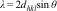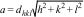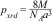Nanocrystalline copper ferrite shows distinct photocatalytic properties, but it suffers from a high recombination rate of photogenerated electrons (e−) and holes (h+) due to its narrow bandgap. Herein, Al3+ doping is shown to reduce the (e−/h+) recombination rate and improve the charge carriers’ availability in doped CuFe2−xAlxO4 (0≤x≤1) nanoparticles produced by a solid-state, mechanochemical process. CuFe2−xAlxO4 (0≤x≤1) nanoparticles exhibit the growth of a nanocrystalline cubic spinel lattice when annealed at 1000°C. The lattice parameter is reduced by Al3+ doping due to the smaller ionic radius of Al3+ ions substituting bigger Fe3+ ions. However, a higher degree of sintering and greater crystallite size are observed for Al3+ doped samples. The surface morphology and topography also reveal an increase in the particle size, but significantly narrow size distribution and greater homogeneity. The effect of Al3+ doping on the optical properties of CuFe2−xAlxO4 (0≤x≤1) nanoparticles is demonstrated by a decrease in the photoluminescence signal that is attributed to the lower rate of (e−/h+) recombination. Thus, Al3+ doping increases transition time and improves the availability of charge carriers for potential photocatalytic applications.
La ferrita de cobre nanocristalino muestra propiedades fotocatalíticas distintas, pero sufre de una alta tasa de recombinación de electrones fotogenerados (e−) y agujeros (h+) debido a su estrecha banda prohibida. Aquí, se muestra que el dopaje Al3+ reduce la tasa de recombinación (e-/h+) y mejora la disponibilidad de los portadores de carga en nanopartículas de CuFe2-xAlxO4 (0≤x≤1) dopadas producidas por un proceso mecanoquímico de estado sólido. Las nanopartículas de CuFe2-xAlxO4 (0≤x≤1) exhiben el crecimiento de una red de espinela cúbica nanocristalina, cuando se recocen a 1000°C. El parámetro de red se reduce por el dopaje Al3+ debido al radio iónico más pequeño de los iones Al3+ que sustituyen los iones Fe3+ más grandes. Sin embargo, se observa un mayor grado de sinterización y un mayor tamaño de cristalito para las muestras dopadas con Al3+. La morfología y la topografía de la superficie también revelan un aumento en el tamaño de partícula, pero una distribución del tamaño significativamente más estrecha y una mayor homogeneidad. El efecto del dopaje Al3+ sobre las propiedades ópticas de las nanopartículas de CuFe2-xAlxO4 (0≤x≤1) se demuestra por una disminución en la señal de fotoluminiscencia que se atribuye a la tasa más baja de recombinación (e-/h+). Por lo tanto, el dopaje Al3+ aumenta el tiempo de transición y mejora la disponibilidad de portadores de carga para posibles aplicaciones fotocatalíticas.
Cubic spinel ferrites are photoactive materials that can be easily photoexcited under visible light to generate electrons (e−) and holes (h+): the active sites for the redox processes [1]. Due to their excellent photocatalytic properties, spinel ferrites have attracted massive research in different fields of catalysis [2–5]. Their photocatalytic efficiency is mainly a consequence of their narrow bandgap ∼2.0eV [6]; which makes them efficient photocatalysts capable of utilizing visible solar energy. Among these spinel ferrites, nanocrystalline copper ferrite (CuFe2O4) has the lowest bandgap 1.32eV [1]. Therefore, spinel CuFe2O4-based photocatalytic materials have found applications in solar water splitting [7,8], CO2 reduction [9,10], and degradation of contaminants [11,12].
However, CuFe2O4 nanostructures also suffer from a high (e−/h+) recombination rate due to their narrow bandgap, which inhibits charge availability and photo-response [10]. To improve the photoactivity of CuFe2O4, nanocomposites are developed by combining CuFe2O4 nanoparticles with quantum dots, polymers, graphene, or ceramics [9,10,12,13]. The synergistic combination of CuFe2O4 nanoparticles with different materials improves the charge separation and reduces the (e−)/(h+) recombination rate in CuFe2O4. The processing of nanocomposites, however, requires additional steps, materials, and cost.
An alternative, cost-effective, and uncomplicated approach involves doping of CuFe2O4 nanoparticles with different di- or trivalent metals such as Zn2+ and Ce3+[14,15]. Doping can substantially alter the crystal structure, ionic distribution, and optical properties of CuFe2O4 nanoparticles [16]. This article presents a straightforward route for mechanochemical synthesis of Al3+ doped CuFe2O4 solid solutions with a chemical formula: CuFe2−xAlxO4 (where, x=0, 0.2, 0.4, 0.6, 0.8, and 1.0; thus, written as (0≤x≤1)). These CuFe2−xAlxO4 (0≤x≤1) nanoparticles are prepared via a solvent-free and additive-free, solid-state method using a high-energy ball milling (HEBM).
HEBM is a dry powder processing technique that uses mechanical collision and friction energy to produce fine particles and solid solutions [17]. CuFe2−xAlxO4 (0≤x≤1) nanoparticles prepared via HEBM are annealed at 1000°C to yield nanocrystallites. The crystal growth and sintering effects on the lattice parameter, crystallite size, and density are determined by X-ray diffraction analysis. The surface morphology and chemical composition are characterized by a scanning electron microscope equipped with energy-dispersive X-ray spectroscopy. The optical properties are studied by photoluminescence characteristics of CuFe2−xAlxO4 (0≤x≤1) nanoparticles.
Materials and methodsSynthesis of CuFe2−xAlxO4 (0≤x≤1) nanoparticlesTo prepare CuFe2−xAlxO4 (0≤x≤1) nanoparticles, iron(III) oxide (Fe2O3 nanopowder, <50nm particle size (BET)), aluminum oxide, (Al2O3 nanopowder, <50nm particle size (TEM)), and copper(II) oxide (CuO nanopowder, <50nm particle size (TEM)) obtained from Sigma-Aldrich are used. HEBM is used to synthesize CuFe2−xAlxO4 (0≤x≤1) nanoparticles with different compositions. A SPEX™ 8000M Mixer/Mill™ equipped with a 500-cc stainless steel vessel containing stainless steel balls (8mm diameter) is used for mechanical milling of the stoichiometric proportions of Fe2O3, Al2O3, and CuO. Table 1 shows the balanced chemical equations to determine the stoichiometric amount of CuO, Fe2O3, and Al2O3 that are used to produce 1g of CuFe2–xAlxO4 nanoparticles with (x=0, 0.2, 0.4, 0.6, 0.8, and 1.0). The steel balls and chemical powders mass ratio is 20 and HEBM is carried out for 5h at 600rpm. Finally, the powdered samples are thermally annealed at 1000°C for 5h and characterized.
