Introducción: Se ha mencionado que tener un familiar directo con enfermedad renal es un factor de riesgo para el padecimiento. El objetivo del estudio fue conocer la prevalencia de enfermedad renal temprana en niños familiares de pacientes con enfermedad renal crónica terminal (ERCT).
Métodos: Se realizó un estudio de tamiz en niños aparentemente sanos, familiares en primer o segundo grado de pacientes con ERCT en programa reemplazo renal (hemodiálisis o trasplante renal). Previa firma de consentimiento informado se realizó el examen físico completo. Se tomó una muestra de sangre para la determinación de creatinina y electrolitos séricos, así como examen general de orina.
Resultados: Se incluyeron 45 sujetos, mediana de edad 9.6 años, 24 (53%) fueron varones. Se encontraron alteraciones urinarias/enfermedad renal en 11 niños (24.4%). La alteración urinaria más frecuente fue hematuria, encontrada en seis sujetos, seguida de microalbuminuria, encontrada en cuatro. Siete estaban en estadio 2 de enfermedad renal y cuatro en estadio 1.
Conclusiones: El estudio de los familiares de pacientes en terapia sustitutiva renal permite identificar individuos con etapas tempranas de enfermedad renal.
Background: Having a first- or second-degree relative with chronic kidney disease (CKD) has been reported as a risk factor for CKD development. The aim of the study was to determine the prevalence of CKD in children with a first- or second-degree relative undergoing renal replacement therapy (hemodialysis or renal transplant).
Methods: A screening study was performed in asymptomatic children with a family history of CKD in a first- or second-degree relative undergoing renal replacement therapy. Informed consent was obtained in all cases. A clinical examination was performed. Blood and urine samples were obtained for serum creatinine, serum electrolytes, urinalysis, and microalbumin/creatinine ratio.
Results: There were 45 subjects included with a median age of 9.6 years; 24 (53%) were male. Urinary abnormality/CKD was observed in 11 subjects (24.4%). The most common urinary abnormalities were hematuria (6/11) and microalbuminuria (4/11). Stage 2 CKD was found in seven subjects and four subjects with stage 1 CKD.
Conclusions: The study of families of patients undergoing renal replacement therapy is useful to identify children in early stages of kidney disease.
1. Introduction
Irreversible damage that could lead to end-stage kidney disease (ESKD) is considered to be chronic kidney disease (CKD)1. It is defined as kidney damage if it is ≥3 months duration, with the presence of structural and functional abnormalities of the kidney, with or without decrease in the glomerular filtration rate (GFR) and with one or more of the following characteristics: changes in the composition of the urine or blood, changes in imaging tests, changes in renal biopsy or patients with GFR <60 ml/min/1.73 m2 for 3 months or more with or without other symptoms previously described2.
There is little information about the prevalence of the first stages of CKD during childhood because patients tend to be asymptomatic. The majority of the epidemiological information about CKD comes from data available of patients with ESKD when renal replacement therapy (dialysis or transplant) is necessary for maintaining life. It is believed that the numbers of patients in early stages of the disease are 50 times higher than those with ESKD3.
In 2008 a global incidence of the disease of patients 0-19 years of age under treatment with renal replacement was estimated at 9/1,000,000 (age range 4-18 years)1. The etiology of CKD is different in children and adolescents than in adults. In the former, structural disorders of the urinary tract and glomerulonephritis are more common, whereas in adults the principal causes are diabetes mellitus and arterial hypertension4.
It has been mentioned that having a direct family member with kidney disease is a risk factor for the disease5. For these reasons, the objective of the study was to learn the prevalence of early renal disease in children with family members with CKD.
2. Methods
A screening study approved by the Research and Ethics Commission of the Hospital Infantil de Mexico Federico Gómez (HIMFG) was carried out. Children and adolescents <18 years of age who were apparently healthy with first- or second-degree relatives with CKD in hemodialysis programs or with renal transplant and seen in the HIMFG were invited to participate. Informed consent was obtained from the parents or guardian and consent from the minor in subjects >6 years of age was requested.
