With the aim to contrast existing historical documentation with information provided by the own materials, an archeometric study of a representative set of tiles from the Monastery of El Escorial was undertaken. The ensemble, composed of 19 ceramic fragments, covers examples from the 16th and 17th century, either from Talavera or Toledo provenances, and was selected according to stylistic criteria. The goals of the study consisted in determining the average chemical composition of the ceramic body, its textural and microstructural characteristics, the crystalline phases to establish firing temperatures, also in studying these aspects in the enamel or glaze layer, as well as in identifying the chromophores for the coloring of the decorations. Conventional techniques such as binocular magnifying glass, XRF spectrometry, petrographic observation by thin-section, XRD, FESEM with EDS microanalysis and visible spectrophotometry were used. Resulting data indicated the use of calcareous clays for the body (22.0–25.5wt.% CaO), the presence of neoformed phases such as gehlenite, diopside and anortite which suggest a firing temperature between 950 and 1050°C approximately, lead-based glazes (∼25.0wt.% PbO on average) with SnO2-opacifier (∼57.0wt.% on average) and Co2+, Cu2+, Fe3+-ions and lead antimoniate for blue, green, brown and yellow decorative colors, respectively.
Con el objetivo de contrastar la documentación histórica existente con la información proporcionada por los propios materiales se realizó un estudio arqueométrico de un conjunto representativo de azulejos procedentes del Monasterio de El Escorial. El conjunto, compuesto por 19 fragmentos cerámicos, cubre ejemplares de los siglos xvi y xvii, tanto de posible procedencia talaverana como toledana, y se seleccionó según criterios estilísticos. Los objetivos del estudio consistieron en determinar la composición química promedio del cuerpo cerámico, sus características texturales y microestructurales, las fases cristalinas para establecer las temperaturas de cocción, también en estudiar estos aspectos en la capa de esmalte o vidriado, así como en identificar los cromóforos de la coloración de las decoraciones. Se utilizaron técnicas convencionales como lupa binocular, espectrometría de FRX, observación petrográfica mediante lámina delgada, DRX, MEBEC con microanálisis EDS y espectrofotometría visible. Los datos resultantes indicaron el uso de arcillas calcáreas para el cuerpo cerámico (22,0-25,5% en peso de CaO), la presencia de fases neoformadas como gehlenita, diópsido y anortita que sugieren una temperatura de cocción de entre 950 y 1.050°C, aproximadamente, esmaltes de base plomo (promedio ∼25,0% en peso de PbO) con SnO2 (promedio ∼57,0% en peso) como opacificante y presencia de iones Co2+, Cu2+, Fe3+ y antimonio de plomo para los colores azul, verde, marrón y amarillo, respectivamente, de las decoraciones.
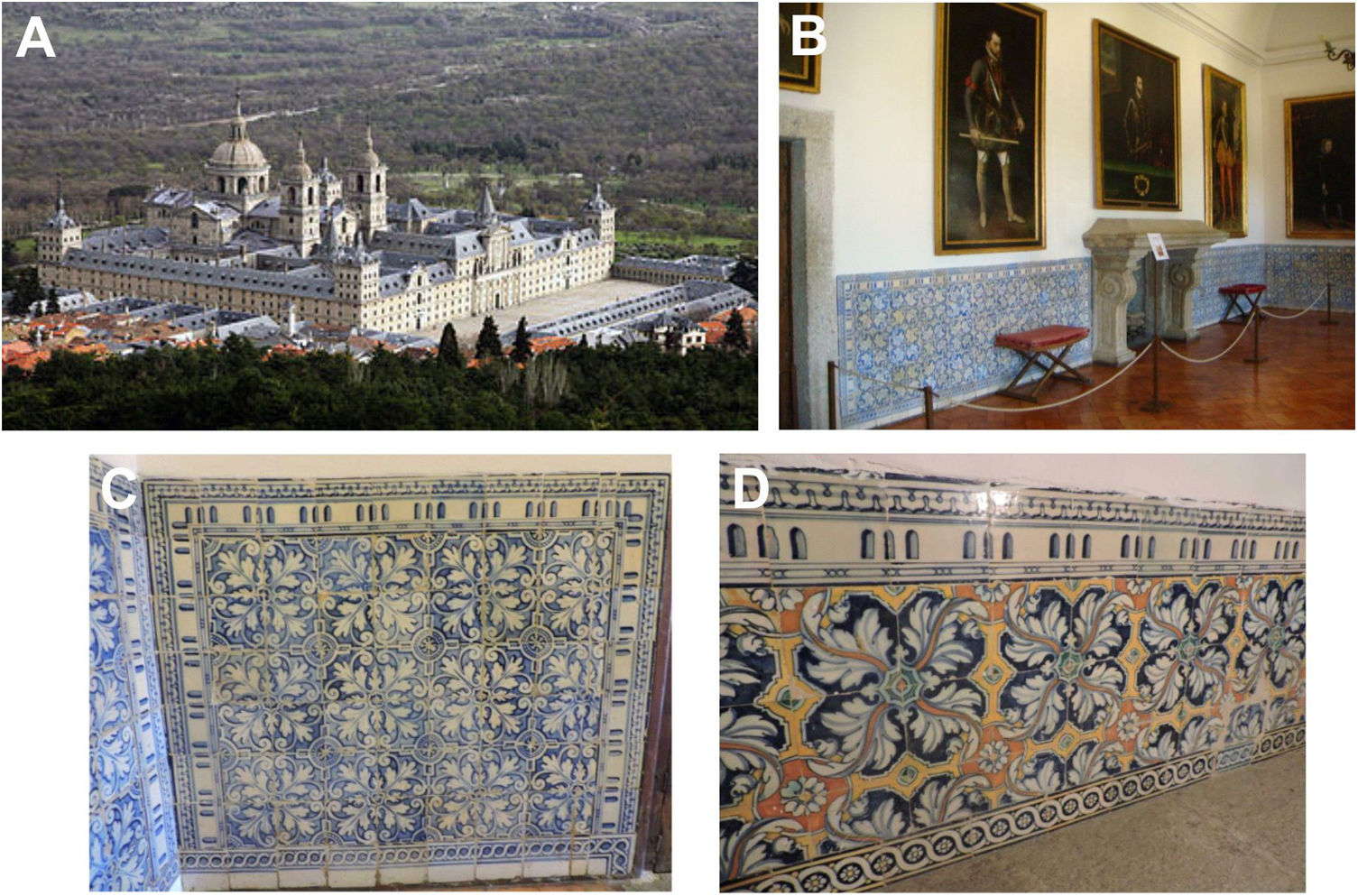
Since the foundation of the Monastery of San Lorenzo de El Escorial by the King Philip II in the 16th century at the foot of the Madrid mountains (Fig. 1A), many of its rooms, corridors and stairs were covered with tile baseboards that were ordered to both Talavera and Toledo workshops. Consequently, apart from flat and brush-painted tiles, there are also many examples of paviments made according to the old technique of border tiles of hispano-moresque tradition that was still practised in Toledo in the second half of the 16th century, although with a Renaissance repertoire. This pre-firing technique consists of the use of a mold, which is pressed in the plastic/viscous state of the ceramic surface prior to the decoration process. The mold leaves a relief which is used to apply the colors of the decorative motif thereby preventing pigments mixing [1]. According to the documentation found, either in the archives of the Royal Monastery of El Escorial or in the archive of Simancas (Spain), on both order contracts and payments upon receipt of the tiles, the first contract dates from 1570 and the last payments of the building phase from 1595. However, at the end of the 17th century and as a consequence of the serious fire that devastated the monastery in 1671, the existing historical documentation indicates that the tile replacement was carried out almost exclusively by Talavera workshops, due to the Toledo industry was then in a situation of crisis and decline [2].
The tile baseboards of El Escorial, usually of approximately 60cm in height, are located in the main dependencies of the Habsburg Palace: the rooms of the King Philip II, his daughter Isabel Clara Eugenia, the Audience room, the Gallery or Anteroom and so on (Fig. 2B), as well as in the main dependencies of the friars living in the monastery, including the priory cell. Most of the tiles are flat and brush-painted tiles made in the Italian style implanted by Francisco Pisano at the beginning of the 16th century [3–5]. The baseboards are conceived as a continuous panel based on the design of four tiles repeated indefinitely. The design is composed of four leaves of acanthus which start from an eight-pointed star. The panels are framed by a string of pearls with two-sided sills at the joints decorated with frets. This decorative scheme follows the Flemish esthetic novelties spread during the reign of the King Philip II. The majority of the tiles are hand brush-painted with cobalt blue color (Fig. 1C), even though some panels located in the baseboard of the stairs, which communicate the Habsburg royal rooms with the basement, also exhibit polychrome decorated tiles (Fig. 1D). The baseboards of El Escorial became well-known in a few years and they will be soon imitated in other contemporary monasteries, churches and civil noble houses, as in the case of the Incarnation Monastery in Madrid. The tiles not only served an ornamental function but were also a durable and cleanliness solution to isolate moisture from the walls, making them more showiness.
Despite El Escorial tiles are precious and well-known ceramic products from Talavera and Toledo workshops they still remain a quite unknown ceramic material from both the technological and productive point of view, since no archaeometric studies have been undertaken to date in which historical documentation was confronted and interrelated with technological information provided by the own ceramic materials. Within the general methodological framework developed by the authors in the study of other historical ceramic materials [6], an archaeometric study of a representative set of tiles from different places of the Royal Monastery of El Escorial (Madrid, Spain) is presented in this paper. The goals of the study consisted in determining the average chemical composition of the tiles ceramic body, their textural and microstructural characteristics, the presence of a variety of grains having different crystalline phases and also neoformed phases to establish their possible firing temperature, also in studying these same aspects in the enamel or glaze layer, as well as in identifying the chemical species (chromophores) responsible for the coloring of the decorations.
