A 7-year-old male patient with gait ataxia and abundant epistaxis.
1.1HistoryMother of 25 years old with secondary schooling, housewife, who denied alcoholism and smoking, apparently healthy. Father of 28 years old with high school education, current smoker, and with occasional alcohol ingestion. Apparently healthy. Six-year-old healthy brother. His grandmother died of cancer (unspecified).
Born and resident of Mexico City. He lived in a rented department, of enduring materials, with all the basic services, with five people. Integrated into the family diet. He started walking at 18 months and developed sphincter control at 24 months. Myelotoxicity exposure denied.
He was the product of the first pregnancy of his mother which occurred without abnormalities. His mother went to prenatal care from the first month of pregnancy, she had seven visits, and three ultrasounds reported as normal. A cesarean section at 40 weeks of pregnancy was prescribed because of transverse presentation. He cried and breathed at birth, weighed 3400g, length 53cm, Apgar score of 9. He was discharged with his mother. Allergic, traumatic and surgical history denied.
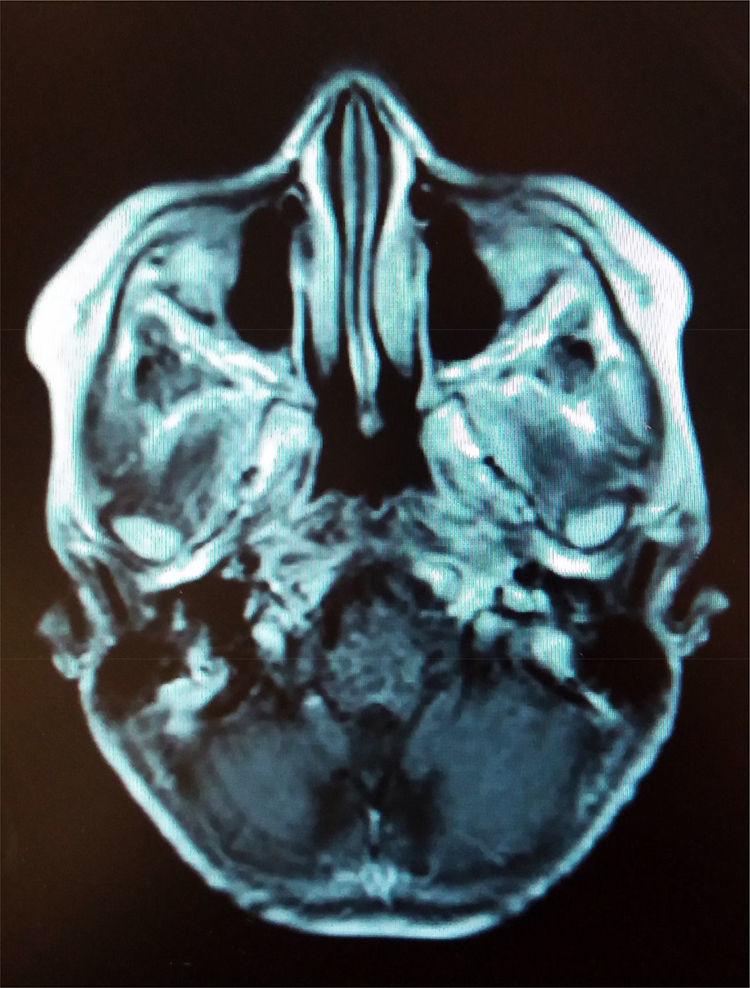
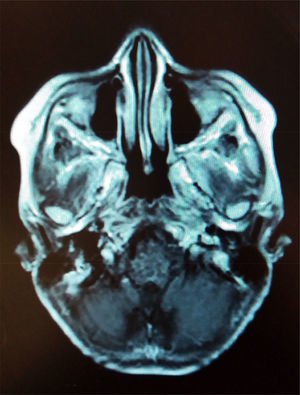
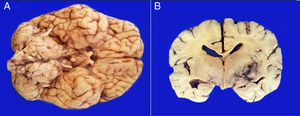
1.2Final illnessThe patient arrived at the hospital for the first time referred from a second-level hospital, with a 6-month evolution of gait ataxia by right lateral lateralization, left parietal oppressive cephalea, abundant epistaxis (three episodes per week), and divergent strabismus at the expense of right eye. On admission, he was found with a cerebellar vermis and hemispheric syndrome secondary to a tumor of the posterior fossa, without hydrocephalus. Magnetic resonance imaging (MRI) showed a brain stem tumor of the pons with a discrete right peduncle mesencephalic extension of 4.1 x 5.2 x 4.2cm, anteriorly displacing the basilar artery. The image suggested a pons glioma (Figure 1).