Synthesis of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles: The stoichiometric amounts of CuO, Fe2O3, and Al2O3 are used to produce 1g of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles.
| x | Balanced chemical equation for CuFe2−xAlxO4 (0≤x≤1.0) synthesis |
|---|---|
| x=0 | CuO+Fe2O3→CuFe2O4 |
| x=0.2 | 10CuO+9Fe2O3+Al2O3→10CuFe1.8Al0.2O4 |
| x=0.4 | 5CuO+4Fe2O3+Al2O3→5CuFe1.6Al0.4O4 |
| x=0.6 | 10CuO+7Fe2O3+3Al2O3→10CuFe1.4Al0.6O4 |
| x=0.8 | 5CuO+3Fe2O3+2Al2O3→5CuFe1.2Al0.8O4 |
| x=1.0 | 2CuO+Fe2O3+Al2O3→2CuFeAlO4 |
The crystal structure of the as-milled and thermally-annealed CuFe2−xAlxO4 (0≤x≤1) nanoparticles is studied with a STOE STADI P X-ray diffractometer (XRD) using a Cu Kα irradiation source (λ=1.54056Å). The samples are scanned in the range of 20–80° with a scan rate of 2°/min. The crystallite size, lattice parameter (a), inter-planar spacing (d311), and X-ray density (ρxrd) are calculated from the XRD data of annealed CuFe2−xAlxO4 (0≤x≤1) nanoparticles [18,19]; that is discussed later.
The microstructure and surface morphology of CuFe2−xAlxO4 (0≤x≤1) nanoparticles is studied with a JEOL JSM-6510 scanning electron microscope (SEM). The micrographs are further analyzed with WSxM 4.0 Beta 9.3 and ImageJ 1.52a programs to study the surface topography and measure mean particle/aggregate diameter [20,21]. The elemental composition of CuFe2−xAlxO4 (0≤x≤1) nanoparticles is determined with the energy-dispersive X-ray spectroscopy (EDS). The optical properties of CuFe2−xAlxO4 (0≤x≤1) nanoparticles are examined by Perkin Elmer LS 45 fluorescence spectrophotometer.
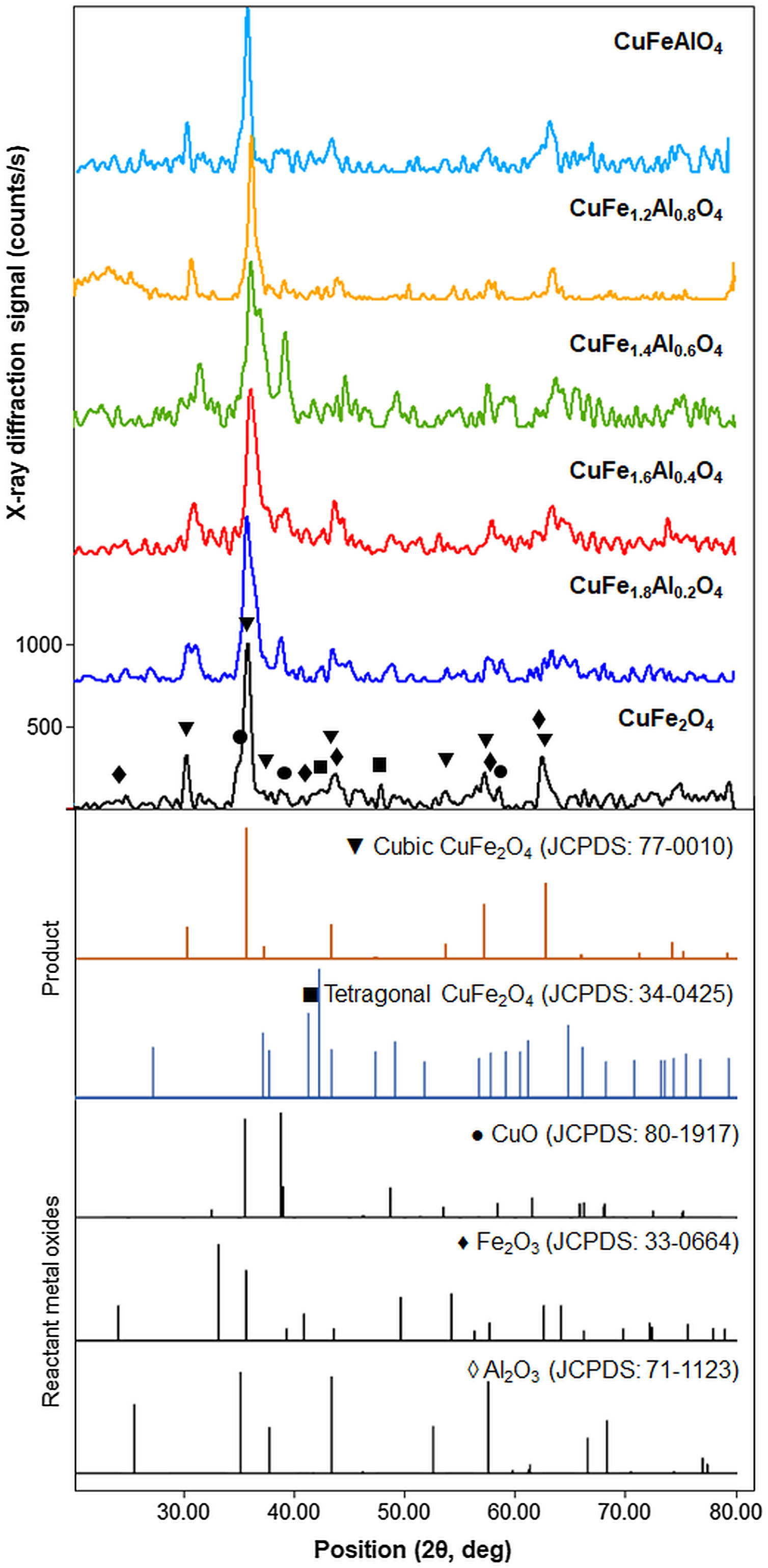
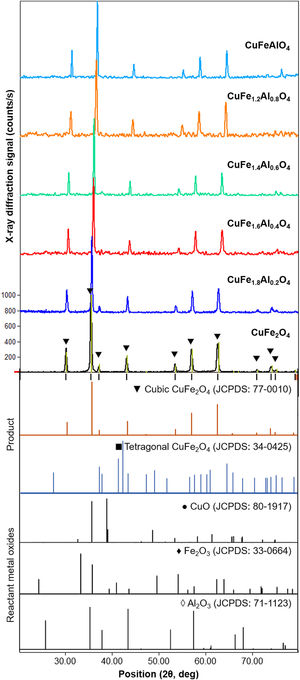
Results and discussionStructural characterizationThe crystal structure of the as-milled CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles is characterized by XRD. Fig. 1 shows the XRD patterns of the as-milled CuFe2−xAlxO4 (0≤x≤1) nanoparticles along with the JCPDS reference patterns for cubic (card 77-0010 [22]) and tetragonal (card 34-0425 [23]) CuFe2O4 and reactant metal oxides, i.e. CuO (card 80-1917 [24]), Fe2O3 (card 33-0664 [25]), and Al2O3 (card 71-1123 [26]). All samples exhibit a characteristic diffraction peaks around 30.5°, 35.8°, and 62.6° 2θ positions, which correspond to the hkl (220), (311), and (440) Miller indices of a spinel ferrite with the Fd3m space group (JCPDS card 77-0010 [22]), respectively. Thus, as-milled CuFe2−xAlxO4 (0≤x≤1) nanoparticles indicate the formation of a spinel lattice structure. However, the as-milled CuFe2−xAlxO4 (0≤x≤1) nanoparticles are semicrystalline in nature with a significant fraction of amorphous content that is revealed by the noisy pattern. Moreover, the characteristic diffraction peaks are considerably broad with a shoulder or doublet indicating the presence of impurities originating from the unreacted oxides, e.g. CuO and Fe2O3, as shown in Fig. 1. Berbenni et al. [27] noticed that CuFe2O4 crystallized into a tetragonal lattice at room temperature and they recorded tetragonal to cubic transition at 398°C.