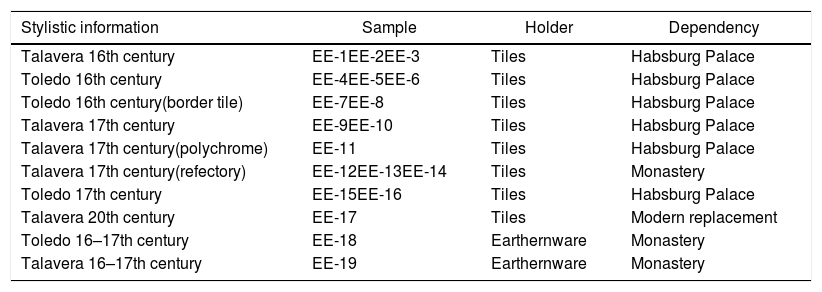
ExperimentalSamples selectedThe representative set of samples selected for this study is composed of 19 ceramic fragments and encompasses examples from the 16th and 17th century, either from Talavera or Toledo provenances, and was mainly selected according to stylistic and decorative criteria. It should be highlighted that some times it is difficult to make a stylistic attribution to one or another center since a great part of designs of Talaveran origin were assimilated by craftsmen from Toledo. Most of the fragments have been found in recent works of renovation and reform and come from different dependencies. The ensemble of 19 ceramic samples consists of 17 tiles and 2 earthernware shreds. Descriptive information of all the samples is given in Table 1 and Fig. 2 displays a photograph of each of them. Most of the tile samples come from dependencies of the Habsburg Palace (samples EE-1/EE-11 and EE-15/EE-16), while others (samples EE-12 to EE-14) come from the refectory of the monastery. Within the tile samples there are also border tiles of the hispano-moresque tradition made in Toledo (samples EE-7 and EE-8), as well as polychrome ones (sample EE-11). An additional modern tile sample made in Talavera in the 20th century (sample EE-17) used in replacement works was also included for comparison with ancient tiles. Finally and also for comparison purposes, two earthernware shreds (samples EE-18 and EE-19) from tablewares used by the friars living in the monastery were also selected for the study.
Descriptive information of the samples selected for this study.
| Stylistic information | Sample | Holder | Dependency |
|---|---|---|---|
| Talavera 16th century | EE-1EE-2EE-3 | Tiles | Habsburg Palace |
| Toledo 16th century | EE-4EE-5EE-6 | Tiles | Habsburg Palace |
| Toledo 16th century(border tile) | EE-7EE-8 | Tiles | Habsburg Palace |
| Talavera 17th century | EE-9EE-10 | Tiles | Habsburg Palace |
| Talavera 17th century(polychrome) | EE-11 | Tiles | Habsburg Palace |
| Talavera 17th century(refectory) | EE-12EE-13EE-14 | Tiles | Monastery |
| Toledo 17th century | EE-15EE-16 | Tiles | Habsburg Palace |
| Talavera 20th century | EE-17 | Tiles | Modern replacement |
| Toledo 16–17th century | EE-18 | Earthernware | Monastery |
| Talavera 16–17th century | EE-19 | Earthernware | Monastery |
Macroscopic observations were accomplished with a Motic SMZ 168 binocular magnifying glass, fitted with a Moticam 2500 digital camera. The elemental chemical composition of the samples was determined by X-ray fluorescence (XRF) spectrometry with a PANalytical Axios wavelength dispersion X-ray spectrometer, equipped with a tube of rhodium of 4kW and 60kV, using pressed samples. Analytical determinations were undertaken through the standard-less analytical software IQ+(PANalytical) based on fundamental patterns of synthetic oxides and natural minerals. The sample was analyzed in powder (less than 30μm in diameter) obtained from the ceramic fragment ground in a mortar and pestle of agate with the external sides of the glazed layer removed by polishing. Chemical data obtained by XRF were submitted to a multivariate statistical technique: principal components analysis [7], using the Systat v. 10.2 package. Oxide concentrations were transformed into logarithmic base 10 values to compensate for large magnitudes of difference between major and minor elements [8].
Petrographic observations of thin-sections were carried out with a Kyowa Bio Pol 2 transmitted light optical microscope fitted with a polarization device. Thin-sections were prepared from a section obtained with a diamond cutting disk from the ceramic fragments, polished with silicon carbide sheets of different grain sizes up to approximately 30μm in thickness. Micrographs were made with the digital camera mentioned above. X-ray diffraction (XRD) analyses were carried out with a PANalytical X’Pert MPD diffractometer, using Kα of copper radiation (1.54056Å) under set conditions of 45kV of voltage and 40mA of intensity. Diffractograms were recorded between 2θ=5–60°, with an angle step of 0.03° and 2s of time per step. The powder method (less than 30μm in diameter) was used. It was obtained in the same way as in XRF. Field emission scanning electron microscopy (FESEM) observations were undertaken with a Hitachi S-4800 cold cathode electron microscope under acceleration voltages of 15kV on samples coated with carbon as conductive medium, using a JEOL JEE4b spputter. The energy dispersive X-ray (EDS) microanalysis system coupled to the electron microscope was an Oxford X-Max of 20mm2 with resolution of 125eV (Mg Kα). For microanalyses of the base enamels EDS acquisitions were always made with the same window size (areas of approximately 20μm2).
The identification of the chemical species (chromophores) responsible for the coloring of the decorations was carried out by means of visible spectrophotometry with an Ocean Optics model HR 4000 CG equipment of optical fiber. Spectra were recorded in the 200–1000nm wavelength range under the reflectance mode on a surface area of 1mm in diameter, since it was not possible to prepare mirror-polished plane-parallel samples for recording spectra under the absorbance mode.
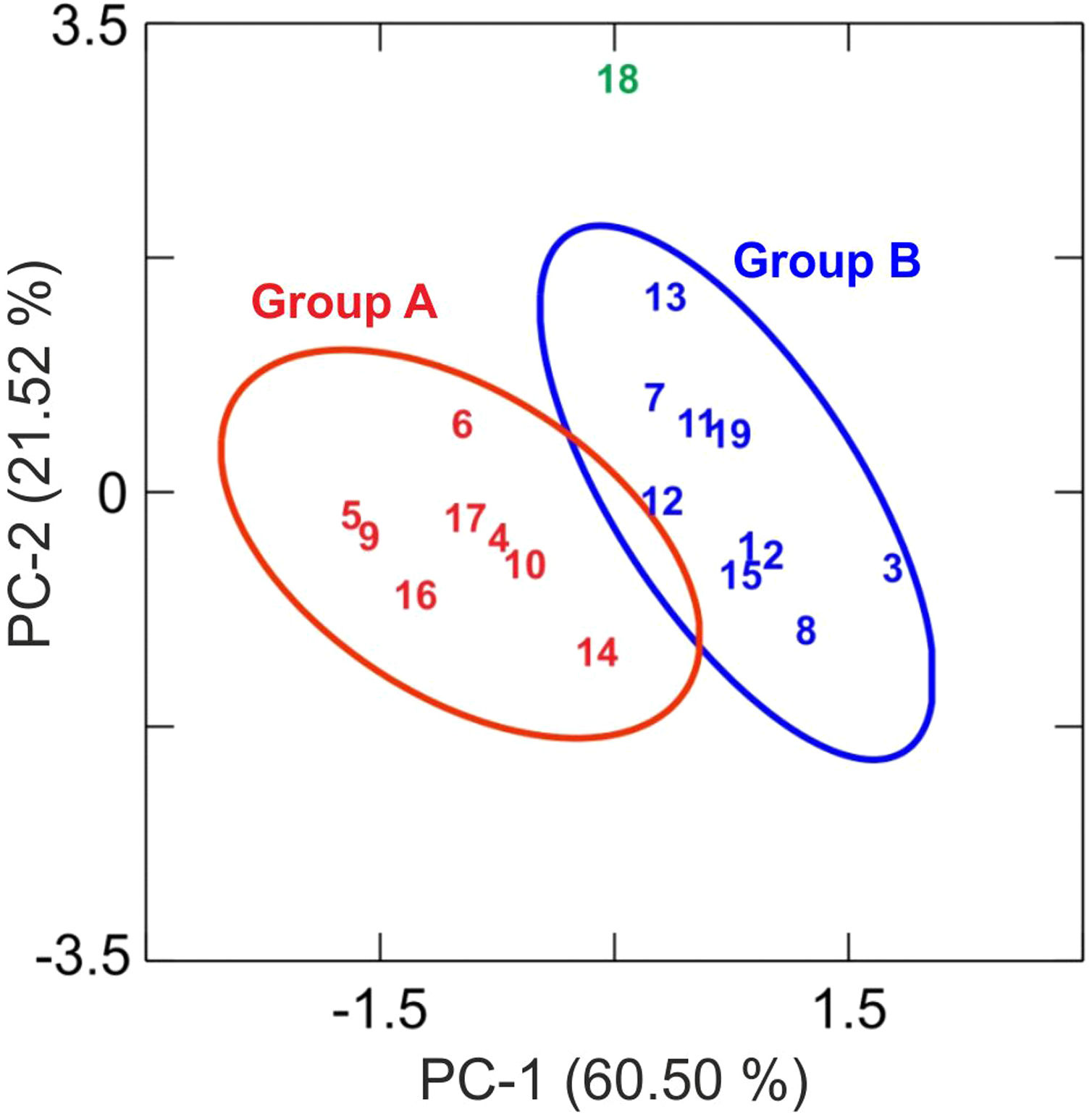
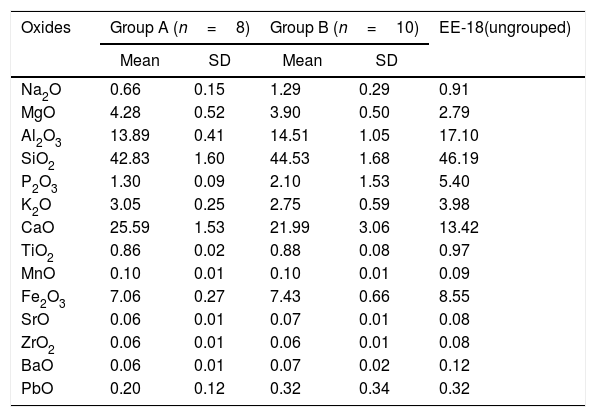
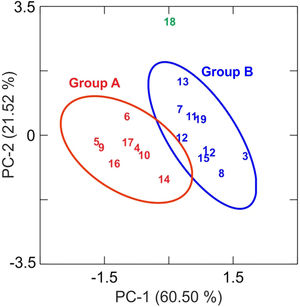
Results and discussionChemical analysis by XRFThe results of chemical analysis carried out on the ceramic body of the fragments showed that all the samples are calcareous ceramics since the concentration of calcium oxide (CaO) is higher than 5.00wt.%. A plot of the first two components derived from a principal components analysis undertaken from a variance-covariance matrix, which is displayed in Fig. 3, shows that the set of samples can be assigned to two groups of different chemical composition plus an ungrouped sample (EE-18). The first two components summarized 82.02% of the total variation in the data. The first one scores 60.50% of variation and the second one 21.52%. The concentration of sodium, calcium and potassium oxides contributes the most to the separation of the two groups in component one, while component two is mainly contributed by aluminum, potassium, calcium and magnesium oxides. Mean and standard deviation values for both groups are given in Table 2.