Two months later, he received the first course of carboplatin 450mg / m2 of body surface area (BSA), etoposide 100mg / m2 of BSA and ifosfamide 1.9g / m2 of BSA. Ten cycles were administered, as well as radiotherapy with a total dose of 55.8Gy associated with temozolomide 200mg per 3 days. He concluded after eight sessions because he presented chickenpox and severe malnutrition. Oral candidiasis was found, and it was managed with acyclovir 1500mg / m2 of BSA / day for ten days and fluconazole 3mg/kg / day for seven days. He was discharged after clinical improvement 11 days after admission. Subsequently, an MRI showed a decrease in tumor size, which measured 3.9 x 3.7 x 3.6cm.
A few months later, he was admitted to the emergency room due to epistaxis, fever, and neutropenia and a nasal packing was placed. Subsequently, he presented epistaxis and an anemic syndrome with a hemoglobin of 7.7mg/dl. Six months later, he presented ataxia syndrome with lateropulsion, star gait, dysarthria, aphasia, vertigo, and altered mental state. MRI showed an increase in the size of the tumor to 4.7 x 3.9 x 4.1cm. Chemotherapy was started again with carboplatin 450mg / m2 of BSA, etoposide 100mg / m2 of BSA and ifosfamide 1.9g / m2 of BSA.
Two months later, he presented cerebral edema, which was managed with hypertonic solutions and steroids. He responded favorably to management. He presented nosocomial sepsis with a pulmonary focus, and he was discharged with palliative care.
Four months later, he was admitted to the emergency room with respiratory distress, fever, tachycardia, systemic inflammatory response, severe metabolic acidosis, hypokalemia, and hypocalcemia. He was intubated, and ampicillin and amikacin were administered as well as comfort and support management. Palliative analgesia was administered with buprenorphine. In blood culture, Staphylococcus epidermidis was isolated and in urine, Klebsiella pneumoniae. He presented respiratory deterioration and systemic inflammatory response. Clinical death was declared.
2Case presentationWe discuss the case of a seven-year-old patient with a malignant pontine glioma, with extensive infiltration to other structures of the brain stem, cerebellum, and meninges. We also address surgical complications and changes in the new World Health Organization (WHO) classification of central nervous system (CNS) tumors in children.
This case describes with a seven-year-old patient with a pons tumor, which is always a challenge for the medical team formed by pediatricians, neurologists, neurosurgeons, and oncologists to reach at an accurate diagnosis and give an appropriate treatment. Multiple diseases and alterations affect the brain stem in children. These include vascular alterations, such as hemorrhage, infarction, vasculitis, arteriovenous malformations; metabolic and neurodegenerative diseases, such as Leigh's disease, maple syrup disease, glutaric aciduria or Wilson's disease; infections by viruses, fungi or parasites; and tumors, of which the most frequent are gliomas, in addition to the medulloblastoma, ependymoma or atypical rhabdoid teratoid tumor. Imaging studies are indispensable for evaluating these lesions. Although some lesions may have overlapping characteristics, studies are indispensable to locate the lesion and know its approximate size.
Gliomas account for more than 40% of neoplasms in the infratentorial portion, where tumors are more common in children. Pons gliomas are approximately 10-20% of all intracranial tumors. Most of them occur in the first decade of life and cause cerebral edema and, occasionally, hydrocephalus. Most are hypointense or isointense in T1 and hyperintense in T2-weighted MRI. Symptomatology in the brain stem is related to the location and size of tumors. In a study at the Hospital Infantil de México Federico Gómez (HIMFG), the most frequent clinical manifestations were intracranial hypertension signs and cerebellar or pyramidal signs, in addition to the affection of different cranial nerves.1
The WHO classifies astrocytomas in low grade (I and II) and high-grade (grade III and IV), and includes anaplastic astrocytoma and glioblastoma.
The location of these lesions in vital regions of the CNS limits surgery feasibility. Therefore, radiotherapy is chosen for the treatment, although it does not provide an alternative cure, only palliative results. Treatment with different chemotherapeutic agents has not demonstrated differences compared with exclusively radiotherapy treatment and has shown to increase morbidity and decrease the quality of life of the patient.
Furthermore, the use of the stereotactic biopsy is controversial. The HIMFG reviewed 50 cases of patients with brain stem tumors. The findings showed that the most frequent are low-grade astrocytomas (30 cases), followed by high-grade astrocytomas (13 cases), primitive neuroectodermal tumor (2 cases), atypical rhabdoid teratoid tumor (2 cases), one case of ependymoma and two cases in which it was not possible to classify the tumor with the biopsy sample.2 The probability of finding tumors other than astrocytomas would justify the practice of stereotactic biopsy. However, the high resolution of modern imaging studies allows determining the procedure to follow for each patient, including the decision of a biopsy. Biopsy complications are minimal in experienced hands; no complications were reported in the study previously mentioned. Moreover, from a research point of view, this decline in practice conditions the tumor to its postmortem study; thus, stereotactic biopsies still should be practised more frequently.3
Data from patients with glioblastoma multiforme, who were diagnosed and treated at the HIMFG, were published in 2009.4 Sixteen patients were documented, with an average age of presentation of 8.8 years. More complications were present in younger patients, and the patients with greater survival were those in whom a greater surgical resection of the tumor volume was achieved.