XRD patterns of the as-milled CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles. The reference patterns for cubic and tetragonal CuFe2O4 nanoparticles and reacting metal oxides are also included. The existence of the cubic spinel lattice structure as the predominant phase is indicated by the characteristic (220), (311), and (440) diffractions around 30.5°, 35.8°, and 62.6° (2θ position), respectively. The presence of unreacted oxides is also indicated.
Marinca et al. [28] studied the structure evolution in ball-milled CuFe2O4 samples annealed at 600–1000°C and observed that after 4h of reactive milling a solid solution of CuO and Fe2O3 and a spinel phase is obtained. However, the single cubic spinel phase CuFe2O4 was formed by annealing at> 600°C. In this case, XRD patterns of the as-milled samples demonstrate the formation of cubic spinel lattice as the predominant structure, which is well in agreement with the relevant literature [28]. According to a recent study using in situ XRD analysis of CuFe2O4 nanocrystals, the cubic spinel structure truly evolves at ∼1000°C [29]. Therefore, the as-milled CuFe2−xAlxO4 (0≤x≤1) nanoparticles are thermally annealed at 1000°C to improve the crystallization and purity and their structure is examined after annealing.
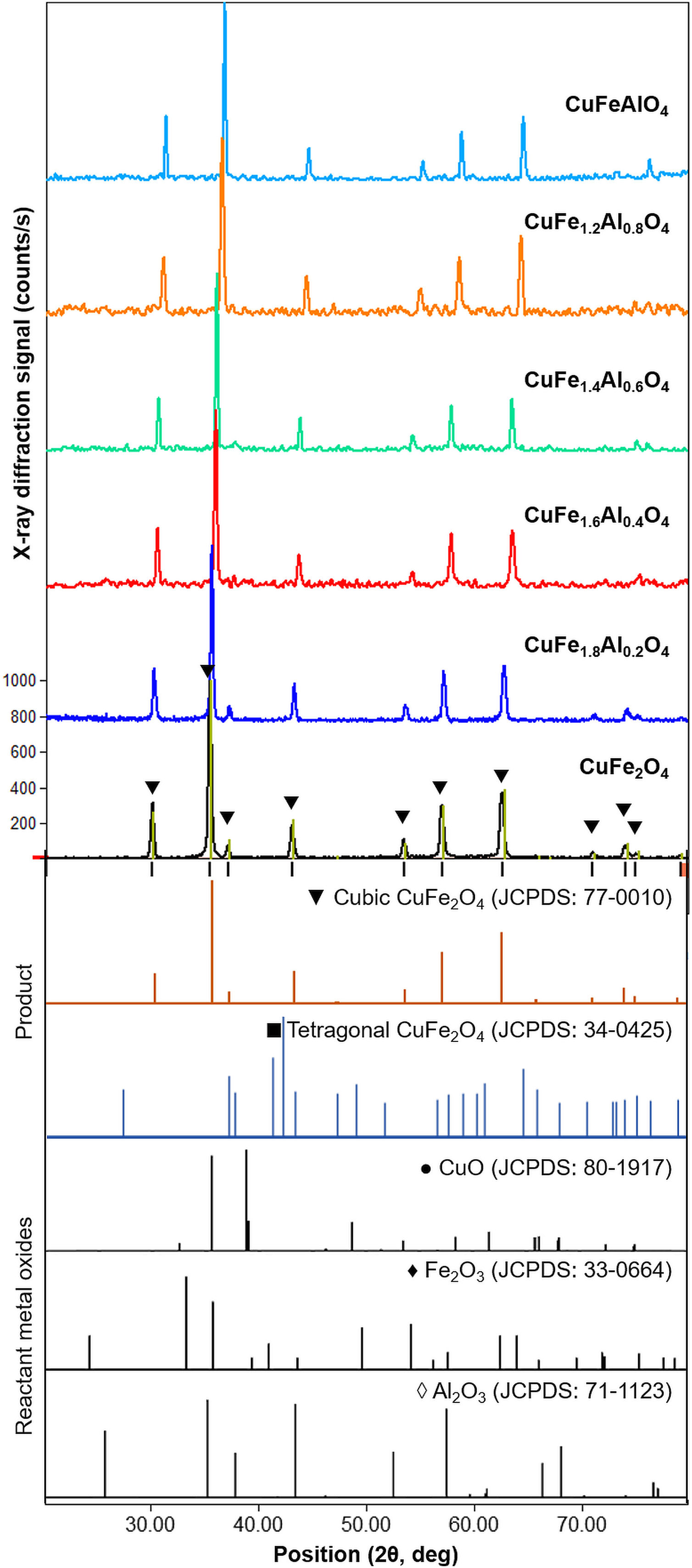
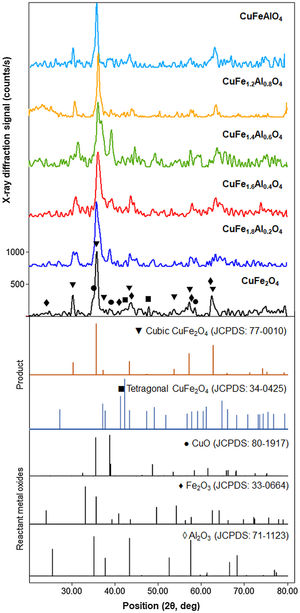
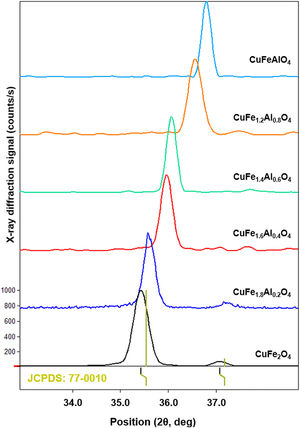
XRD patterns of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles annealed at 1000°C for 5h are shown in Fig. 2. The reference patterns are also included. The patterns for all CuFe2−xAlxO4 (0≤x≤1.0) samples are similar with slight variations in the peak positions except CuFe1.8Al0.2O4. The samples exhibit good crystallinity as indicated by the sharp diffraction peaks. These diffraction peaks are recognized as (220), (311), (222), (400), (422), (511), and (440) planes of the cubic lattice and match well with the cubic spinel copper ferrite (JCPDS card 77-0010) [22]. The data also aligns well with the reported literature for cubic CuFe2O4 nanoparticles [30,31]. Although all CuFe2−xAlxO4 (0≤x≤1.0) samples demonstrate good crystallinity and the formation of a cubic spinel lattice, CuFe1.8Al0.2O4 is an exception. In the case of the CuFe1.8Al0.2O4 sample, broad diffraction peaks with shoulders or superposition of other peaks are observed that may be due to the presence of impurities or unreacted oxides, as shown in Fig. 2. The existence of a secondary phase, i.e. tetragonal CuFe2O4 is not detected. Thus, it could be attributed to experimental error or poor annealing. However, for other types of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles, there is no detectable peak corresponding to oxide-based impurities, which substantiates the formation of the cubic spinel structure of Al3+-doped copper ferrite nanoparticles.