Means and standard deviations (SD) of the two groups derived from principal components analysis of XRF data (wt.%) obtained in the ceramic body. The ungrouped sample EE-18 is also displayed.
| Oxides | Group A (n=8) | Group B (n=10) | EE-18(ungrouped) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Na2O | 0.66 | 0.15 | 1.29 | 0.29 | 0.91 |
| MgO | 4.28 | 0.52 | 3.90 | 0.50 | 2.79 |
| Al2O3 | 13.89 | 0.41 | 14.51 | 1.05 | 17.10 |
| SiO2 | 42.83 | 1.60 | 44.53 | 1.68 | 46.19 |
| P2O3 | 1.30 | 0.09 | 2.10 | 1.53 | 5.40 |
| K2O | 3.05 | 0.25 | 2.75 | 0.59 | 3.98 |
| CaO | 25.59 | 1.53 | 21.99 | 3.06 | 13.42 |
| TiO2 | 0.86 | 0.02 | 0.88 | 0.08 | 0.97 |
| MnO | 0.10 | 0.01 | 0.10 | 0.01 | 0.09 |
| Fe2O3 | 7.06 | 0.27 | 7.43 | 0.66 | 8.55 |
| SrO | 0.06 | 0.01 | 0.07 | 0.01 | 0.08 |
| ZrO2 | 0.06 | 0.01 | 0.06 | 0.01 | 0.08 |
| BaO | 0.06 | 0.01 | 0.07 | 0.02 | 0.12 |
| PbO | 0.20 | 0.12 | 0.32 | 0.34 | 0.32 |
The group A is composed of the following eight tile samples: three tiles from Toledo 16th century (EE-4, EE-5 and EE-6), three tiles from Talavera 17th century (EE-9, EE-10 and EE-14), one tile from Toledo 17th (EE-16), and another one from Talavera 20th century (EE-17). It is characterized by having a high concentration of CaO (25.50wt.%), while contents of SiO2 and Al2O3 are 42.83 and 13.89wt.%, respectively. The weight percentage of Fe2O3 is around 7.00 and K2O is in the range of 3.00wt.%. The concentration of MgO (4.28wt.%) is relatively high and Na2O (0.66wt.%) is slightly low. This group is exclusively formed by tiles and, from the point of view of provenance and chronology, quite diverse since it is composed of either tiles from Talavera or Toledo, and either from 16th or 17th century. It should be highlighted that the Talavera tile from 20th century (EE-17) lies in this group, which suggests that production in Talavera during the 20th century follows the ancient traditions since it does not show notable differences in terms of chemical composition of raw materials.
The group B is composed of ten samples, nine tiles and one earthernware: three tiles from Talavera 16th century (EE-1, EE-2 and EE-3), the two border tiles from Toledo 16th century (EE-7 and EE-8), three tiles from Talavera 17th century including the polychrome example (EE-11, EE-12 and EE-13), one tile from Toledo 17th century (EE-15), and the earthernware sample from Talavera (EE-19). It is characterized by having a little bit lower concentration of CaO (∼22.00wt.%) in comparison with the Group A, while contents of SiO2 (44.53wt.%) and Al2O3 (14.51wt %) are slightly higher. The weight percentages of Fe2O3 (7.43), K2O (2.75) and MgO (3.90) are almost the same, even though in this case the concentration of Na2O (1.29wt.%) is practically double. Except for, surprisingly, the two 16th century border tile samples and another tile from 17th century (this latter probably misclassified), from Toledo in both cases, the remaining samples belonging to this group are all from Talavera, including the earthernware sample, which suggests that the Group B is mostly formed by productions from Talavera. It also indicates that in Talavera either the tiles or the earthernware were made with similar raw materials. An additional argument that may support this suggestion is that compositional data published on Talavera earthernware of the same chronology [9] are closer to this Group B than with the Group A.
The earthernware sample from Toledo (EE-18) are completely outside of the two aforementioned groups as can be seen in Tab. 2, which means that this ceramic material is nothing to do with the rest of materials here analyzed. This suggests that in Toledo the earthernware could be made from a distinct raw materials than that used for tiles. On the other hand, the statistical analysis of XRF chemical data seems that clearly separate 16th century tile productions from Talavera and that from Toledo. However, data obtained also indicate a more complex situation for 17th century tiles, which could be due to, on the one hand, the replacement of tiles in this century was a more complicated process than that shown by the historical documents or, on the other hand, that nowaday panels can be composed of multiple replacements of which there is no documentation.
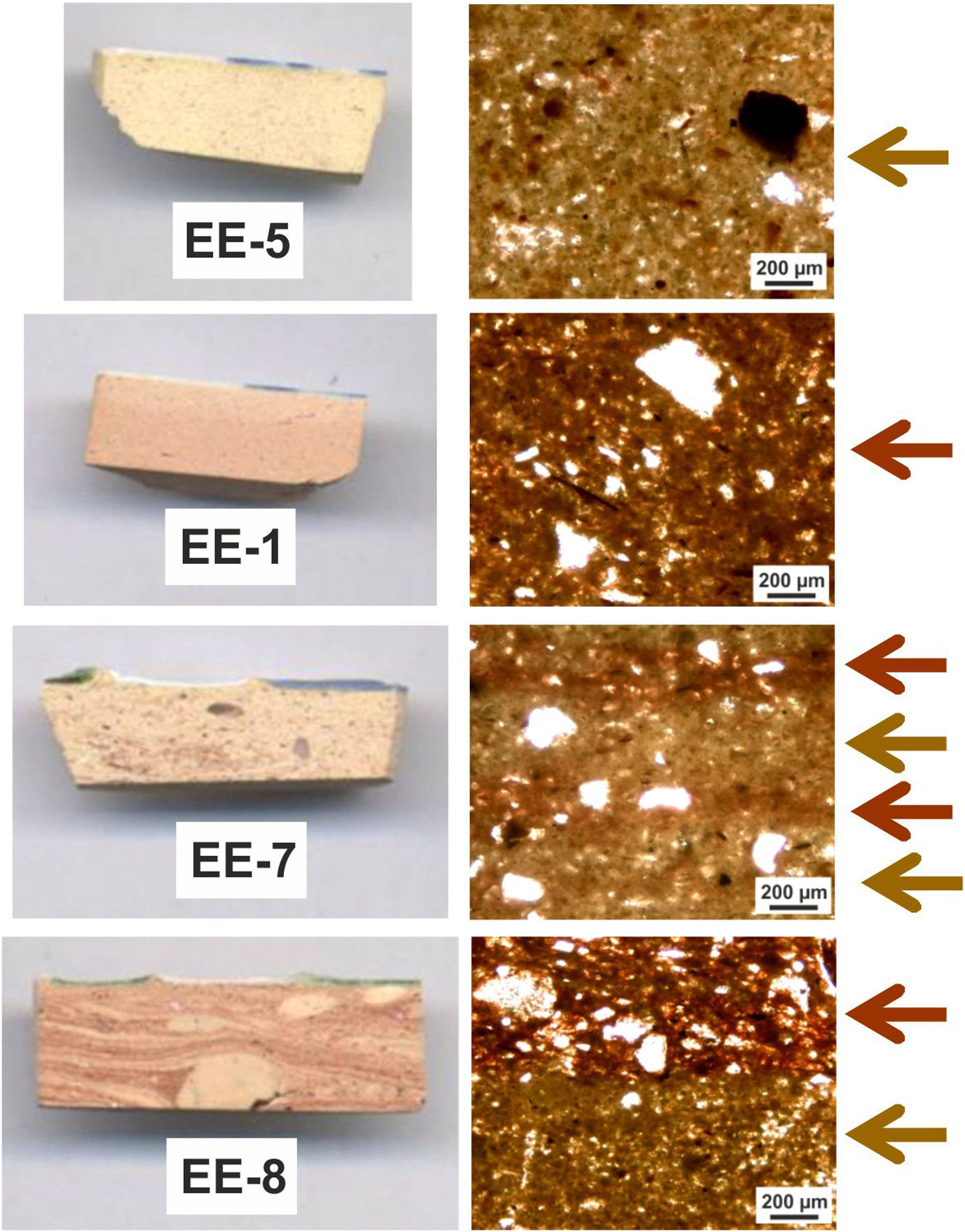
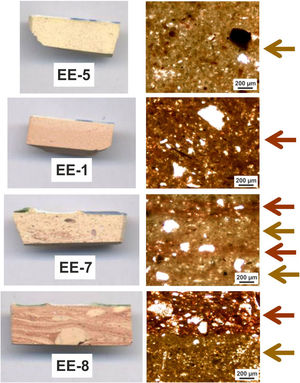
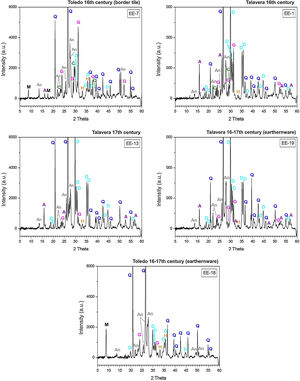
Macroscopic sections and petrographic observation of thin-sectionsSections of compositional Group A show a homogeneous and relatively well sorted cream colored fabric in which a good shrunken degree and a general grain size lower than 200μm can be appreciated by thin-section observation. Higher size opaque nodules of iron oxides are also present (Fig. 4, sample EE-5). Sections of Group B also show a homogeneous and relatively well sorted fabric but more brown-reddish in color. General grain size is similar to the former, even though in this case some larger grains of quartz, of up to 400μm, are observed (Fig. 4, sample EE-1). On the contrary, of the whole set, two samples showed a distinct pattern: border tiles EE-7 and EE-8 (Fig. 4). Both samples show a probable mixing of clay raw materials. In the case of the sample EE-7, the mixing is inferred by the distinct colors and texture of the clay which appeared in layers of different thickness in thin-section observation, while in the sample EE-8 the mixing is better appreciated in the section. In this latter sample the thin-section shows layers of larger thickness (pointed out by colored arrows in Fig. 4). The color of clay layers is quite similar to those shown by samples of compositional groups A and B as can be appreciated in Fig. 4. Probably for this reason, the mixing of clay raw materials, the border tiles were assigned to compositional Group B even though this group seems to be mainly formed by productions from Talavera.