Despite the treatment described, the patient in the present case deteriorated due to sepsis and healthcare-associated pneumonia.
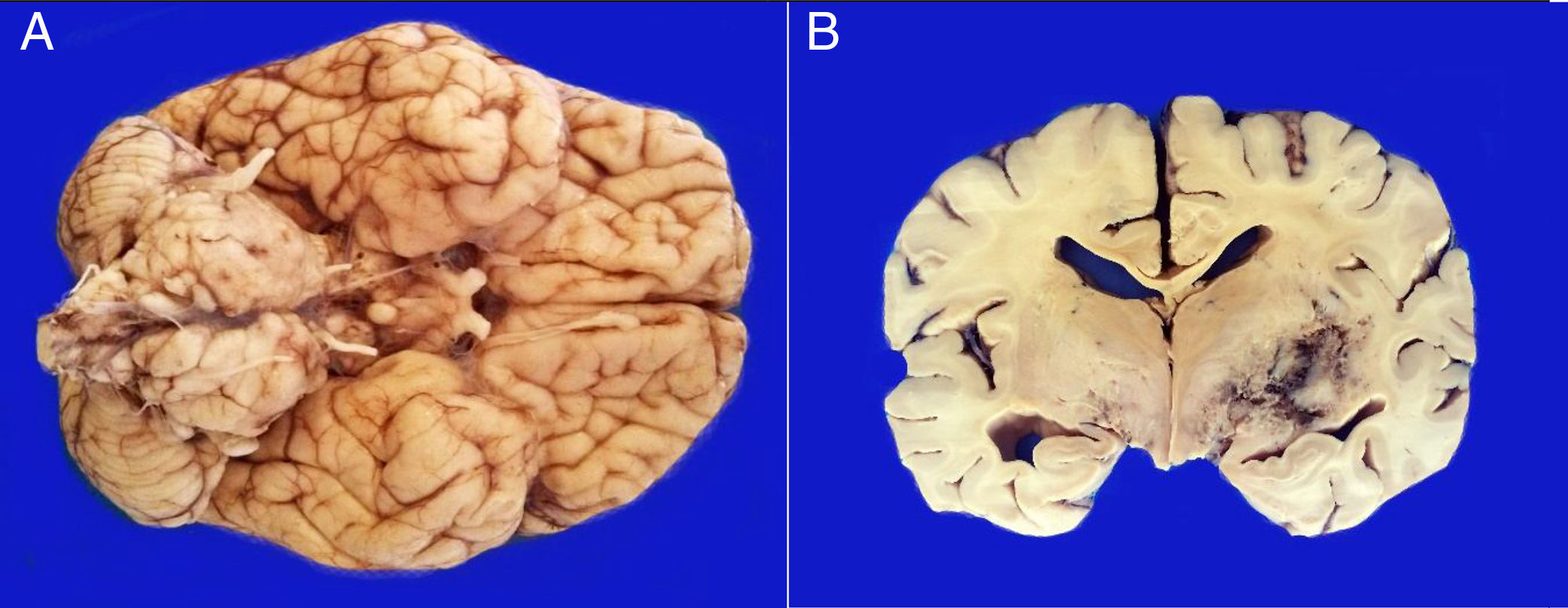
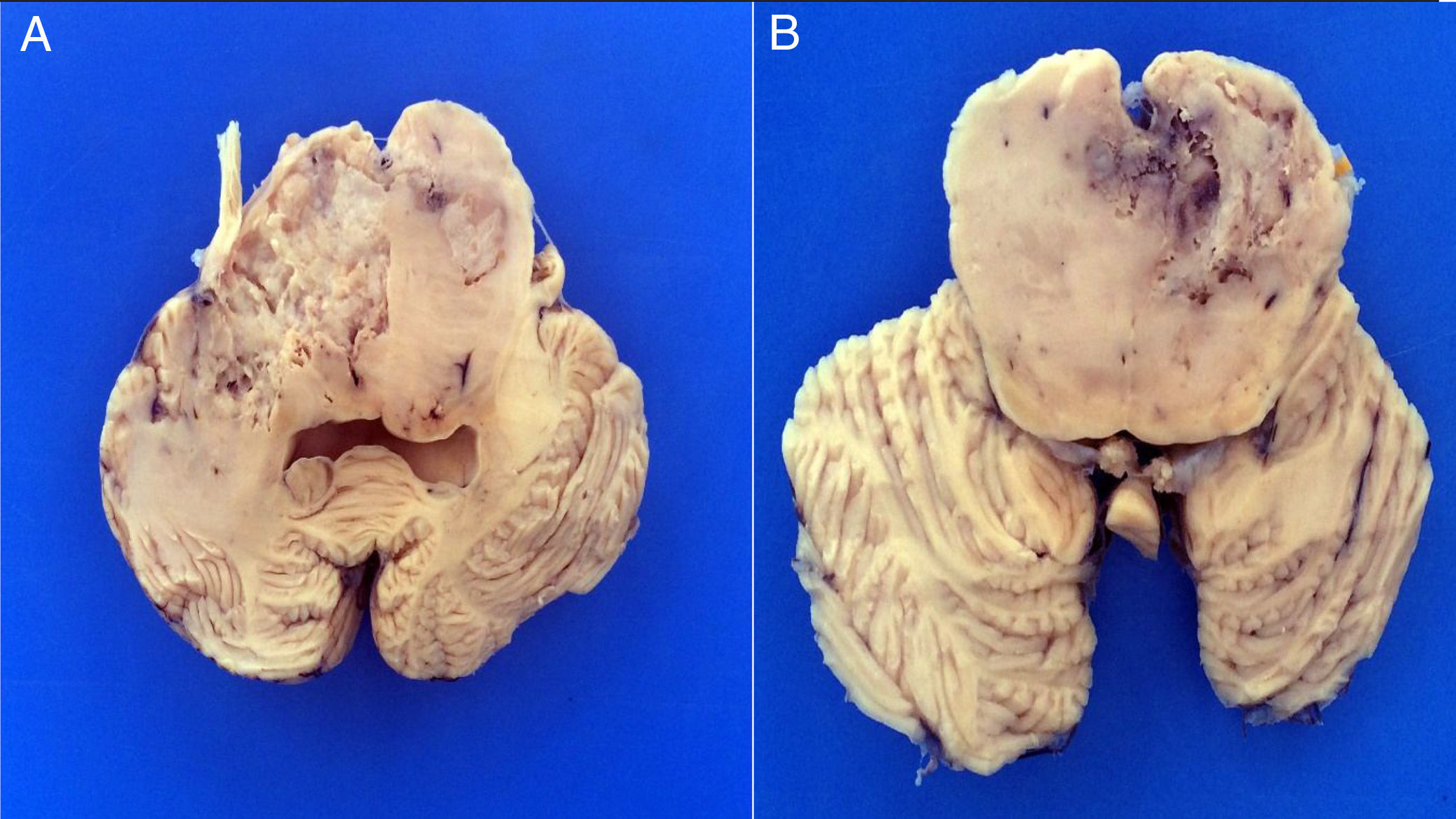
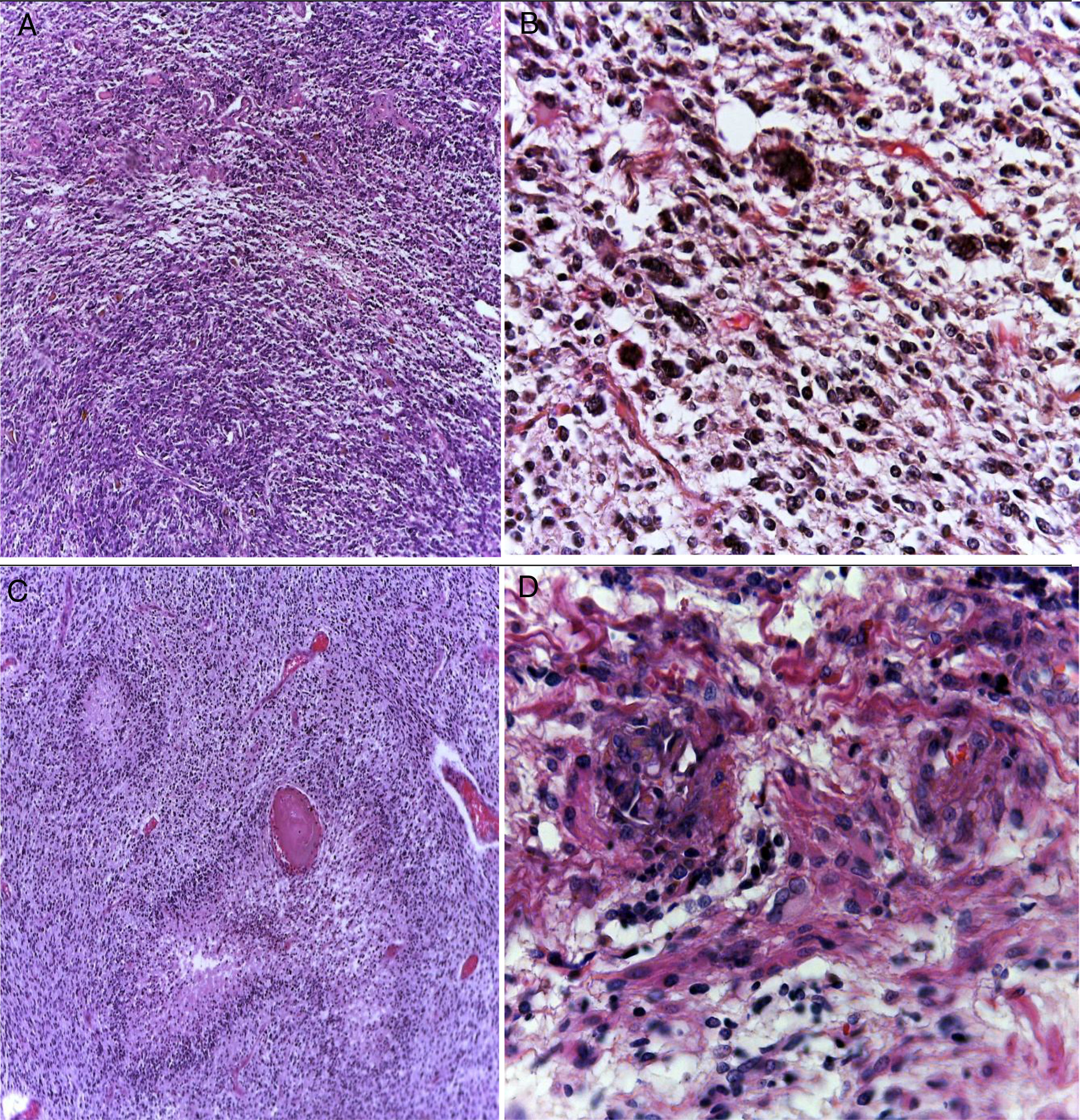
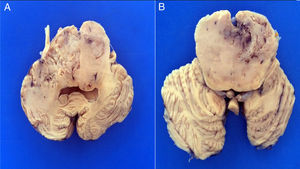
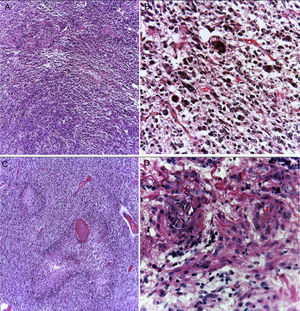
3PathologyThe postmortem study was carried out in a seven-year-old child. Increased brain weight was found: an observed weight of 1263g with an expected of 1100g according to his age and height. The brain showed a normal convoluted pattern and congestion of the meningeal vessels. At the base, a cystic-like tumor that bilaterally widened the pons, with most of it at the right side was found (Figure 2A). Serial sections showed predominantly left hydrocephalus, right uncal herniation, compression of the third and fourth ventricles, and cerebral edema. We