2.1. Study Procedures
Participants were scheduled for outpatient consultation in the Nephrology Department. A clinical history along with physical examination with anthropometry (weight, height, BMI) was done and blood pressure was taken. A blood sample was obtained for determination of creatinine and serum electrolytes. Urinalysis was also done.
2.2. Definition of Values
Microalbuminuria: 30-300 mg/g.
Albuminuria: >300 mg/g.
Hematuria: ≥1+.
Glycosuria: traces or more.
Nitrites: ≥1+.
Leucocyturia in males: ≥1+; in females: ≥2+ or ≥1+ in those with other urinary disorders (nitrites, microalbuminuria, glycosuria or hematuria).
Pre-hypertension: systolic blood pressure (SBP) or diastolic blood pressure (DBP) >90th percentile and <95th percentile for height and gender6.
Hypertension: SBP or DBP >95th percentile for height and gender6.
The stages of CKD are classified according to the KDOQI2 guidelines based on the GFR as follows:
Stage 1: GFR >90 ml/min/1.73 m2
Stage 2: GFR 89-60 ml/min/1.73 m2
Stage 3: GFR 59-30 ml/min/1.73 m2
Stage 4: GFR 29-15 ml/min/1.73 m2
Stage 5: GFR <15 ml/min/1.73 m2.