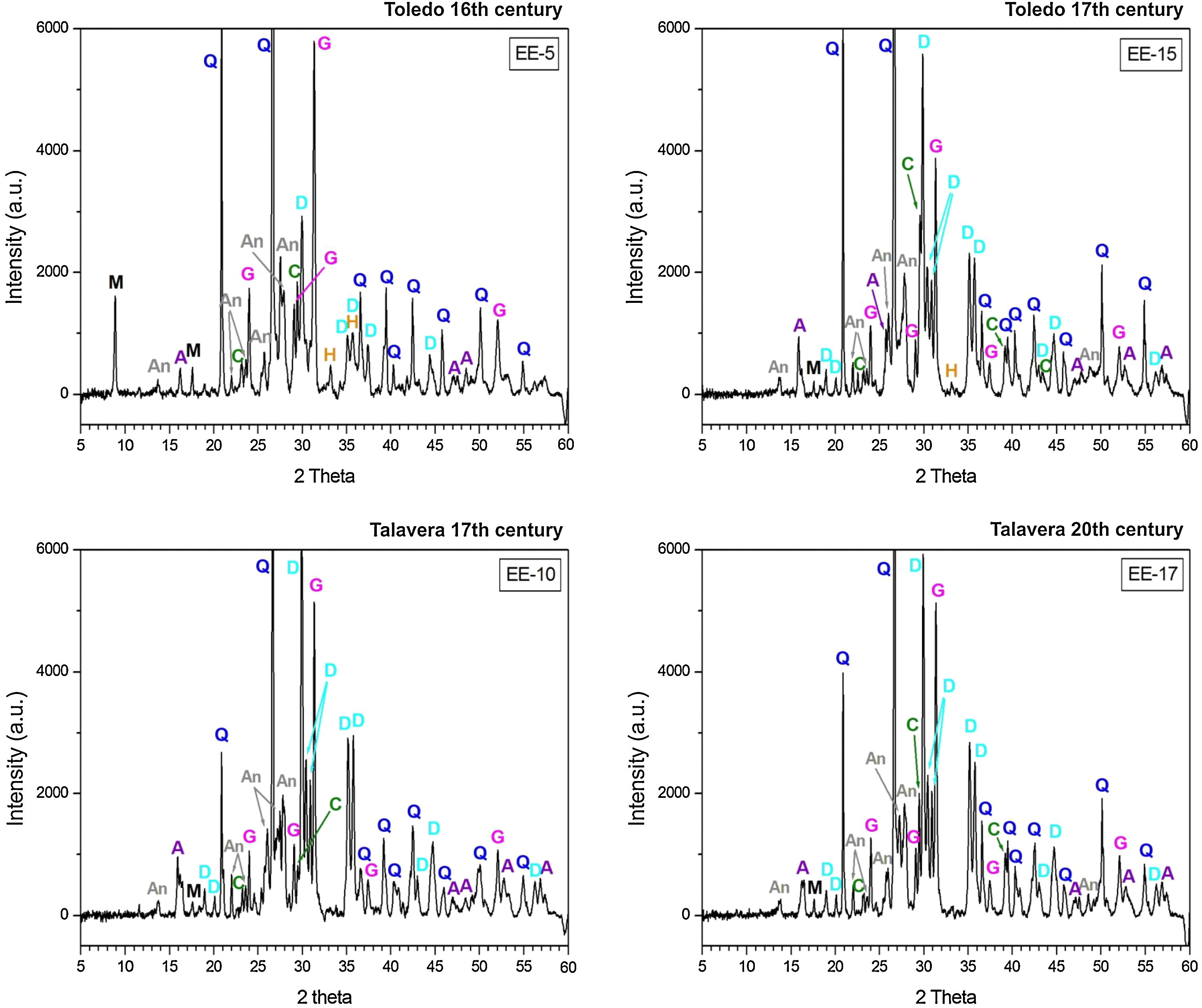
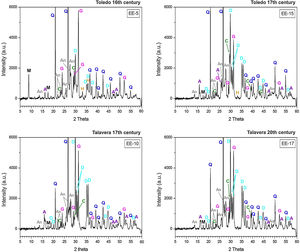
Characterization of crystalline phases by XRD and estimation of firing temperatureX-ray diffractograms derived from samples of Group A are displayed in Fig. 5. All of them show thermically neoformed phases and absence of mica and phyllosilicates as a result of firing temperature, except the sample EE-5 in which reflections of mica and phyllosilicates are still present. Such neoformed phases are the phases commonly developed from calcination of highly calcareous ceramic products and mainly consist of gehlenite, diopside and anortite. Gehlenite is an alumino-silicate of calcium which is formed from the CaO reaction produced by decomposition of calcium carbonates such as calcite with the alumino-silicates of clay at temperatures nearly 850–900°C. Diopside is a calcium and magnesium silicate from the family of pyroxenes which is formed from the CaO and MgO reaction also produced by decomposition of carbonates with the alumino-silicates of clay at temperatures of about 900–950°C. The formation of this silicate is undoubtedly due to relatively high content of MgO in samples of Group A (4.28wt.% on average, Table 2). Anortite is likewise an alumino-silicate of calcium formed from the reaction of CaO from carbonates with the alumino-silicates of clay at temperatures higher than 1000°C [10,11]. Taking into account the presence of these three thermo-formed phases along with the absence of reflections of phyllosilicates, which on the whole experience a full dehydroxylation above 850–900°C [12], an equivalent firing temperature between 950 and 1050°C can be roughly estimated. In the case of the sample EE-5 the equivalent firing temperature could have been lower, around 950°C, since it still shows reflections of mica and phyllosilicates and a low reflection of calcite at 3.03Å, not present in the rest of the samples, which means that a full decomposition of this phase has not taking place yet. On the other hand, the sample EE-5 also displays reflections of hematite, barely present in the rest of the samples of this group. Hematite is a ferric oxide which begins to crystallize at temperatures above 750–800°C from iron oxides released by decomposition of clay minerals [12]. However, in CaO-rich environments its crystallization is considerably inhibited [13]. As the rest of the samples were fired at a higher temperature and, therefore, a probably higher amount of free CaO was produced by decomposition of carbonates, hematite reflections were then poorly developed. In addition, all the samples of this group present analcime, which is a hydrated sodium alumino-silicate commonly formed during burial in calcareous ceramics fired at temperatures around 1000°C or higher [14]. As the sample EE-5 was fired at a lower temperature, the phase analcime appears barely crystallized.
X-ray diffractograms derived from samples of Group B are displayed in Fig. 6. Except the samples of the border tiles (EE-7 and EE-8), which still present phases of mica and phyllosilicates, all the samples of this group show the same thermo-formed phases as those samples of Group A, that is: gehlenite, diopside and anortite. They also show the inhibition of the grow of hematite due to the CaO-rich environment and the presence of analcime. Therefore, a similar equivalent firing temperature between 950 and 1050°C can be also estimated for Group B. It must be highlighted that border tiles EE-7 and EE-8, due to the presence of reflections of mica and phyllosilicates, were in all probability fired at a lower temperature of about 950°C, as in the case of the sample EE-5 of former Group A. These samples display as well more developed reflections of hematite and poorly crystallized analcime due to effects of a lower firing temperature.
The ungrouped sample EE-18 (Fig. 6) is the sample with the higher reflections of mica and phyllosilicates and with the lower development of thermically neoformed phases such as gehlenite, diopside and anortite. It also presents well defined reflections of hematite and has no analcime. All of these data, along with the fact of its low content of CaO (13.42wt.%) in relation to Groups A (25.59wt.%) and B (21.99wt.%), indicate a lower equivalent firing temperature between 850 and 950°C.