found a poorly delimited grayish yellow pons tumor, with destruction of the parenchyma, areas of hemorrhage and necrosis (Figure 2B), infiltration to the bulb, right superior cerebellar peduncle, midbrain, cerebral peduncles, predominantly to the right gray nuclei of the base and right hippocampus with extensive infiltration to leptomeninges (Figure 3). Histological sections stained with hematoxylin and eosin showed a highly cellular malignant glial neoplasia composed of poorly differentiated, pleomorphic cells with atypia and multiple mitoses (Figure 4A). A few multinucleated cells were observed (Figure 4B). This neoplasm characteristically shows areas of necrosis surrounded by atypical nuclei palisades and glomeruloid vascular endothelial proliferation. It is accompanied by recent ischemic necrosis and changes by chemotherapy and radiotherapy (Figure 4C and 4D). This histological aspect corresponds to the astrocytoma grade IV or glioblastoma multiforme according to WHO classification.
A. The base shows a tumor that bilaterally increases the volume of the pons. The tumor is light yellow and solid in appearance. B. Coronal sections of the brain show a poorly delimited neoplasm of yellow color with areas of necrosis and hemorrhage and loss of parenchyma affecting the gray nuclei of the right side base.
Glioblastoma is a highly cellular glial neoplasm (A) composed of poorly differentiated, pleomorphic cells with abundant atypia and mitosis. There may be multinucleated elements (B). There are areas of necrosis that may vary in size; are characteristically surrounded by viable tumor cells in the periphery, delimiting them, and forming a palisade (C). There is vascular proliferation with vascular wall hyalinization and glomeruloid appearance (D).