XRD patterns of the annealed CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles. The reference patterns for cubic and tetragonal CuFe2O4 nanoparticles and reacting metal oxides are also included. The formation of the cubic spinel lattice with the Fd3m space group is confirmed by the characteristic diffractions corresponding to JCPDS card 77-0010. An exception is the CuFe1.8Al0.2O4 sample that indicates the presence of unreacted oxides.
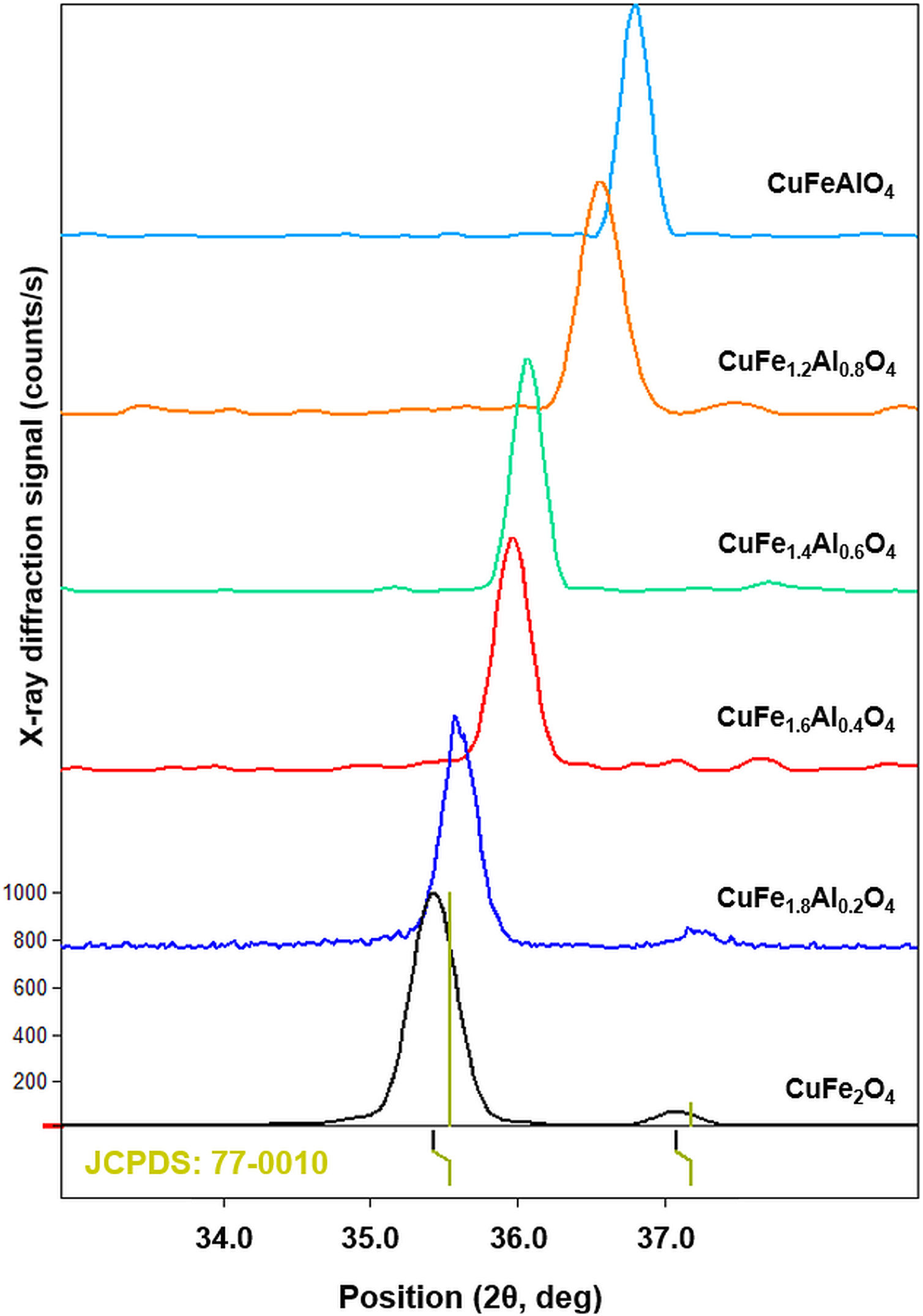
As shown in Fig. 2, the most intense diffraction corresponds to the (311) plane of the cubic lattice at ∼35.8° 2θ position. Fig. 3 shows (311) peak position and width for different types of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles. The position of (311) diffraction varies with the increasing Al3+ ratio. The peak shifts to a higher 2θ position as Al3+ content increase from 0 to 1, which may be attributed to the smaller size of Al3+ ions compared to Fe3+ ion [32,33]. The ionic radii of Al3+ and Fe3+ ions are 0.53 and 0.65Å, respectively [34]. XRD analysis of the annealed CuFe2−xAlxO4 (0≤x≤1) nanoparticles is used to calculate the crystallite size (D), lattice parameter (a), inter-planar spacing (d311), and X-ray density (ρxrd) of all samples [18]. Table 2 shows the experimentally obtained structural parameters and properties of the annealed CuFe2−xAlxO4 (0≤x≤1) nanoparticles.
The structural propertiesa of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles calculated from the X-ray diffraction (XRD) analysis.