The samples fired at lower temperatures: EE-5 (16th century tile) from Group A, EE-7 and EE-8 (16th century border tiles) from Group B, and the ungrouped sample EE-18 (16–17th century earthernware), are from Toledo in all cases. This fact suggests that a lower temperature could be a characteristic trait of ceramic products from Toledo in comparison with productions from Talavera. It could suggest also that indeed the sample EE-15 (17th century tile from Toledo), as already pointed out by chemical data, is a probably misclassified sample since it has the same features, in terms of crystalline phases present, than those shown by the rest of Talavera productions.
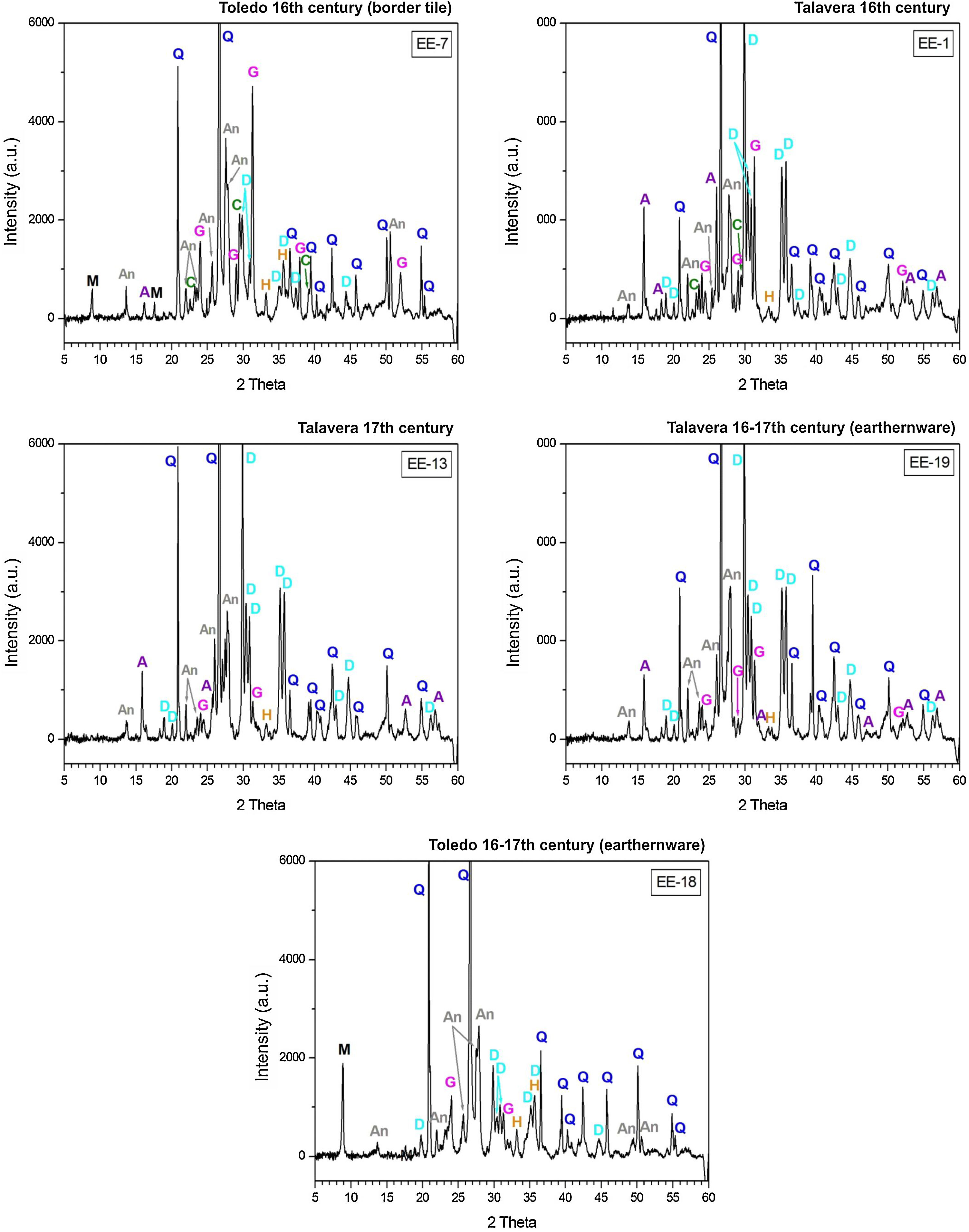
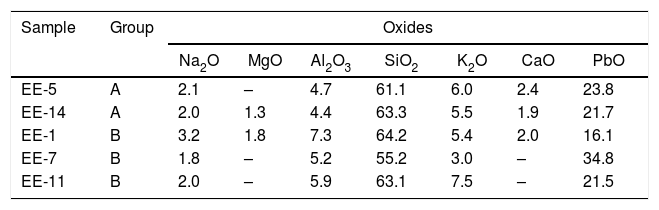
Observations by FESEM and EDS microanalyses of enamels or glazed layersAn enamel of homogeneous aspect, regular surface, abundant spherical bubbles of different sizes and approximately 400μm in thickness is observed in a polished section of the sample EE-5 from Group A (Fig. 7A). It shows a good adherence with the ceramic body, which suggests that when it was applied it had an adequate fluidity. The EDS microanalyses carried out on the enamel body (Fig. 7B, analysis 1) indicate that it is a leaded enamel with a PbO content of ∼24wt.%. Apart from PbO, SiO2 (61.1wt.%) and Al2O3 (4.7wt.%) were also detected, as well as K2O (6.0wt.%), Na2O (2.1wt.%) and CaO (2.4wt.%). It can be therefore classified as a lead/alcaline-earth enamel [15]. The enamel also presents unmelted crystals of quartz of different sizes (Fig. 7B, analysis 2), with rounded edges without evidences of reaction, along with concentrations of lighter crystals with a high content of SnO2 (56.8wt.%; Fig. 7B, analysis 3), which indicate that tin oxide was added as opacifier to obtain the base white enamel. These lighter crystals are made up of an assembly of small particles that seem to be relics of former grains, which can be compatible with the addition of grains of the mineral cassiterite as tin oxide contributor.
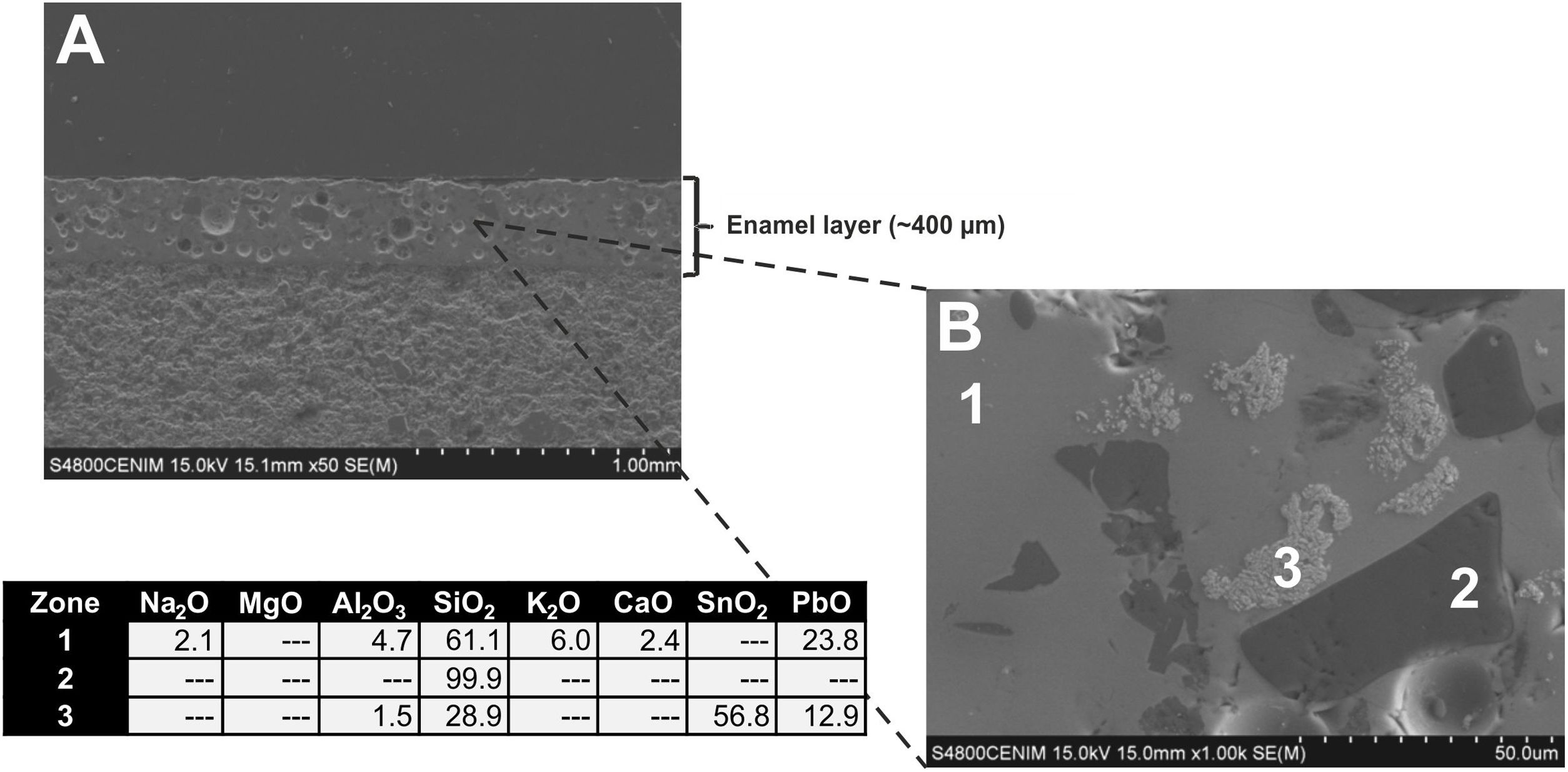
The enamel of the sample EE-14, likewise belonging to Group A (Fig. 8A), also shows an homogeneous aspect, regular surface, somewhat less abundant spherical bubbles and a lower thickness (∼325μm) in comparison with the previous one. It shows as well a good adherence with the ceramic body and the content of PbO (21.7w.%; Fig. 8B, analysis 1) indicates that it is also a leaded enamel which can be evenly classified as a lead/alcaline-earth enamel. The only notable difference in comparison with the previous one is the detection of a small percentage of MgO (1.3wt.%; Fig. 8B, analysis 1). The enamel presents unmelted crystals of quartz of different sizes (Fig. 8B, analysis 2), with rounded edges and signs of reaction; unmelted fragments of felspars, possibly potassic feldspars due to the relatively high concentration of K2O (13.8wt.%; Fig. 8B, analysis 3) determined in these fragments; and less concentrated lighter crystals of SnO2 (52.4wt.%; Fig. 8B, analysis 4), which also indicate the use of tin oxide as opacifier.
