This work suggests a method to evaluate quantitatively the effect of doping oxide on the microstructure phase coexistence, mobility of charge carriers of non-polarized ceramic PZT doping by different amounts of Fe2O3 and Cr2O3. Besides the analysis of XRD patterns, the electrical properties measurement showed that chromium and iron oxide doping furthers the tetragonal structural phase and disadvantages the rhombohedral structure to a cubic form. Besides, chromium oxide causes more rapidly a mobility of charge carriers of the non-polarized material, compared to iron oxide. Furthermore, the addition of both chromium and iron oxides promotes increase in the size of the tetragonal phase crystal. On the other hand, the rhombohedral phase crystal size increases due to the addition of iron oxide and decreases by doping with chromium oxide.
Este trabajo sugiere un método para evaluar de manera cuantitativa el efecto del óxido impurificado sobre la coexistencia de fase de la microestructura, la movilidad de los portadores de carga de impurificación de PZT cerámico no polarizado por diferentes cantidades de Fe2O3 y Cr2O3. Además del análisis de los patrones XRD, la medición de las propiedades eléctricas mostró que la impurificación con óxido de cromo y óxido de hierro fomenta la fase estructural tetragonal y desfavorece a la estructura romboédrica por una forma cúbica. Además, el óxido de cromo genera una movilidad más rápida de los portadores de carga del material no polarizado en comparación con el óxido de hierro. Además, la adición de óxido de cromo y óxido de hierro promueve el aumento de tamaño del cristal de fase tetragonal. Asimismo, el tamaño del cristal de la fase romboédrica aumenta debido a la adición de óxido de hierro y disminuye por la impurificación con óxido de cromo.
Lead zirconate titanate (PZT) with perovskite structure is widely studied due to its excellent piezoelectric properties [1,2], and largely used in electronic industry because of its useful piezoelectric properties. Various devices, such as transducers and integrated circuits for microelectronic applications use this type of ceramics [3,4]. The material was intensively investigated since the miscibility of lead titanate and lead zirconate was discovered in the 1950s [5]. Adding different dopants within the basic matrix (PZT) is the way to vary different properties, in order to synthesize new ceramic materials for different applications. For this purpose, many researchers studied the composition of PZT modified by appropriate substitution at sites A and B [6–8], using dopants to improve the electrical and mechanical properties of piezoelectric materials. The properties of PZT samples being highly sensitive to additives, composition and synthesis methods [9].
Electrical conductivity, which directly relates to power losses or charge leakage of piezoelectric devices, is a key physical parameter and appreciably restricts the utilization of several properties of ferroelectrics [10]. An increase in conductivity hinders the determination of the Curie point of known ferroelectrics, using dielectric measurements. Electrical conductivity of ferroelectric ceramics is also essential to the poling efficiency and in turn, the domain configurations [11], which influence the dielectric and piezoelectric properties. For pristine ferroelectric polycrystals, the piezoelectric effect cannot be observable at a macroscopic scale owing to the absence of net polarization. The net polarization, which brings out piezoelectricity, can be developed in polycrystals through the poling process. The poling process depends on the local spontaneous polarization in the unit cells, and can also be reoriented when an adequately strong electric field is applicable, i.e. the ferroelectric effect [12]. Reorientation of spontaneous polarization appears in the microstructure, due to either the domain wall motion, or the boundaries separating adjacent domains [13]. Many factors, such as the poling field, poling temperature and poling time, may affect the degree of poling and consequently the piezoelectric properties [14].
A literature survey showed that few groups have reported the temperature-dependent electrical properties of PZT [15]. Basu et al. studied the effect of temperature-dependent electrical properties (dielectric constant, field-induced displacement, electromechanical coupling factor) of PZT wafers made by tape casting. Maiwa et al. [16] investigated the temperature-dependent electrical and electromechanical properties of PZT thin films and concluded that thinner films possess higher Curie temperature. Yimnirun et al. [17] found that the hysteresis area and the remanent polarization of bulk PZT follows the power law temperature-dependent relationship, while the coercivity field scales linearly with temperature. Gubinyi et al. [18] reported the effect of temperature on the electrical properties of PZT bulk. Glaum et al. [19] studied the temperature and field dependent degradation properties of bulk PZT under a unipolar electric field. Chen et al. [20] studied the effect of lead excess on polarization fatigue, and also reported the deterioration of fatigue with increased temperature.