| Sample | M (g/mol) | a (Å) | V (Å3) | d311 (Å) | ρxrd (g/cm3) | D (nm) |
|---|---|---|---|---|---|---|
| CuFe2O4 | 239.4 | 8.397 | 592.2 | 2.532 | 5.371 | 26.33±1.96 |
| CuFe1.8Al0.2O4 | 233.6 | 8.354 | 583.0 | 2.519 | 5.323 | 28.69±1.38 |
| CuFe1.6Al0.4O4 | 227.8 | 8.271 | 565.8 | 2.494 | 5.349 | 35.16±1.79 |
| CuFe1.4Al0.6O4 | 222.0 | 8.249 | 561.3 | 2.487 | 5.256 | 43.93±2.33 |
| CuFe1.2Al0.8O4 | 216.2 | 8.144 | 540.2 | 2.456 | 5.318 | 32.03±0.87 |
| CuFeAlO4 | 210.5 | 8.093 | 530.0 | 2.440 | 5.275 | 48.29±1.72 |
The interplanar spacing (d311) is calculated from the Bragg's equation (1)[35,36]:
where λ is the wavelength of the X-rays and θ is the Bragg's angle. The interplanar spacing decreases from 2.512Å for pure CuFe2O4 nanoparticles to 2.440Å for CuFeAlO4 nanoparticles, i.e. an increase in Al3+ doping reduces the interplanar spacing due to smaller ionic radius of Al3+ ions.The lattice parameter can be calculated from the following Eq. (2):
where a is the lattice parameter of a cubic (a=b=c) lattice, and dhkl is the interplanar spacing in the successive crystallographic planes (h, k, l) of the lattice. The lattice parameter of pure CuFe2O4 (0≤x≤1) nanoparticles is calculated as 8.397Å that is comparable to the values reported in the literature [37,38]. Al3+ doped ferrites exhibit a decrease in the lattice parameter with the increasing Al3+ content that is again related to the differences in the ionic radii of Al3+ and Fe3+ ions. Quinzeni et al. [39] also demonstrated that at increasing Al3+ content, a more compact unit cell was formed with a smaller lattice parameter. Thus, CuFeAlO4 nanoparticles show significantly smaller a value of 8.093Å, as shown in Table 2.Lattice volume (V) shows a similar trend along the series of CuFe2−xAlxO4 (0≤x≤1) nanoparticles as it is calculated from the lattice parameter as (V=a3). The lattice volume of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles is reduced from 592.2Å3 to 530.0Å3 with the increasing Al3+ content. The density (ρxrd) of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles can also be determined from the XRD analysis as given by the following equation (3)[33,40]:
where M is molecular weight and NA is Avogadro's number. The density is a measure of mass per unit volume, while both molecular weight and lattice volume decrease with the addition of Al3+ ions as the dopant. However, the decrease in density is not uniform because it depends on the magnitude of the decrease of molecular weight and lattice volume. Hence, due to the different extent of decrease in lattice volume of thermally annealed CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles, the density does not follow a clear pattern and does not correlate with the Al3+ content.The crystallite size (D) of the annealed CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles is determined by the Scherrer's formula [41], equation (4):
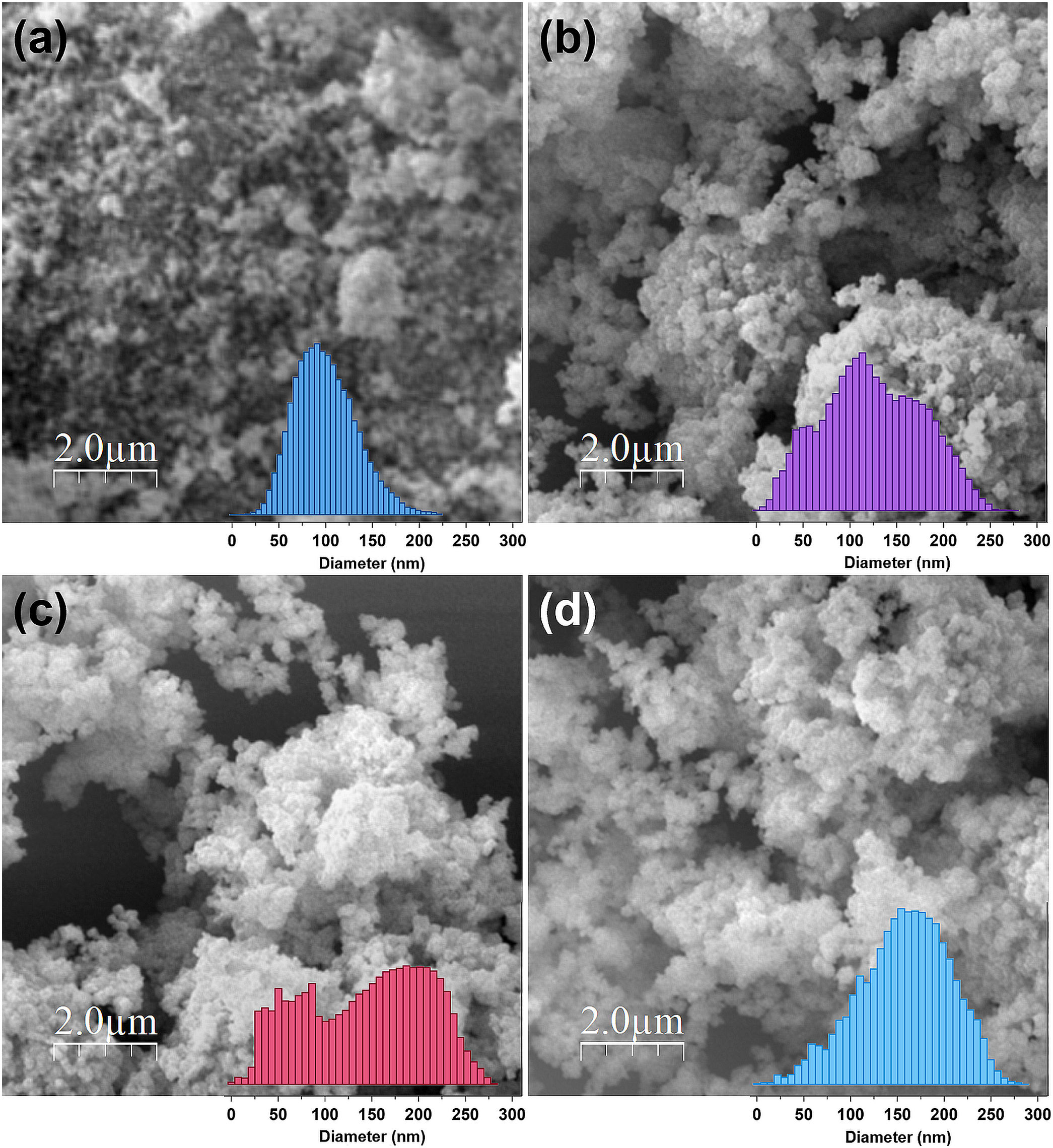
where K is a numerical factor (K=0.89) referred to as the crystallite-shape factor and B is full-width at half-maximum of the diffraction peaks in radians. For the crystallite size calculations, three of the most intense diffraction peaks corresponding to (220), (311), and (440) planes are evaluated and the data are reported along with standard deviation in Table 2. Unlike the lattice parameter (a), the value of D in general increases from 26.33nm to 48.29nm with the increase in Al3+ dopant from (x=0→1). However, an exception is also recorded as CuFe1.2Al0.8O4 nanoparticles exhibit small crystallite size (32.03nm) compared to other samples. Nonetheless, the increase in crystallite size is attributed to the sample's tendency to fuse at high temperatures. The smaller, strained unit cells demonstrate a higher rate of sintering that leads to an increase in the crystallite size [42]. Thus, XRD analyses provide significant information about the structural properties of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles.MorphologyThe SEM images of the CuFe2−xAlxO4 nanoparticles with (x=0.2, 0.6, and 1.0) are shown in Fig. 4. Pure CuFe2O4 nanoparticles show a uniform microstructure with the smallest particle size and narrow size distribution, as shown in Fig. 4a. The micrographs of doped CuFe2−xAlxO4 (0≤x≤1) nanoparticles show similar microstructure and surface morphology. The clusters of CuFe2−xAlxO4 (0≤x≤1) nanoparticles can be seen in these micrographs (Fig. 4b–d). The respective histograms obtained via image analysis tools provide detailed insight into the microstructural differences among different types of CuFe2−xAlxO4 (0≤x≤1) nanoparticles. Pure CuFe2O4 nanoparticles exhibit the smallest size and narrow size distribution compared to doped samples. The aggregate size of doped CuFe2−xAlxO4 (0≤x≤1) nanoparticles increases with the Al3+ content. However, the size distribution in CuFeAlO4 nanoparticles is narrow compared to CuFe1.8Al0.2O4 and CuFe1.4Al0.6O4 samples.