Glomerular Filtration Rate (GFR): The formula by Schwartz7 was used with creatinine measured by the Jaffe method.
2.3. Statistical Analysis
Measures of central tendency and dispersion for continuous variables and frequencies and percentages for categorical variables were calculated. We calculated differences of means and ratios between groups (with urinary disorders/ CKD or normal). GraphPad Prism v. 5.0 for Mac OS X was used.
3. Results
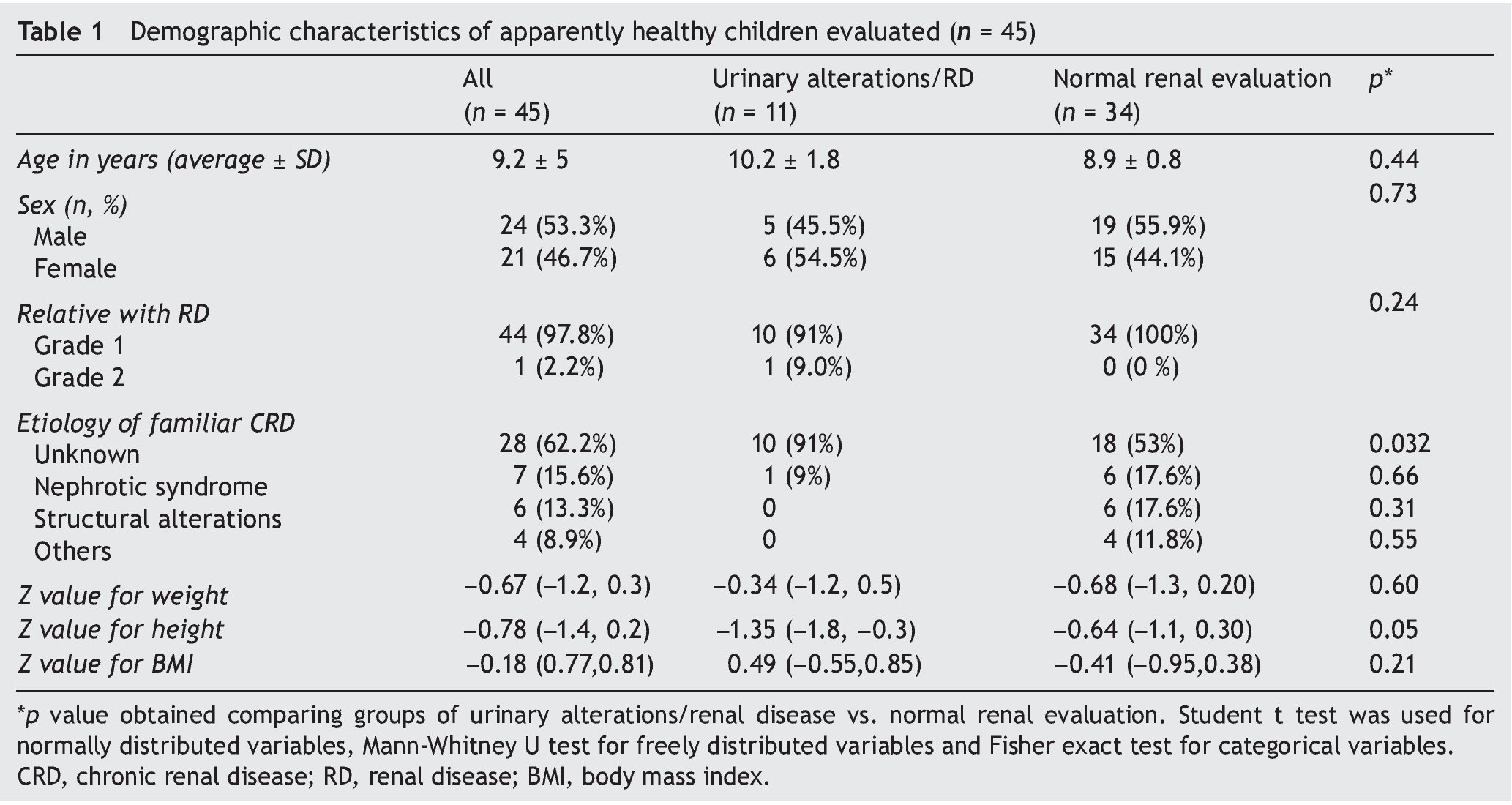
Forty five subjects were included, 24 males (53%) with an average age of 9.6 years. Demographic and anthropomorphic characteristics are described in Table 1.
Urinary disorders/renal disease were found in 11 children (24.4%). Most subjects presented for having a first-degree family member with CKD (father/mother, brother/sister), and only one by having a second-degree family member (cousin). In 62% of the participants the cause of the primary kidney disease in the relative was unknown, but this prevalence was 91% for those in whom urinary disorders/renal disease were documented, and from 53% for those with normal renal values (p = 0.032).