The present work aims to characterize the structural and electrical properties of unpolarized PZT doped with Cr2O3 and Fe2O3. The microstructure properties, resistivity and conductivity were investigated as a function of the dopant nature, composition and temperature.
ExperimentalIn the present study, from high purity oxide powders namely, PbO (99.90%), ZrO2 (99.90%), TiO2 (99.80%), Cr2O3 (99.90%), and Fe2O3 (99.90%), samples were prepared by the conventional ceramic process using the formulae (1−x) Pb (Zr0.48, Ti0.52) O3-x Cr2O3 and (1−x)Pb(Zr0.48,Ti0.52)O3-x Fe2O3, respectively (x=2%, 4%, 6%, 8%). The metal stoichiometric amount in the designated (1−x) PZT-x Cr2O3 and (1−x) PZT-x Fe2O3 composition was mixed for 2h as grinding media and ethanol as solvent. After milling for 6h, the resulting slurry was dried in an oven and the powder was calcined in a furnace at 900°C for 120min. Then, the calcined powder was ball milled for 6h to make sure a fine particle size is obtained. After drying, the powder was pressed into disk-like shapes and sintered at 1200°C in a closed alumina crucible, in a PbO vapor enriched atmosphere, using PbZrO3 powder. An X-ray diffractometer with CuK radiation was used to reveal the phases of the sintered samples. The electrical properties (resistivity and conductivity) were measured using an LCR-meter and programmable oven.
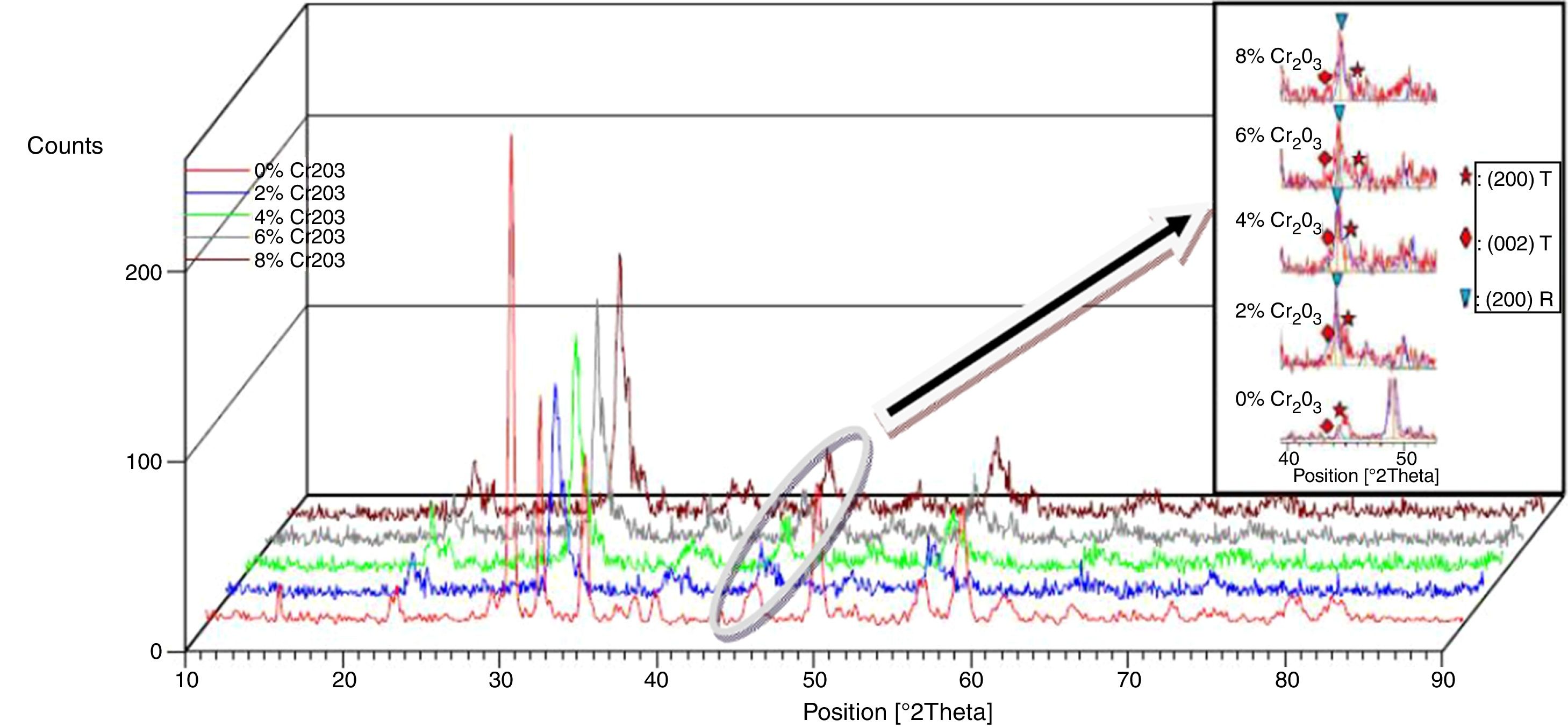
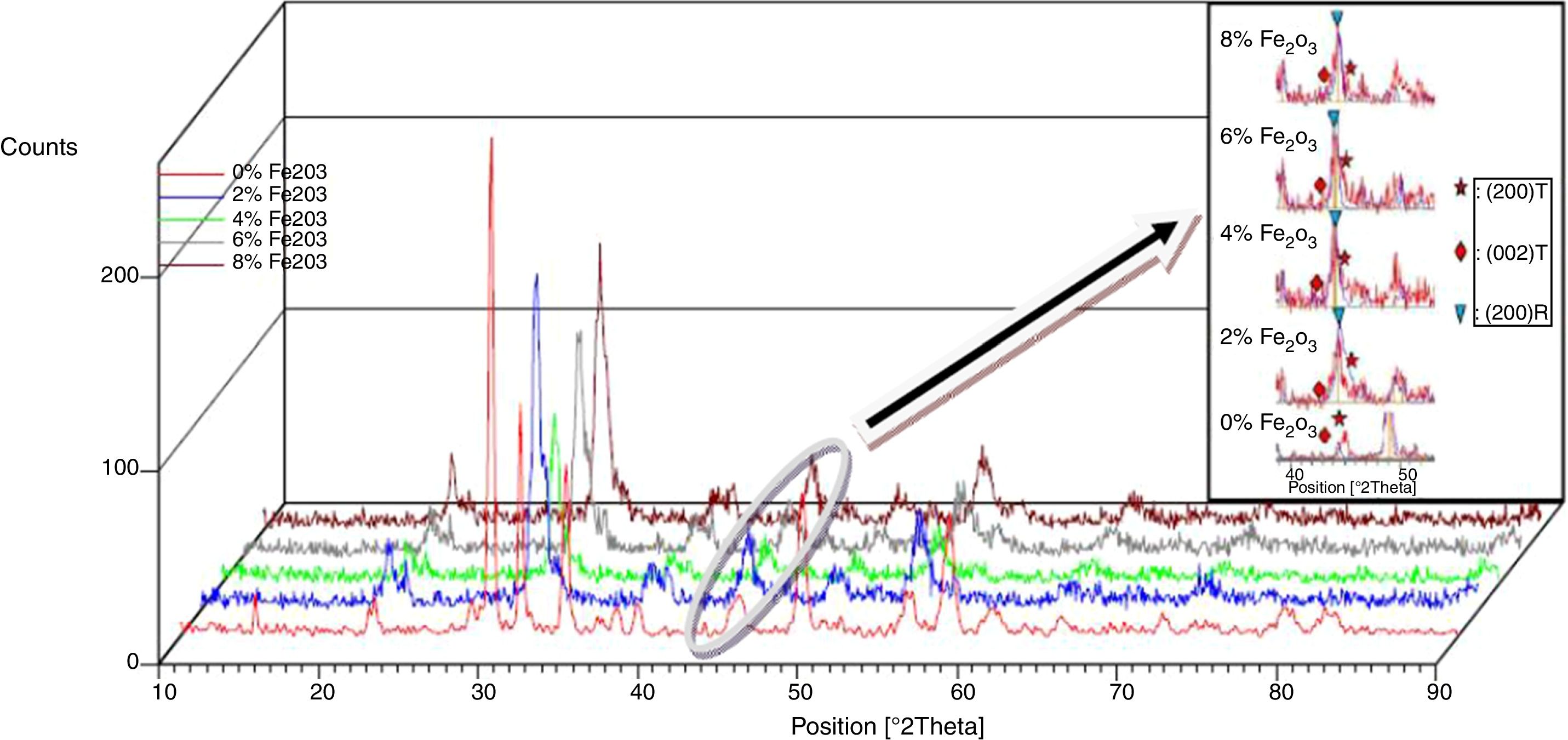
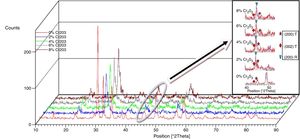
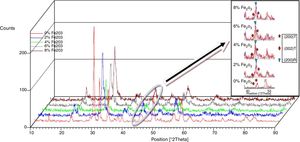
Results and discussionMicrostructure propertiesFigs. 1 and 2 show the XRD patterns of (1−x) Pb (Zr0.48, Ti0.52) O3-x Cr2O3 and (1−x) Pb (Zr0.48, Ti0.52) O3-x Fe2O3 respectively, for various contents (2, 4, 6 and 8%). The tetragonal, tetragonal–rhombohedral phases were identified by analyzing the peaks 002 (tetragonal), 200 (tetragonal) and 200 (rhombohedral), in the 2θ range of 43°–47°.