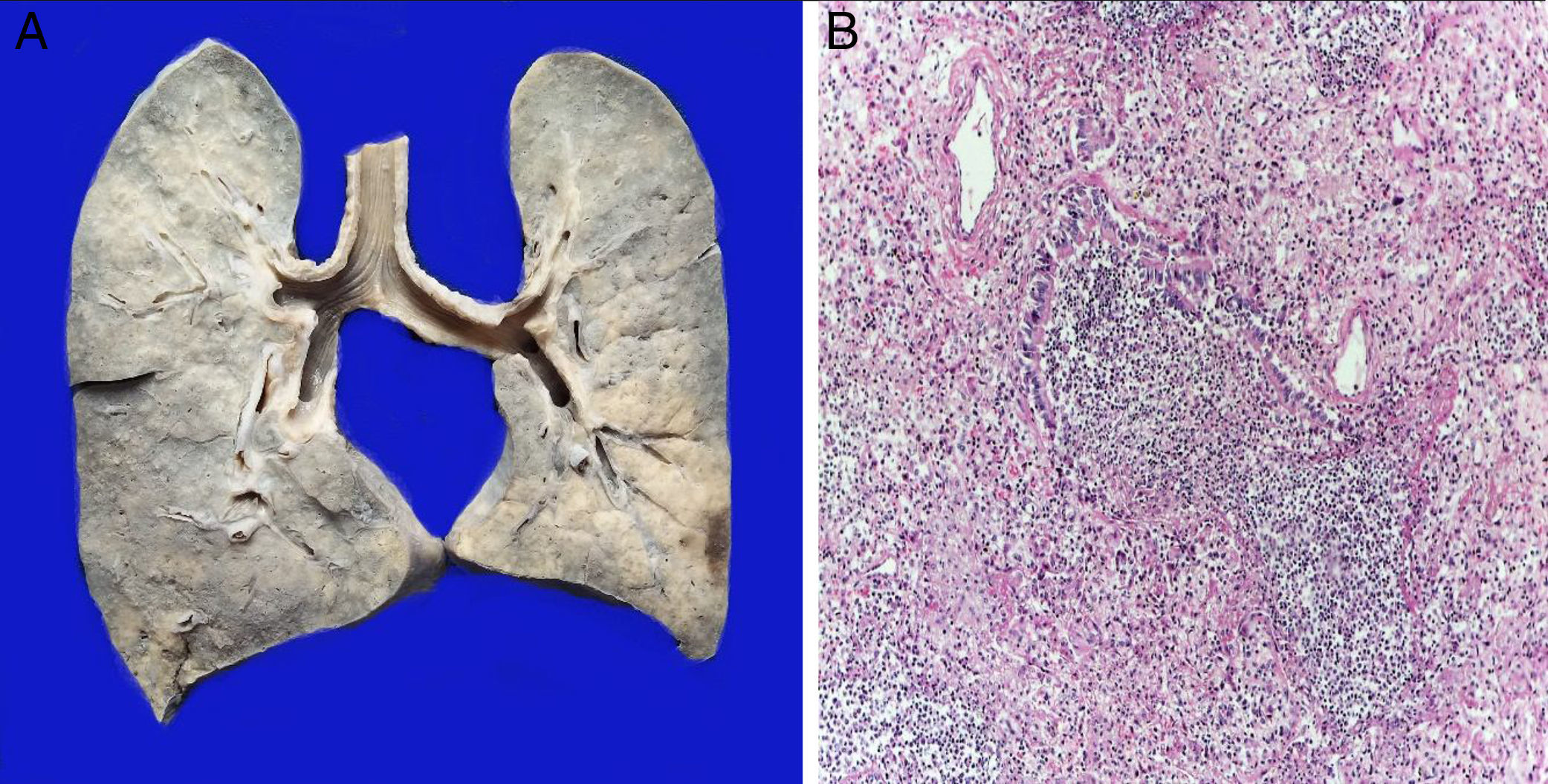
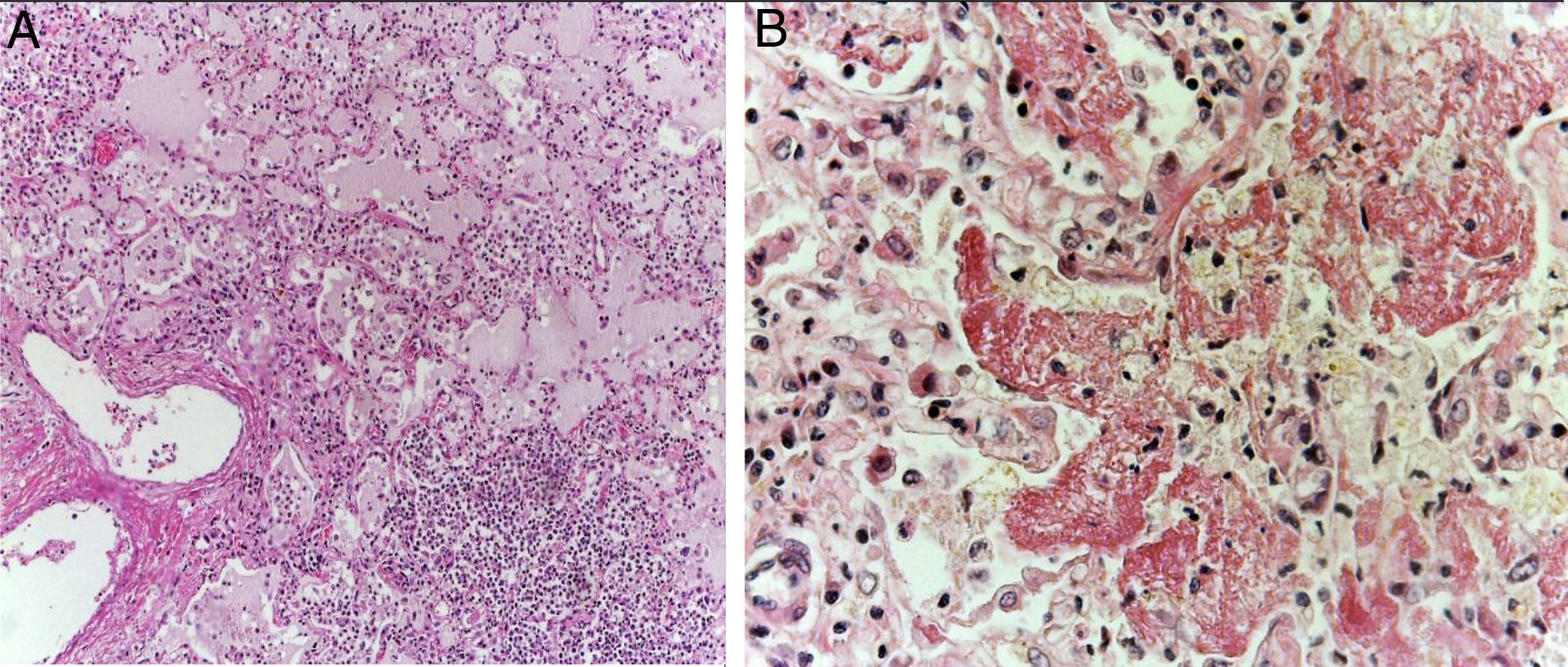
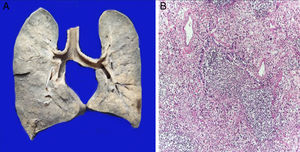
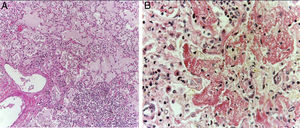
The lungs were firm, enlarged in size and weight, with a gray surface and white-yellow colored areas of consolidation, which mainly affected the right lung, approximately 40% of the sliced surface (Figure 5A). Histological sections showed areas with extensive and abundant infiltration of polymorphonuclear leukocytes that focally affected the wall of terminal bronchioles and alveolar walls (Figure 5B). In other areas, the inflammatory component was formed by lymphocytes, plasma, and