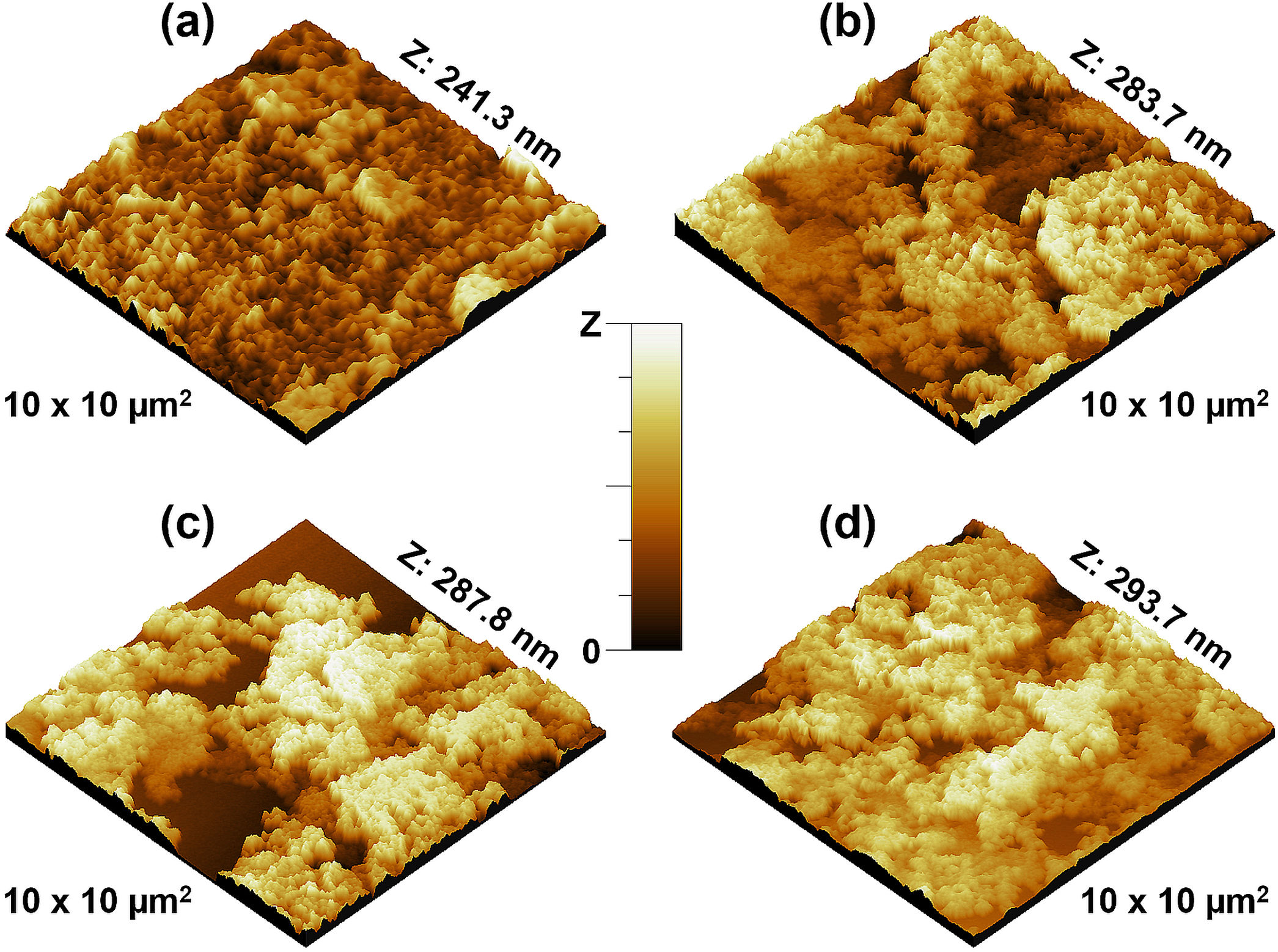
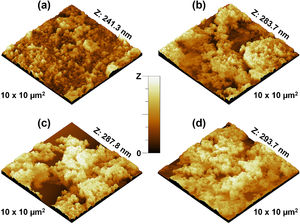
Further analysis of the micrographs and 3D imaging shows the surface topography and roughness. Fig. 5 shows 3D SEM images of the CuFe2−xAlxO4 nanoparticles with (x=0, 0.2, 0.6, and 1.0). The mean particle or aggregate size is calculated with an SEM image analysis program: ImageJ 1.52a by the National Institute of Health [21]. The mean particle sizes for CuFe2O4, CuFe1.8Al0.2O4, CuFe1.4Al0.6O4, and CuFeAlO4 nanoparticles are 104.5, 126.2, 149.0, and 160.5nm, respectively. Thus, mean particle or aggregate size increases with the increasing Al3+ content, and CuFeAlO4 nanoparticles exhibit the highest values. This is attributed to the higher degree of sintering exhibited by the strained smaller lattices containing more Al3+ ions [42].
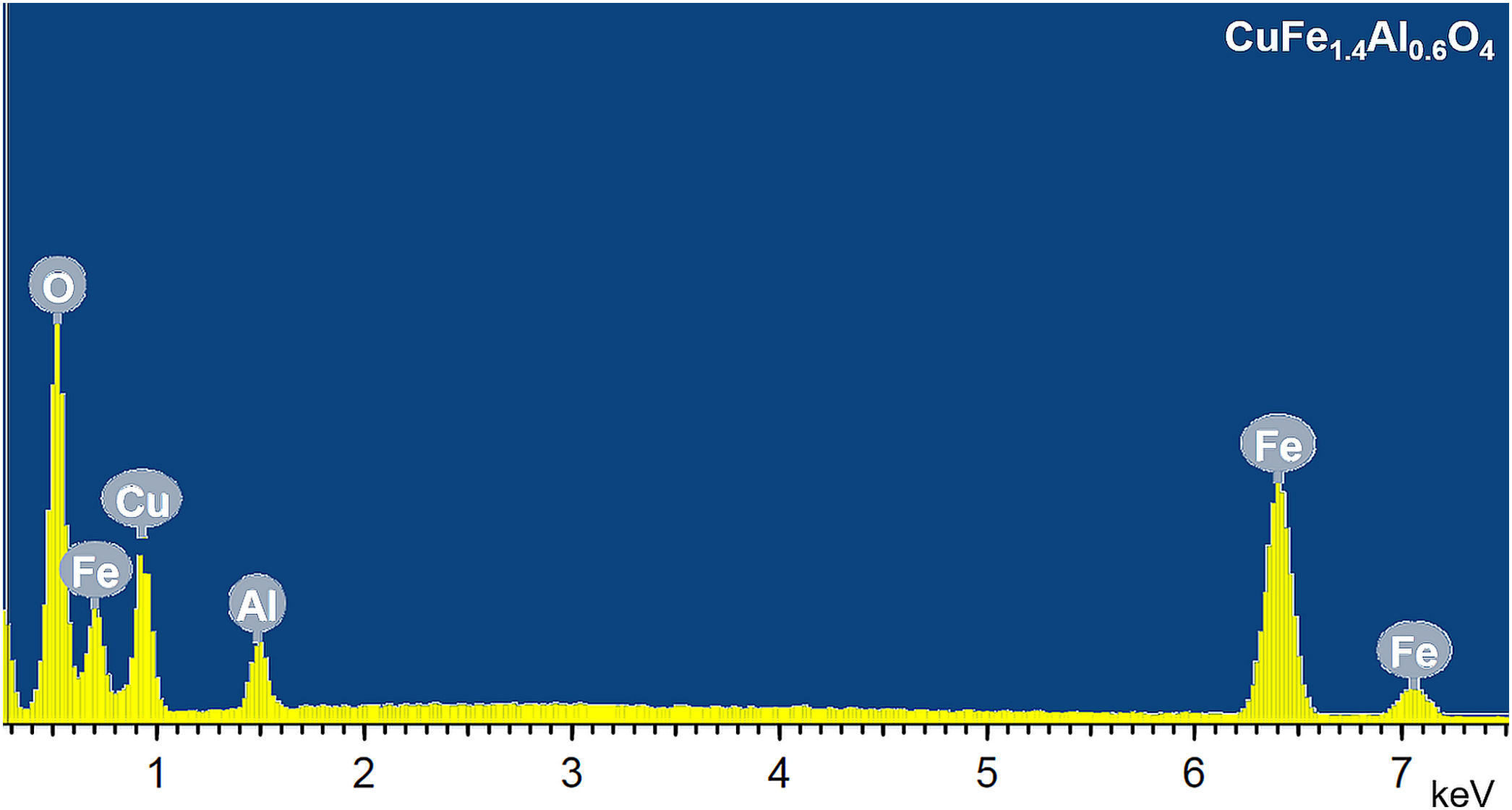
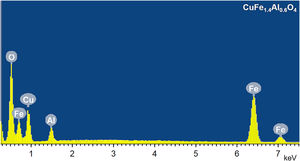
The chemical composition of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles is determined from the EDS analysis. Fig. 6 shows a typical EDS spectrum of CuFe1.4Al0.6O4 nanoparticles. The results of EDS analysis are given in Table 3, which compares theoretically calculated elemental percentage (at%) with the experimentally observed (at%) for the tested samples. The analyses reveal significant differences in the calculated and experimentally-found chemical composition of these samples. The major differences lie in the amount (at%) of oxygen that is significantly lower than the expected values, while the amount (at%) of metals (Cu, Fe, and Al) is generally higher than the theoretically calculated amount. This trend can be explained by the presence of oxygen vacancies in the annealed CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles. According to the literature [43–45], the loss of oxygen is inevitable during thermal annealing of oxides at temperatures above 700°C and the concentration of oxygen vacancies generally increases with the