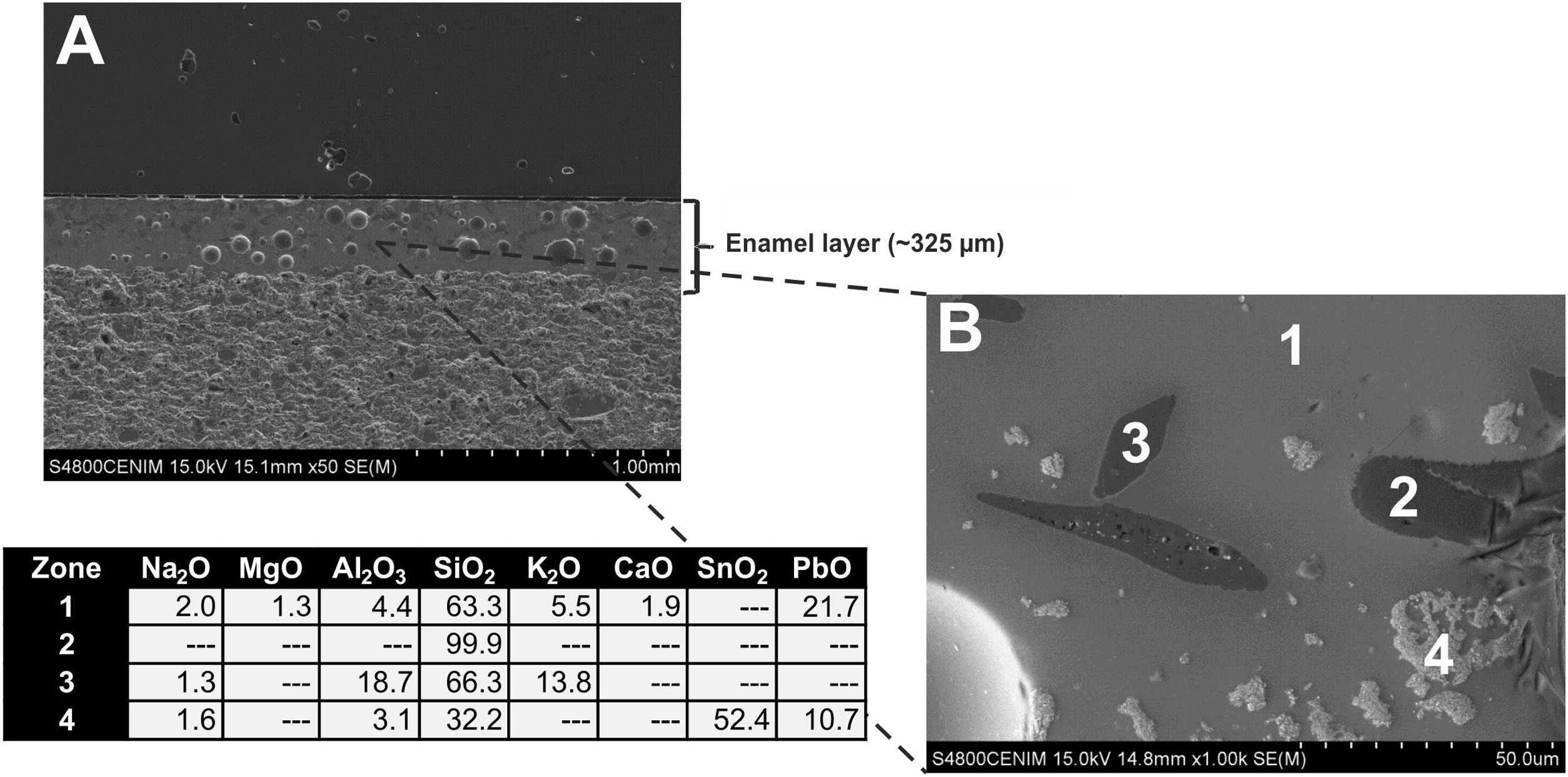
Fig. 9A displays the enamel of the sample EE-1 from Group B in which big spherical bubbles distributed for all the section can be observed. The enamel shows a regular surface and has an average thickness of about 450μm. It was surely applied with an adequate fluidity since it also shows a good adherence with the ceramic body. The EDS microanalyses also indicate a leaded enamel, even though in this case the concentration of PbO (16.1wt.%; Fig. 9B, analysis 1) is lower in comparison with previous examples of Group A. The concentration of both SiO2 (64.2wt.%) and Al2O3 (7.3wt.%) is a little bit higher than in those enamels of Group A, while the contents of K2O (5.4wt.%), Na2O (3.2wt.%) and CaO (2.0wt.%) are roughly in the same order of magnitude. A small percentage of MgO (1.3wt.%) was also detected. This composition is therefore compatible with a lead/alcaline-earth enamel as in the previous tile samples. In this case the enamel does not show any unmelted crystal of quartz, it only has unmelted and large fragments of potassic felspars according to the relatively high concentration of K2O (12.8wt.%; Fig. 9B, analysis 2), some of them with incipient reactions at their edges. In addition, lighter and quite dispersed crystals of SnO2 (61.4wt.%; Fig. 9B, analysis 3) were observed and microanalyzed, which evenly indicate that tin oxide was also used to opacify the enamel.
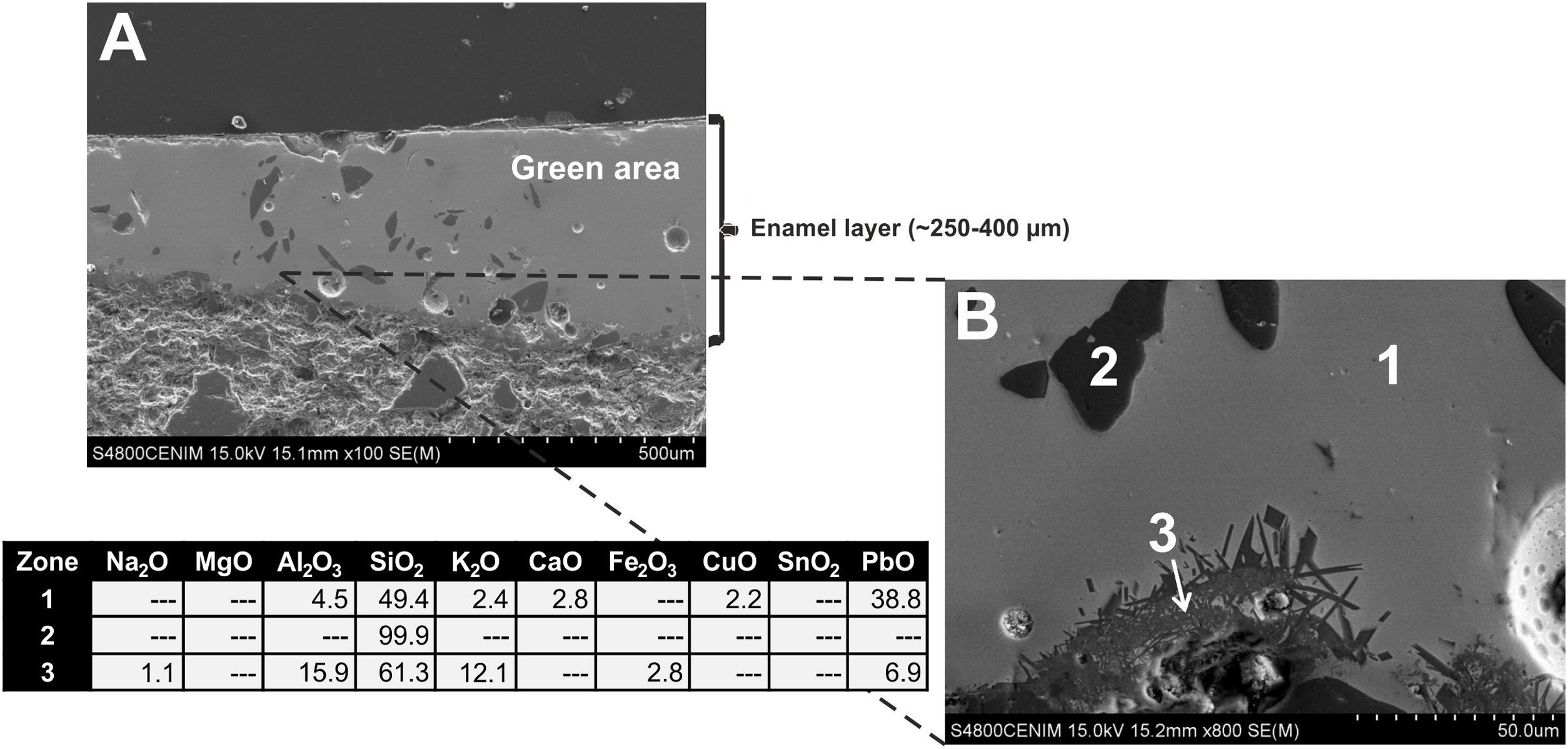
Border tiles belonging to Group B also showed a homogeneous aspect, with regular surfaces but with much less spherical bubbles. Fig. 10A-B displays micrographs and EDS results of the most interesting area, the green colored one, from the sample EE-7. White and blue areas looked very similar and are not shown for space reasons. The EDS microanalysis determined as well a notable PbO concentration (38.8wt.%; Fig. 10B, analysis 1), which is characteristic of a leaded enamel. In fact, it is the example with the highest concentration of PbO and, therefore, the contents of alumino-silicates (49.4wt.% of SiO2 and 4.5wt.% of Al2O3) and alkaline (2.4wt.% of K2O) and alkaline-earth (2.8wt.% of CaO) oxides are consequently lower in relative terms. Moreover, Na2O was not detected, which mean in addition that probably the enamel has undergone a small dealkalinization process. Along with these oxides, a concentration of 2.2wt.% of CuO was also determined, which must be connected with the green color of the enamel as is discussed later in the next section. Although white and blue areas do present SnO2, the tin oxide opacifier was not used in the green area. Nonetheless, unmelted crystals of quartz of different sizes (Fig. 10B, analysis 2) were also identified in this green area. Furthermore, at the interface between the enamel and the ceramic body, a devitrification attacked zone was identified. It is characterized by having sets of small needle shaped crystals in which high amounts of SiO2 (61.3wt.%), Al2O3 (15.9wt.%) and K2O (12.1wt.%) were determined (Fig. 10B, analysis 3). This kind of phenomena uses to be produced when the thermal densification of the enamel is prolonged for a too long period of time [16].