macrophages with brown pigment in their cytoplasm. Also, there was mild peribronchial fibrosis that expanded the contiguous alveolar septa. There was hyperplasia of type II pneumocytes. Pulmonary edema (Figure 6A) and alveolar damage with the formation of hyaline membranes were also observed (Figure 6B).
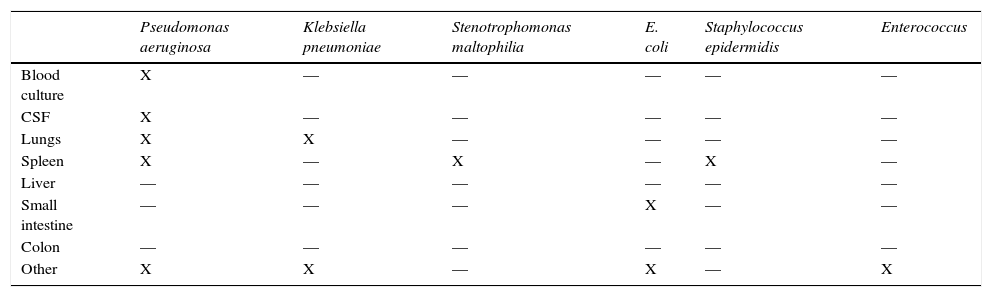
Shock signs were found in the digestive tract, bladder, kidneys, and heart. Anatomical final diagnoses are presented in Table 1. The patient had a clinical history of sepsis by Klebsiella pneumoniae and Pseudomonas aeruginosa. The results of postmortem cultures are shown in Table 2.