annealing temperature. Thus, CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles annealed at 1000°C reveal the formation of oxygen vacancies as indicated by the reduced amount (at%) of oxygen and an increased amount (at%) of metal ions (Cu2+, Fe3+, and Al3+) compared to theoretically calculated values.
The chemical composition of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles: Theoretically calculated and experimentally obtained composition (in at%) of CuFe2−xAlxO4 (0≤x≤1) nanoparticles with different Al3+-dopant content.
| Sample | Cu (at%) | Fe (at%) | Al (at%) | O (at%) | ||||
|---|---|---|---|---|---|---|---|---|
| Calc. | Exp. | Calc. | Exp. | Calc. | Exp. | Calc. | Exp. | |
| CuFe2O4 | 14.29 | 13.90 | 28.57 | 29.73 | 0.00 | 0.00 | 57.14 | 56.36 |
| CuFe1.8Al0.2O4 | 14.29 | 14.58 | 25.71 | 25.51 | 2.86 | 4.17 | 57.14 | 55.74 |
| CuFe1.4Al0.6O4 | 14.29 | 14.37 | 20.00 | 20.40 | 8.57 | 8.20 | 57.14 | 57.04 |
| CuFeAlO4 | 14.29 | 14.85 | 14.29 | 14.75 | 14.29 | 13.86 | 57.14 | 56.53 |
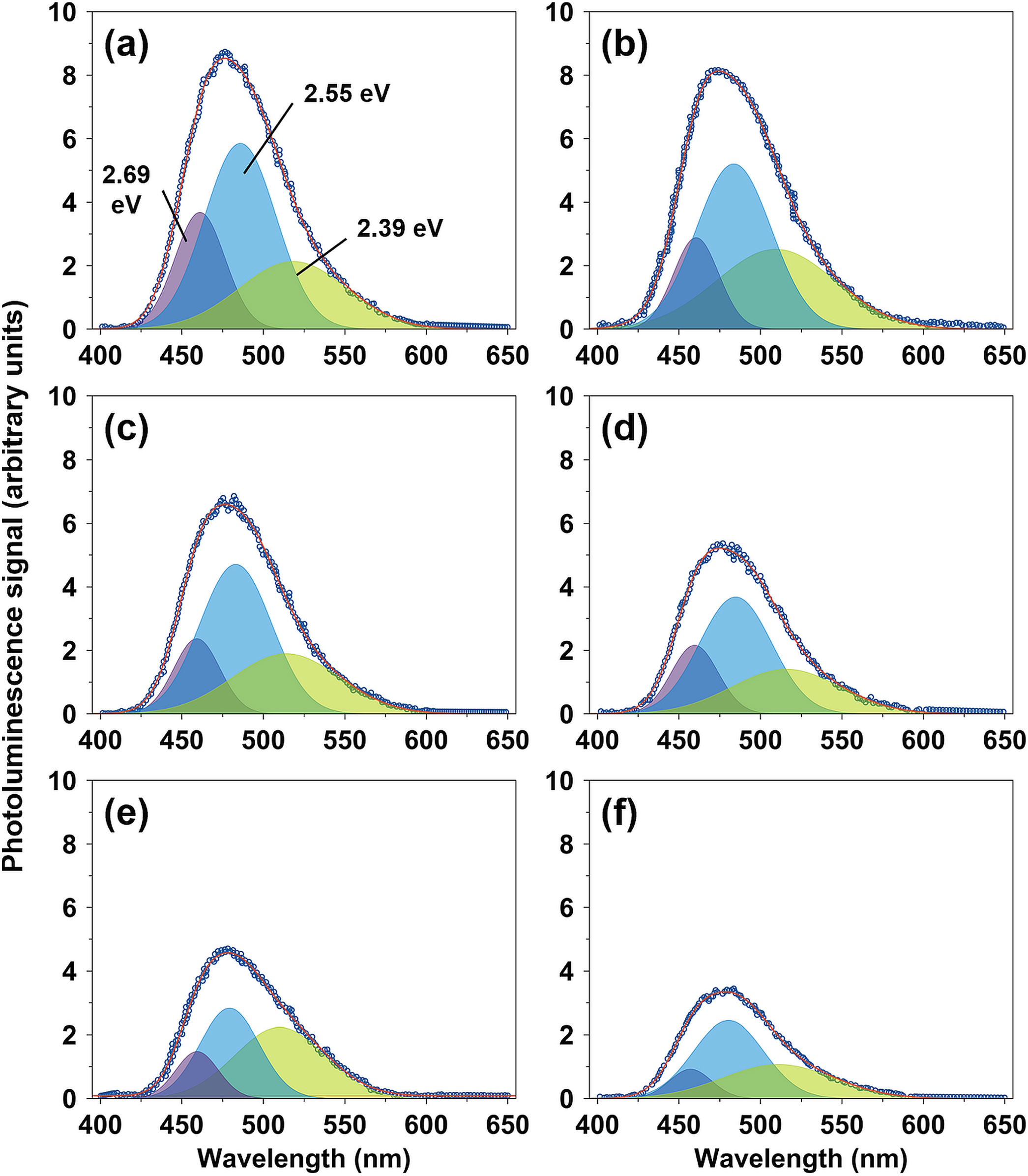
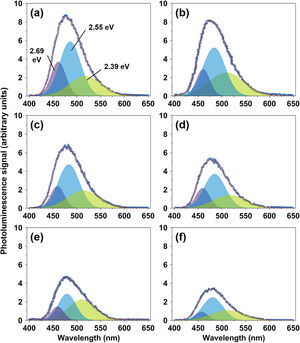
The optical properties of the spinel CuFe2−xAlxO4 (0≤x≤1) nanoparticles are studied by examining their photoluminescence emissions at room temperature. The emission spectra reveal significant information about the excitation, immigration, and recombination of the photogenerated electron (e−) and hole (h+) pairs in a sample [46]. Fig. 7 shows the PL spectra of different CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles excited at 254nm. The PL emission spectra are deconvoluted into multiple Gaussian components to obtain the best-fit approximation of the experimental data, which indicates the surface defects and electronic structure of the sample. CuFe2O4 nanoparticles exhibit three major PL emissions: violet emission at 461.1nm (2.69eV), the most intense blue emissions at 485.9nm (2.55eV), and the least intense green emission at 518.2nm (2.39eV).