A general overview of the enamels observed and microanalyzed indicates that all of them are quite homogeneous and show a good adherence with the ceramic support, which means that they were applied with an adequate fluidity. On the contrary, they commonly show varied amounts of spherical bubbles, which means that the cooling process of the enamels was not suited enough since the enamel prolonged its viscosity during cooling, not allowing gases to scape, and thereby producing bubbles [17]. The presence of bubbles in the enamel is compatible with a possible co-firing of the powder enamel and the ceramic body at the same time. Table 3 shows the chemical composition of the enamels. All of them are tin opacified lead based enamels, even though tin is usually not detected by EDS in the body of the enamel due to it mainly concentrates in relics of cassiterite grains. A chronological evolution in composition recipes cannot be stated. However, it can be tentatively established a notorial difference between tiles from Toledo and tiles from Talavera. Tiles from the former only contain unmelted crystals of quartz, while tiles from the latter contains either quartz crystals or fragments of K-feldspars. Such difference is also observed in border tile samples.
Chemical composition of enamels derived from EDS analyses (wt %).
| Sample | Group | Oxides | ||||||
|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | PbO | ||
| EE-5 | A | 2.1 | – | 4.7 | 61.1 | 6.0 | 2.4 | 23.8 |
| EE-14 | A | 2.0 | 1.3 | 4.4 | 63.3 | 5.5 | 1.9 | 21.7 |
| EE-1 | B | 3.2 | 1.8 | 7.3 | 64.2 | 5.4 | 2.0 | 16.1 |
| EE-7 | B | 1.8 | – | 5.2 | 55.2 | 3.0 | – | 34.8 |
| EE-11 | B | 2.0 | – | 5.9 | 63.1 | 7.5 | – | 21.5 |
Not detected.
Lead base tin-opacified enamels should be connected with the hispano-moresque tradition of making glazed ceramics. The presence of this kind of enamels is known in hispano-moresque (mudéjar) ceramic productions of Paterna (Valencia, Spain) from the 14th century onwards [18], as well as in hispano-moresque ceramics and tiles from the Alcázar Palace in Seville until the 15th century [19], among other important sites. This hispano-moresque tradition was also widespread in Portugal since the 15th and the 16th centuries and is known in different sites such as the Palácio Nacional (Sintra) [20] or the Monastery of Santa Clara-a-Velha (Coimbra) [21]. Nevertheless, the geometric hispano-moresque designs of Islamic tradition evolved to more complex figurative Renaissance motifs influenced by the Italian style first implanted in Spain at the beginning of the 16th century by Francisco Pisano [4]. For Toledo and, especially, Talavera production centers this meant that, since the 16th century onwards, they elaborated tiles using lead base tin-opacified enamels within the hispano-moresque tradition but with a Renaissance repertoire of motifs of Italian influence painted on their white backgrounds.
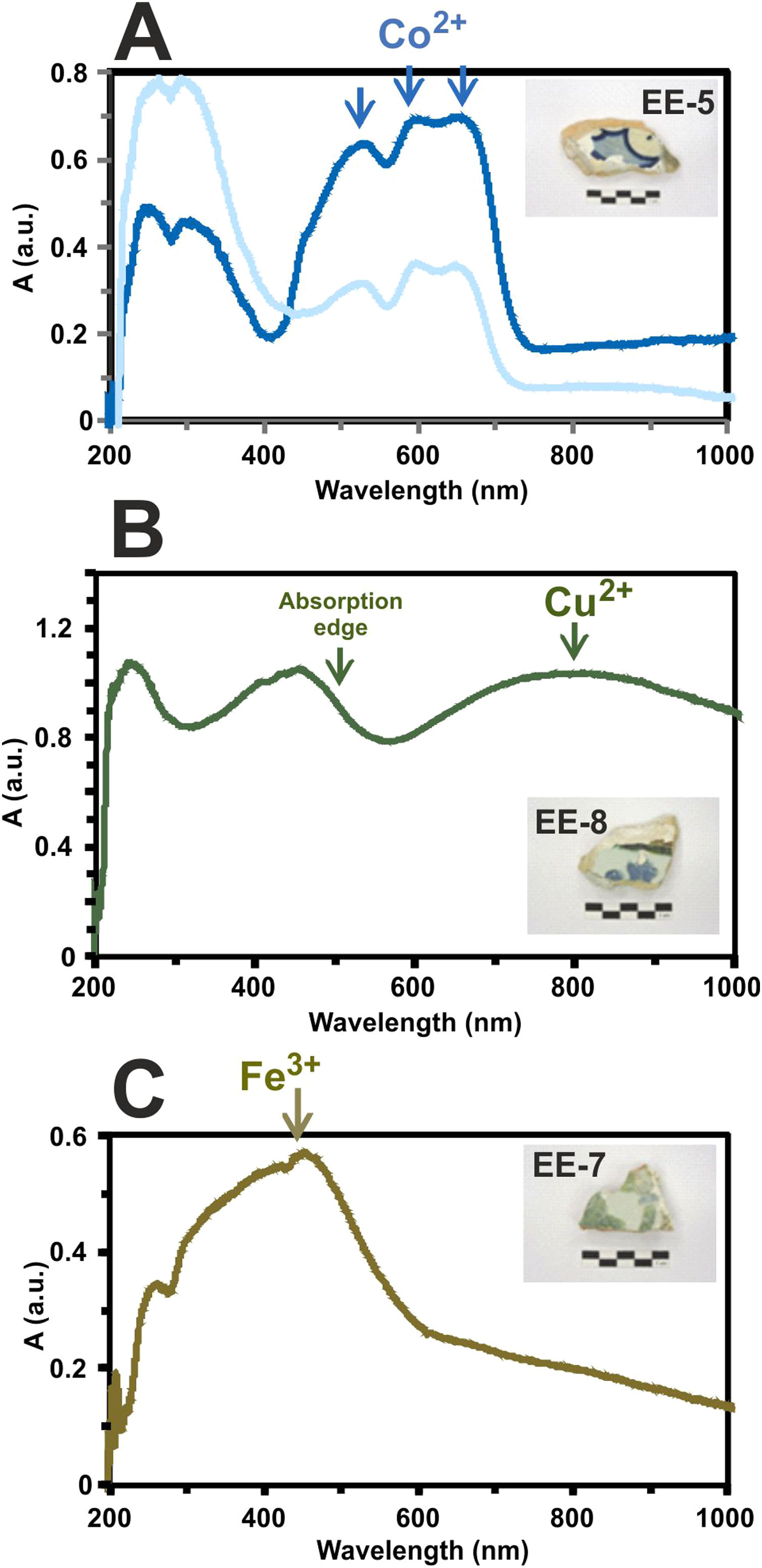
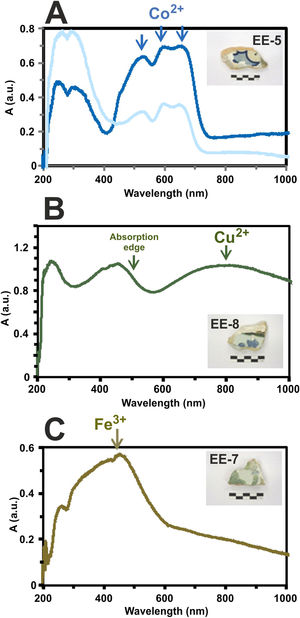
Determination of chromophores through Vis spectrophotometry in the enamels or glazed layersThe blue color presents a great intensity triplet at 530, 590 and 650nm, respectively, which is due to the absorption bands of Co2+-ions (Fig. 11A). These ions impart a very intense coloration to the glasses even at very low concentrations (less than 0.005wt.%) [22]. The difference in the intensity of the blue color applied to the tiles decoration is also recorded in the intensity of these bands in the absorption spectra as can be observed in the tile sample EE-5 of Fig. 11A.