In relation to the anthropometry, no differences were found in Z-value for weight of the children with or without urinary disorders/kidney disease. However, affected children had a Z-value for height significantly less (median -1.35 vs. -0.64, p = 0.05).
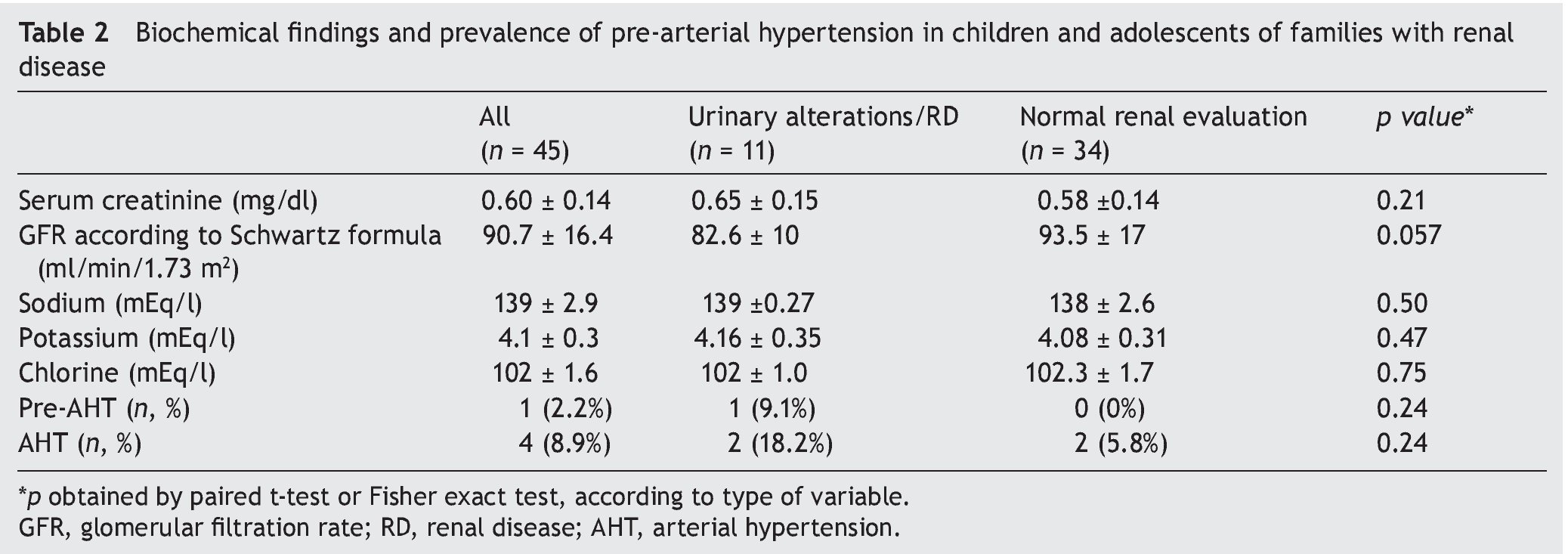
Biochemical findings, GFR and prevalence of arterial hypertension in the participants are shown in Table 2. GFR was 82.6 ± 10 ml/min/1.73 m2 in children with urinary disorders/ renal disease in contrast with 93.5 ± 17 ml/min/1.73 m2 in those with normal evaluation (p = 0.057). There were no statistically significant differences found in serum electrolytes and serum creatinine. There was a higher prevalence of arterial hypertension in those patients with urinary disorders/renal disease (18% vs. 5.8%), but without statistical significance.
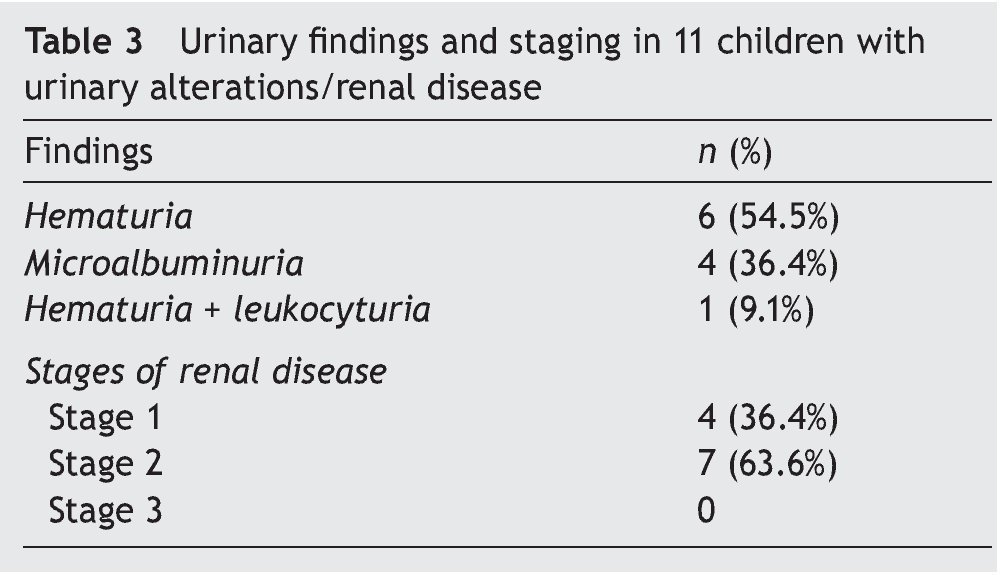
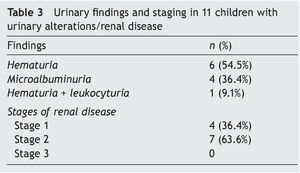
Urinary findings and stratification of renal disease in the 11 affected children is shown in Table 3. The most common urinary disorder was hematuria (six subjects) followed by microalbuminuria (four subjects). Seven were in stage 2 renal disease and four in stage 1.
