclusion criteria were assigned to one of two groups (A or B) according to a random number table, if they gave their consent to take part after receiving verbal information about the study. Group A patients received infiltration via a posterior approach (1 cm below the outer edge of the posterior spine of the scapula) of 1 ml triamcinolone acetonide and 1 ml 2% mepivacaine delivered with a 21 G intramuscular needle. Group B patients were given a set of 5-mg nitroglycerin patches to be placed over the area of worse pain (lateral face of the shoulder) for 3 days. Each patient was trained in the proper way to apply the patch daily. During the treatment period no oral analgesics or antiinflammatories were allowed.
After 7 days of treatment we evaluated the dependent variables pain and adverse events. Complete improvement was considered a reduction in pain of more than 5 points on the VAS; partial improvement was considered a reduction of 3-5 points, and treatment failure was recorded when there was no improvement in pain or when the decrease was less than 3 points. Adverse effects were also recorded. Patients whose pain did not show complete improvement were given an appointment 15 days after treatment to receive nitroglycerin or infiltration depending on their initially assigned treatment, and pain and adverse events were again recorded one week later. This procedure was repeated up to 3 times. Scheme illustrates the overall design of the study.
The results were analyzed statistically by comparison of proportions with the SPSS. The results were expressed together with 95% confidence intervals (CI).
Results
A total of 48 patients (33 women, 69%, and 15 men, 31%) were studied. Mean age was 61 years. There were no significant differences between the groups in age or sex.
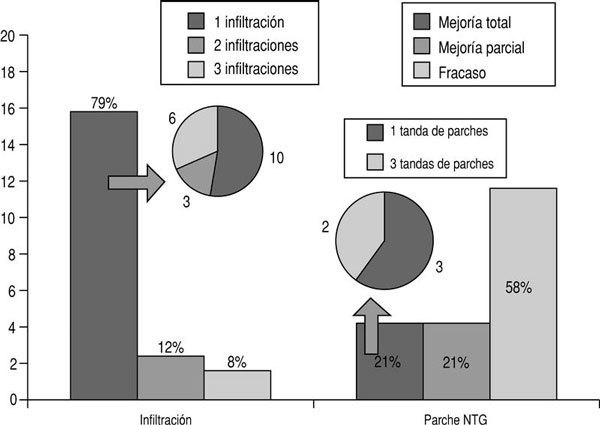
In group A complete improvement was seen in 19 patients (79%), partial improvement in 3 (12%) and treatment failure in 2 (8%). Of the 19 patients with complete improvement, this result was obtained with one infiltration in 10 participants, 2 infiltrations in 3, and 3 infiltrations in 6. In group B complete improvement was seen in 5 patients (21%), partial improvement in 5 (21%) and treatment failure in 14 (58%). Of the 5 patients with complete improvement, this result was obtained with one 3-day set of patches in 3, and with 3 sets of patches in 2 (fig. 1).
Figure 1:Analgesic efficacy of the two treatments.
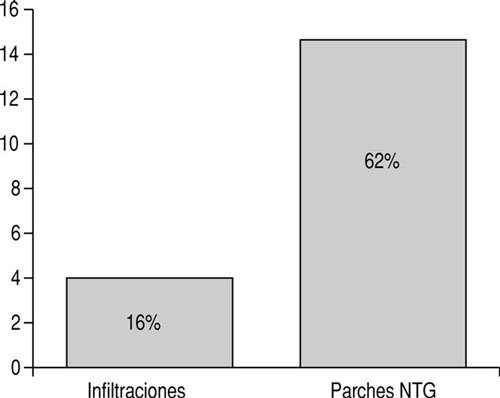
With regard to adverse effects, 4 patients (16%) in group A reported mild pain at the injection site, whereas in group B 15 patients (62%) reported headaches, which led 8 of them to abandon treatment (fig. 2).
Figure 2: Side effects.
When we compared patients who showed complete improvement with the other two outcome groups (partial improvement and treatment failure), we found a difference in the proportion of each treatment group of 0.58 (95% CI: 0.350.81), which was statistically significant. When we compared the proportions of patients with complete and partial improvement, the difference in proportion between the two treatment groups was 0.500 (95% CI: 0.270.72), which was also statistically significant.
Discussion
The conclusions of this study indicate that the analgesic efficacy of corticosteroid injections is greater that that of transdermal nitroglycerin. Moreover, the frequency of adverse effects was higher with the latter treatment. The adverse effects of nitroglycerin were tolerated poorly by our patients, 53% of whom abandoned treatment for this reason. In contrast, the adverse effects of corticosteroid infiltration did not lead to any drop-outs.
A similar study done in 1999 in the Florida Nord basic health care area (L´Hospitalet de Llobregat), in which topical nitroglycerin was used for different locomotor disorders potentially treatable with infiltration, yielded similar results. Of the 31 patients who were treated, 22.6% attained complete improvement, 29% partial improvement, and 48% failed to respond. Moreover, 50% of the patients abandoned treatment because they could not tolerate the adverse effects (mainly headache). These findings are broadly similar to the results of the present study. However, a study by Berrazueta et al. that compared topical nitroglycerin and a placebo for RCT found significant differences in favor of nitroglycerin. It should nonetheless be noted that the statistical power and design of their study were problematic: only 10 patients were included in each group, and the response was evaluated after a very short treatment period (24 and 48 h).
In the present study, randomization bias was significantly reduced by a controlled, random process of assignation; thus our groups were comparable for prognostic factors. However, some limitations should be noted. The variable we used to measure treatment efficacy (decrease or no change in pain) is difficult to measure. It would be worthwhile to obtain information on other parameters such as functional improvement in the joint mobility. In addition, the best way to evaluate treatment is known to be by comparison against a placebo; hence our results should be interpreted with caution, despite the fact that other studies have reported very similar findings9. Finally, the high percentage of drop-outs as a result of adverse events needs to be taken into account in the evaluation of the response to treatment in the group that used transdermal nitroglycerin.
Correspondence: Sandra Pons Cuevas. C/ Carlets, 32, 3.º, 2.ª, 08800 Vilanova i la Geltrú, Barcelona, Spain. Email: 32076spc@comb.es Manuscript accepted for publication 18.06.01.