Border tiles from Toledo present up to four colors in their decorations: white, blue, green and brown. The white color is obtained by tin oxide opacification. The blue color by the presence of Co2+-ions. The green color is due to a wide absorption band at about 800nm, which can be assigned to the presence of Cu2+-ions (Fig. 11B) and, as mentioned above, is in accordance with detection of CuO (2.2wt.%) by EDS in a section of this green enamel. In addition, the high PbO concentration (38.8wt.%) in the enamel displaces the absorption edge up to ∼500nm. This fact is well-known in Islamic emerald-green glasses from Al-Andalus [23] and connects this technological tradition with hispano-moresque glazed ceramics such as border tiles from Toledo here analyzed. The brown color is due to a wide absorption band at 380, 420 and 440nm, respectively, which can be assigned to Fe3+-ions (Fig. 11C). A brown area of border tiles could not be analyzed by FESEM-EDS.
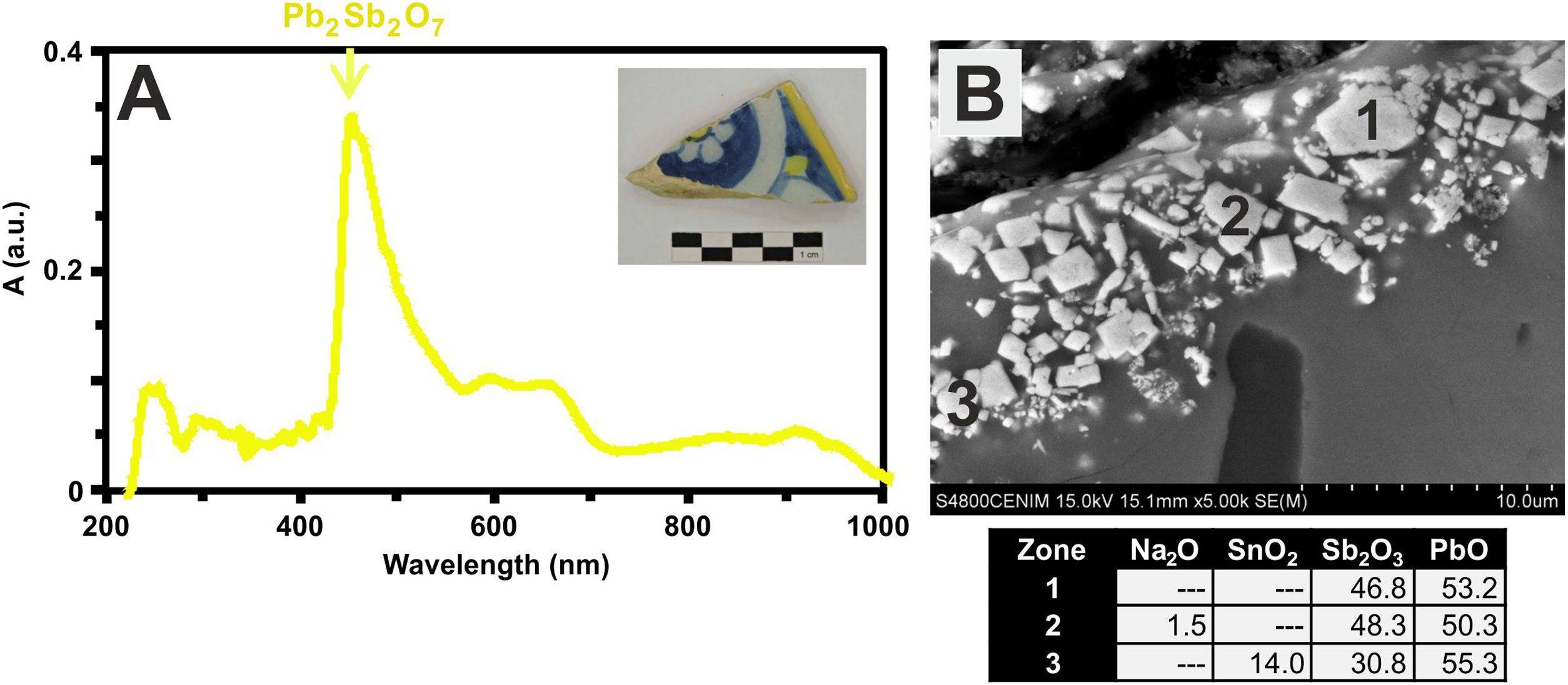
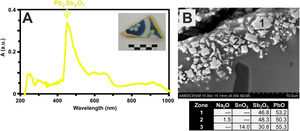
Finally, the yellow color of polychrome decorations was investigated in the sample EE-11 from Talavera. The visible absorption spectrum of this sample shows an intense absorption band at about 450nm, which can be associated to the presence of lead antimoniate (Pb2Sb2O7) (Fig. 12A). This compound was corroborated by FESEM and EDS microanalyses and resulted in the observation of micro-crystals of lead antimoniate concentrated nearly the surface of the yellow area whose microanalyses determined high concentrations of PbO (from 50.3 to 55.3wt.%) and Sb2O3 (from 30.8 to 48.3wt.%) (Fig. 12B, analyses 1–3). The content of SnO2 (14.0wt.%) in the analysis 3 surely comes from the white base on which the yellow color was applied.
ConclusionsThe results of the archaeometric study undertaken on tiles of different dates from the Royal Monastery of El Escorial (Madrid, Spain) has allowed the verification of a great technological homogeneity in the production of 16th and 17th century tiles from Talavera and Toledo. Such technological homogeneity is the result of a preindustrial processing in the making of these productions.
Two groups of different chemical composition in the ceramic body were determined, which did not always coincide with the stylistic and decorative criteria followed in the material selection. Based on chemical data it is possible to separate 16th century tile productions from Talavera of those from Toledo. However, data obtained indicate a more complex situation for 17th century tiles, which could be due to, on the one hand, the replacement of tiles in this century was a more complicated process than that shown by the historical documents or, on the other hand, that nowaday panels can be composed of multiple replacements of which there is no documentation. Chemical data also indicate that in Talavera either the tiles or the earthernware were made with similar raw materials, while in Toledo the earthernware seems to be made with distinct raw materials than tiles. In addition, they also suggest that 20th century production in Talavera follows ancient traditions since there are not notable differences with 16th and 17th century tiles.
The tiles here analyzed were produced from highly calcareous ceramic materials which were mainly fired at a temperature between approximately 950 and 1050°C according to the thermo-formed phases identified. A group of samples from Toledo in all cases, and due to the presence of mica and phyllosilicates among other features, were fired at lower temperatures of about 950°C or even a little bit lower. This fact could be a characteristic trait of ceramic products from Toledo in comparison with productions from Talavera.
All the glazes can be classified as lead/alcaline-earth base enamels which have been opacified by using tin oxide probably added from cassiterite grains. In general they are quite homogeneous and show a good adherence with the ceramic body, which means that they were applied with an adequate fluidity. They also show varied amounts of spherical bubbles, which means that the cooling process was not suited enough since the enamel prolonged its viscosity during cooling, not allowing gases to scape, and thereby producing bubbles. The presence of bubbles is compatible with a possible co-firing of the powder enamel and the ceramic body at the same time. No evidence has been found that can establish a chronological evolution in the recipes of enamel composition. However, it can be tentatively established a notorial difference between tiles from Toledo and tiles from Talavera since tiles from Toledo only contain unmelted crystals of quartz, while tiles from Talavera contains either quartz crystals or fragments of K-feldspars. In any case, lead base tin-opacified enamels should be connected with the hispano-moresque tradition of making glazed ceramics well documented in other parts of Spain and Portugal.
The following chromophores were determined in the flat brush-painted tiles: Co2+-ions in blue decorations, with different intensities, and micro-crystals of lead antimoniate (Pb2Sb2O7) in yellow decorations. In the border tiles the chromophores determined were: Co2+-ions for the blue color, Cu2+-ions for the green color, and Fe3+-ions for the brown color.
The border tiles in all probability from Toledo showed some distinctive technological characteristics: probable mixing of clay raw materials in their making, which misassigned the samples to the chemical group mainly formed by productions of Talavera; lower firing temperature in the range of about 950°C; higher content of PbO in the enamels; devitrification processes at the interface enamel/ceramic body; and greater variety of colors in decorations.
An overall evaluation of the data obtained in this study indicates that not always stylistic and decorative criteria and historical documentation match with information provided by the own materials. This fact means that the issue is probably more complex and, therefore, further analyses and combined studies would be certainly needed to confirm or reject some of the results reached in this first approach to the issue.
FundingProgram TOP Heritage: Technologies in Heritage Sciences, Regional Government of Madrid (Ref. S2018/NMT-4372) and project Challenges of Society from the Spanish Ministry of Science and Innovation (Ref. PID2019-104220RB-I00).
The authors acknowledge funding support from the Program TOP Heritage: Technologies in Heritage Sciences, Regional Government of Madrid (Ref. S2018/NMT-4372), and from the project Challenges of Society from the Spanish Ministry of Science and Innovation (Ref. PID2019-104220RB-I00). They also acknowledge professional support from the TechnoHeritage network of Science and Technology for the Conservation of Cultural Heritage and CSIC Interdisciplinary Thematic Platform Open Heritage: Research and Society (PTI-PAIS). They also gratefully acknowledge Spanish National Heritage (Real Collections Directorate) for providing with the samples to undertake the study.