CuFe2O4 is inherently a low bandgap semiconductor. However, the bandgap energy depends on different factors such as the method of preparation, experimental conditions, annealing temperature and time, microstructure, and chemical composition that is why different values of the bandgap energy ranging from 1.32–3.09eV have been reported in the literature for pure CuFe2O4 nanostructures [1,47–50]. Considering the structure and chemical composition of the annealed CuFe2O4 nanoparticles, the green emission (2.39eV) is attributed to the band to band transitions. The violet and blue emissions are assigned to the luminescence centers that are formed by band edge free excitons, intra-bandgap lattice defects such as oxygen vacancies, and their aggregates [51,52].
Raja et al. [51] observed the emission bands in the 410–460nm region due to the intra-bandgap defects such as oxygen vacancies. Thus, violet emission (2.69eV) is attributed to the lattice defects resulting from oxygen vacancies on the surface of CuFe2O4 nanoparticles. The blue emission (2.55eV) is assigned to the transitions between the defect sites and valence band or between the conduction band and defect sites. Paramasivan et al. [53] also reported sharp emission bands (at approximately 460, 490, 550, and 590nm) for hydrothermally-prepared CuFe2O4 nanoparticles annealed at 400–700°C. Thus, these results are well in agreement with the literature [53–55], albeit there are peculiar differences in the emission spectrum of CuFe2O4 nanoparticles.
The emission wavelengths of CuFe2−xAlxO4 nanoparticles with (x=0.4, 0.6, and 1.0) do not change greatly by Al3+ doping, as shown in Fig. 7b–f. However, the luminescence signal is significantly reduced with the increasing Al3+ content. EDS results have already shown that like pure CuFe2O4, CuFe2−xAlxO4 (0≤x≤1) nanoparticles are oxygen deficient. The decrease in luminescence signal of Al3+ doped CuFe2−xAlxO4 (0≤x≤1) nanoparticles can be attributed to different factors: (a) increase in crystallite size, and (b) the lower rate of recombination of photoexcited (e−) and (h+). Other factors like the presence of impurities or secondary phases are not considered because of their absence or negligible presence in the XRD patterns of CuFe2−xAlxO4 (0≤x≤1) nanoparticles.
Considering the effect of crystallite size, many researchers suggest that smaller particle size increases the quantum efficiency and should improve the luminescence signal due to the enhanced quantum confinement effect [56–58]. For example, Hjiri et al. [59] reveal that the differences in the crystallite size of NiFe2O4 nanoparticles influence the luminescence signal, i.e. an increase in crystallite size decreases the signal. However, Kombaiah et al. [60] argue that the smaller CuFe2O4 particle size means an increased number of dangling bonds combining with the oxygen vacancies, thereby creating non-radiative defects at the surface of nanoparticles and reducing the luminescence signal. The idea is supported by other researchers [49,61]. Therefore, a decrease in the luminescence signal of CuFe2−xAlxO4 (0≤x≤1) nanoparticles may not be due to the increase in their crystallite size compared to pure CuFe2O4. An evidence, in this regard, is presented by the CuFe1.2Al0.8O4 nanoparticles with D=32.0nm (Fig. 7e) that shows lower photoluminescent intensity despite its smaller size compared to CuFe1.4Al0.6O4 nanoparticles with D=43.9nm (Fig. 7d).
On the other hand, it is well known that photoluminescence results from the recombination of photogenerated charge carriers (e−/h+), and slower radiative recombination reduces the emission [62]. Therefore, we believe that lowering of the luminescence signal should be ascribed to the slower (e−/h+) recombination rate [13,46]; that is the direct recombination of excited electrons in Fe3+ conduction band with holes is prevented by Al3+ doping resulting in a lower signal. Al3+ acts as a surface trap for (e−) and delays their recombination with (h+); thereby reducing the luminescence signal. The dopant ions are known to improve the photocatalytic properties of various oxides by diminishing the rate of recombination and expanding the transition time of photoexcited charge carriers [62,63]. Therefore, the higher ratio of Al3+ in CuFe2−xAlxO4 (0≤x≤1) nanoparticles ensures greater charge separation and availability, which is essential for surface redox reactions. Consequently, CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles have great potential for photocatalytic applications.
ConclusionsThis study presents solid-state synthesis of CuFe2−xAlxO4 (0≤x≤1.0) nanoparticles using HEBM technique. CuFe2−xAlxO4 (0≤x≤1) nanoparticles synthesized at room temperature exhibit semicrystalline spinel structure, which transforms into single-phase cubic spinel lattice by annealing at 1000°C. As the dopant (Al3+) concentration increases, the annealed nanoparticles show an increase in crystallite size and particle diameter with narrow size distribution and reduced surface roughness. The smaller unit cells of Al3+-doped nanoparticles show a higher degree of fusion and sintering tendency compared to pure CuFe2O4 nanoparticles. The photoluminescence spectra of CuFe2−xAlxO4 (0≤x≤1) nanoparticles reveal a decrease in the emission intensity with the increasing Al3+ content, which indicates the ability of dopant ions to slow down the recombination of (e−) and (h+). CuFe2−xAlxO4 (0≤x≤1) nanoparticles present greater charge availability for surface redox reactions that could potentially enhance their photocatalytic properties.
Authors’ contributionsConceptualization, F.A.A. and A.A.; methodology, F.A.A.; validation, F.A.A.; formal analysis, F.A.A. and M.A.; investigation, M.A. and F.A.; data curation, M.A., U.Y.Q., and F.A.; writing–original draft preparation, A.A.; writing–review and editing, U.Y.Q. and A.A.; visualization, U.Y.Q. and A.A.; project administration, F.A.A. and A.A.; funding acquisition, F.A.A. and A.A. All authors have read and agreed to the final version of the manuscript.
FundingThis research was funded by King AbdulAziz City for Science and Technology (KACST), grant number 13-NAN467-04.
Conflicts of interestThe authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
The authors would like to acknowledge the support provided by King AbdulAziz City for Science and Technology (KACST) through the Science and Technology Unit at King Fahd University of Petroleum and Minerals (KFUPM) for this work through project No.13-NAN467-04 as part of the National Science, Technology, and Innovation Plan. F.A.A. and A.A. are grateful to S.B. Waje and M.A. Atieh for their help in the characterization of samples and discussions of the outcomes.