Anatomical final diagnoses.
| Main disease |
| Pontine astrocytoma stage IV (WHO) (glioblastoma multiforme) with infiltration to the bulb, upper right cerebellar peduncle, mesencephalon, cerebellar peduncles, basal gray nuclei with right dominance and right hippocampus with extensive infiltration to leptomeninges, recent ischemic necrosis, and changes due to chemotherapy and radiotherapy. |
| Concomitant alterations |
| Hydrocephalus predominantly left-sided |
| Right-sided uncal herniation |
| Compression of the third and fourth ventricles |
| Cerebral edema |
| Acute pneumonia mainly left-sided, associated to healthcare |
| Sepsis due to Klebsiella pneumoniae and Pseudomonas aeruginosa |
| Pleural and costal adhesions |
| Bilateral pleural effusion (right 20 ml, left 20 ml) |
| Shock data |
| Hypoxic-ischemic visceral myopathy |
| Acute tubular necrosis |
| Coaugulative myocytolysis in myocardium |
| Focal acute pancreatitis |
| Congestive hepatomegaly OW 800g / EW 680g |
WHO, World Health Organization; OW, observed weight; EW, expected weight.
Postmortem cultures.
| Pseudomonas aeruginosa | Klebsiella pneumoniae | Stenotrophomonas maltophilia | E. coli | Staphylococcus epidermidis | Enterococcus | |
|---|---|---|---|---|---|---|
| Blood culture | X | — | — | — | — | — |
| CSF | X | — | — | — | — | — |
| Lungs | X | X | — | — | — | — |
| Spleen | X | — | X | — | X | — |
| Liver | — | — | — | — | — | — |
| Small intestine | — | — | — | X | — | — |
| Colon | — | — | — | — | — | — |
| Other | X | X | — | X | — | X |
CSF, cerebrospinal fluid.
The WHO has organized scientific meetings to develop tumor classifications of different organs involving experts in different areas. In the case of CNS tumors, the meeting included neuropathologists, neuro-oncologists, and scientists, who were in charge of updating the classification published in 2007.
The most important innovations introduce the findings of molecular biology to the different entities. In the case of pediatric patients, the classification of glial tumors, medulloblastoma, embryonal tumors, and ependymoma underwent remarkable changes.
Diffuse pediatric astrocytomas used to be classified with their peers in adults. However, in the new molecular age, they were found to have different characteristics. K27M mutations were found in histone H3 of the H3F3A gene, or less frequently in the HIST1H3B gene. These tumors grow diffusely in the midline, thalamus, brain stem and the spinal cord. They were named as diffuse midline gliomas with K27M H3 mutation, and include the pons diffuse astrocytoma.3 As for glioblastoma, it was divided into two groups: one involving the wild-type HDI gene (90% of the cases) and the other involving the mutated HDI (the remaining 10%). These two varieties are mostly found in adults. The epithelioid glioblastoma present in children and adolescents with mutations in the BRAF gene was added and included in the wild-type HDI group. In children, high-grade gliomas, III and IV, anaplastic astrocytoma and glioblastoma were considered as a single group for treatment planning due to their molecular alterations and similar evolution. Unlike glioblastoma in adults, these usually are present in the midline: on the pons, thalamus, and spinal cord and, less frequently, in the cerebellum. The annual incidence in children under 20 years old is 0.14 cases per 100 000 inhabitants; in adults, 4 per 100 000 inhabitants. Almost entirely, glioblastomas in children arise de novo. The genes involved, RAS, MAPK, tyrosine kinase receptors or genes involved in the p53 pathways, encode proteins involved in the transcription and chromatin regulation. The presence of a syndrome associated with cancer, such as the Li-Fraumeni syndrome, type 1 neurofibromatosis or the constitutional deficiency of repair harmonization increases the incidence of high-grade gliomas in children.
Diffuse gliomas of the pons remain a devastating event in pediatric patients without effective treatment. Molecular studies have shown that these pediatric tumors are different from adult high-grade astrocytomas, and have opened new resources for early diagnosis and effective treatment.3,5 Furthermore, it is necessary to reassess the role of stereotactic biopsy in these cases.1
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Chico PdLF, Perezpeña-Diazconti M. Glioblastoma del puente. Gliomas pediátricos en la clasificación actual de los tumores del sistema nervioso central por la Organización Mundial de la Salud. Bol Med Hosp Infant Mex. 2017;74:147–153.