4. Discussion
The present study found that 24% of apparently healthy children with family history of CKD have urinary disorders/kidney disease. In other countries it has been reported that first- or second-degree relatives of patients with ESKD are two to three times more likely to suffer from CKD5,8,9. In adults, it has been observed that individuals with a family history of CKD are also more likely to have diabetes and obesity10.
In children, the leading causes of kidney disease are structural alterations of the kidneys and urinary tract; 25% of direct family members of patients with structural abnormalities of the kidney may also be affected and >20 monogenic causes of structural disturbances have been detected11,12. It is interesting to note that in this series the cause of renal disease in most families of the participants in the study was unknown, but the proportion was highest (91%) in subjects with urinary disorders/kidney disease. They are generally labeled as unknown cause when there is no history of glomerulopathies (edema, proteinuria), no evidence of structural alterations of the kidney or urinary tract in imaging studies and the kidneys are too small for a renal biopsy to be performed.
There are other hereditary nephropathies such as certain variants of nephrotic syndrome, Alport syndrome or mutation of the APOL gene (which has been described in African-Americans)13,14. It has recently been described that children with chronic renal disease, whether or not due to structural alterations, have a higher prevalence of genetic imbalance, such as increase in the number of copies or sizes of genes, duplication of the X chromosome (XXX syndrome) or alternatively deletion of chromosome 17q1215. However, there are no studies in this regard in the Mexican population.
In the study published by Inserra et al. in Argentina, 810 relatives of hemodialysis patients of whom 142 were subjects between 5 and 18 years of age were studied. In that pediatric population a prevalence of renal disease of 14.1% was found16. The age of patients undergoing hemodialysis is not mentioned, but it is assumed that they were older in age because 96% of the pediatric subjects were children of patients. The latter differs from results seen in this study as all patients developed ESKD during the pediatric age and were treated in the HIMFG.
A normal screening result does not rule out that some subjects may subsequently develop renal disease. The recommendation is to continue participating in timely detection such as with the KEEP program for adults17. Early diagnosis offers the opportunity for performing a renal biopsy and to determine the cause of familial CKD.
Despite being asymptomatic, growth in children with uri-nary disorders/renal disease was affected (with a Z-value for height significantly lower than participants with normal renal assessment) as well as lower GFR. The majority of the children detected by screening were found to be in stage 2 of renal disease, i.e., with a GFR between 60 and 89 ml/ min/1.73 m2.
The limitations of the study are the sample size, having only study visit done, not knowing the etiology of uremia in the majority of patients who developed ESKD during the pediatric age and lack of family assessment for obesity, hypertension, and risk behaviors.
It is considered that the study of the relatives of patients on renal replacement therapy allows the identification of individuals with early stage kidney disease. It is important to take this risk factor into account in the strategies for early detection of CKD in children because timely intervention can prevent or delay the progression to ESKD.
Ethical disclosure
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Acknowledgments
The authors appreciate the collaboration of Germán Sosa, Humberto González, Alfredo Mendoza Chávez, Herlinda Reyes and Lourdes Ortiz in the taking and processing of the samples.
Funding
The study was financed by Fondos Federales del Hospital Infantil de México Federico Gómez, protocol HIM/2012/060.
Conflict of interest
The authors declare no conflicts of interest.
Received 28 April 2015;
accepted 28 July 2015
* Corresponding author.
E-mail:medeiro.mara@gmail.com (M. Medeiros).