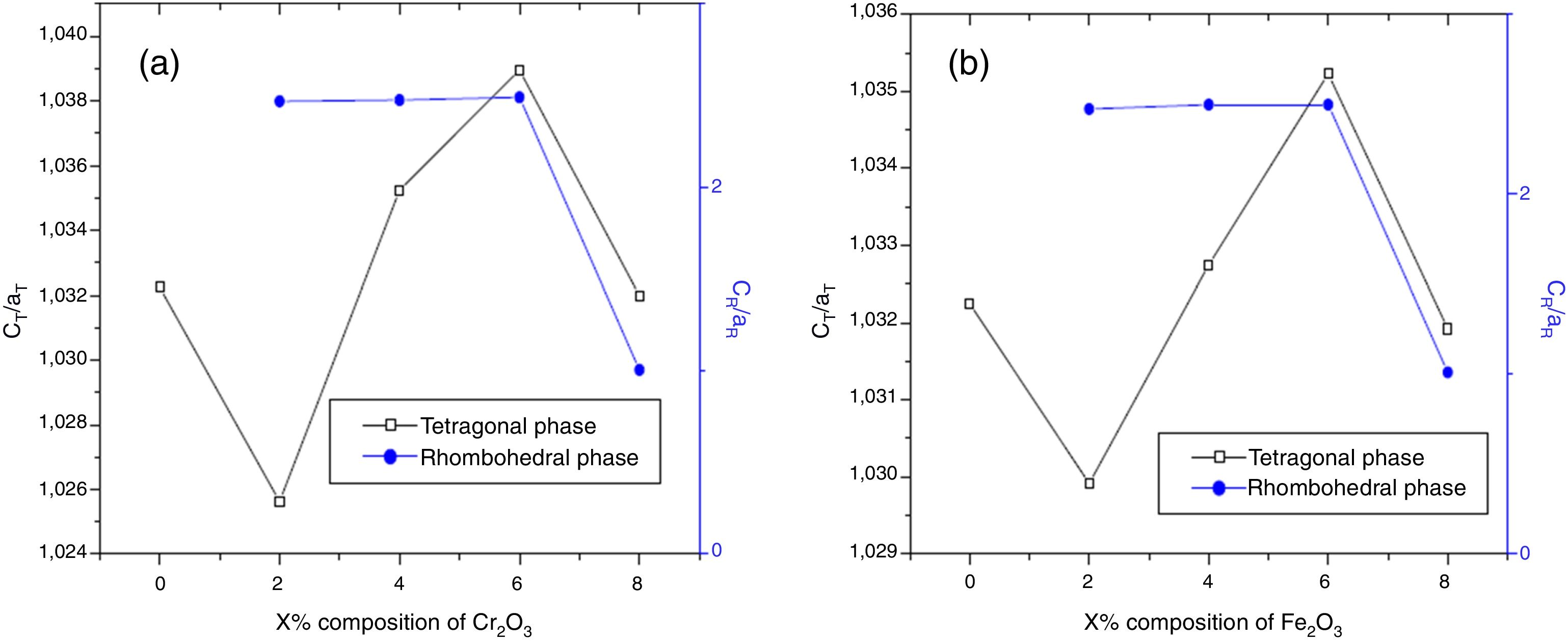
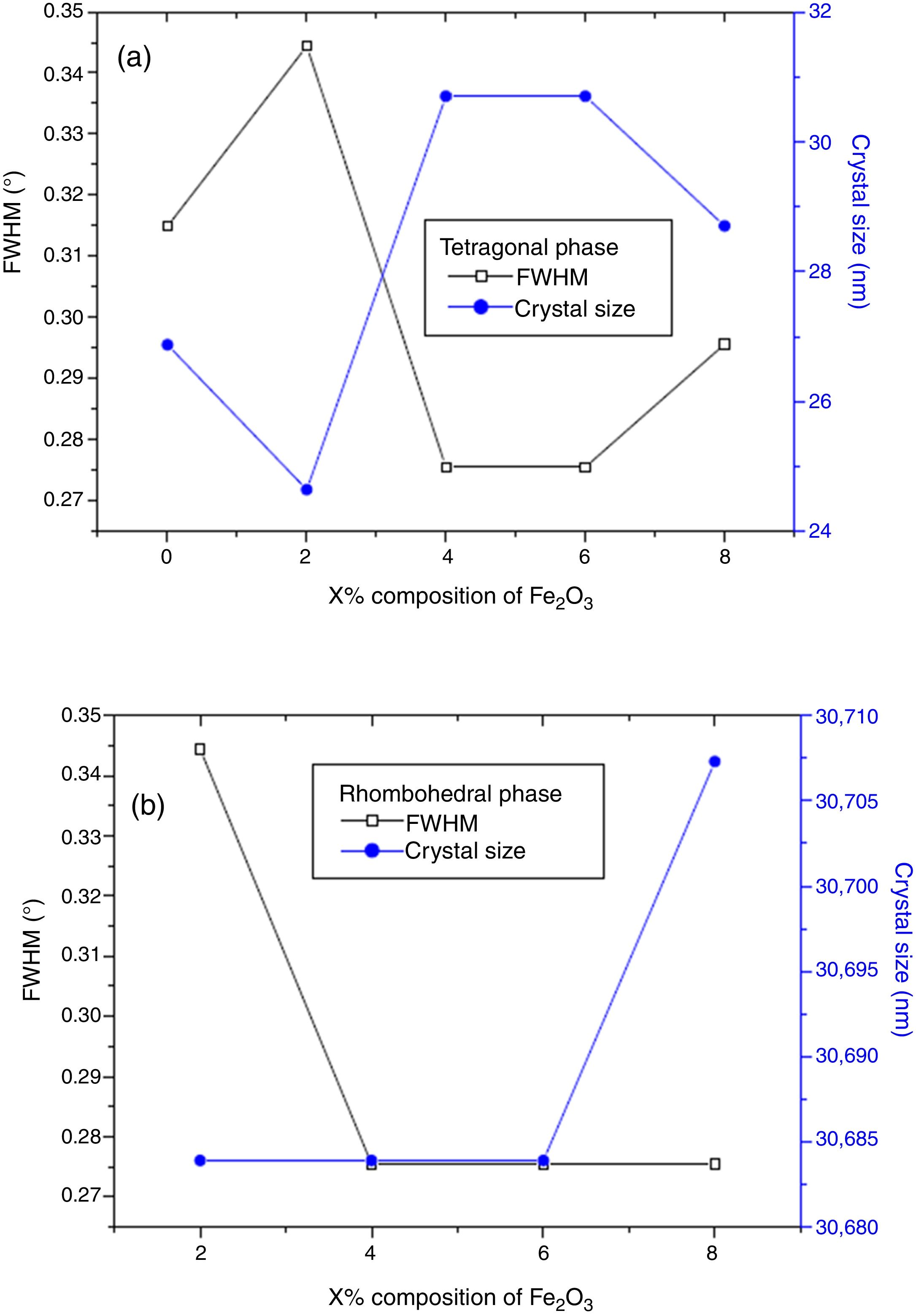
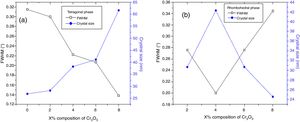
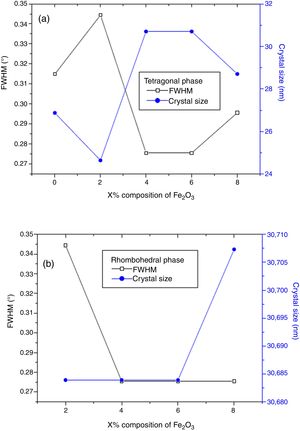
The effect of Cr2O3 and Fe2O3 content on the microstructure is observed in Figs. 3(a, b), 4(a, b) and 5(a, b). The increasing Cr2O3 and Fe2O3 content causes a change in the lattice parameters for tetragonal and rhombohedral phases. Generally, the increase in chromium and iron oxide contents favors tetragonality (until x=0.06) and disadvantages the rhombohedral form toward a cubic structure.
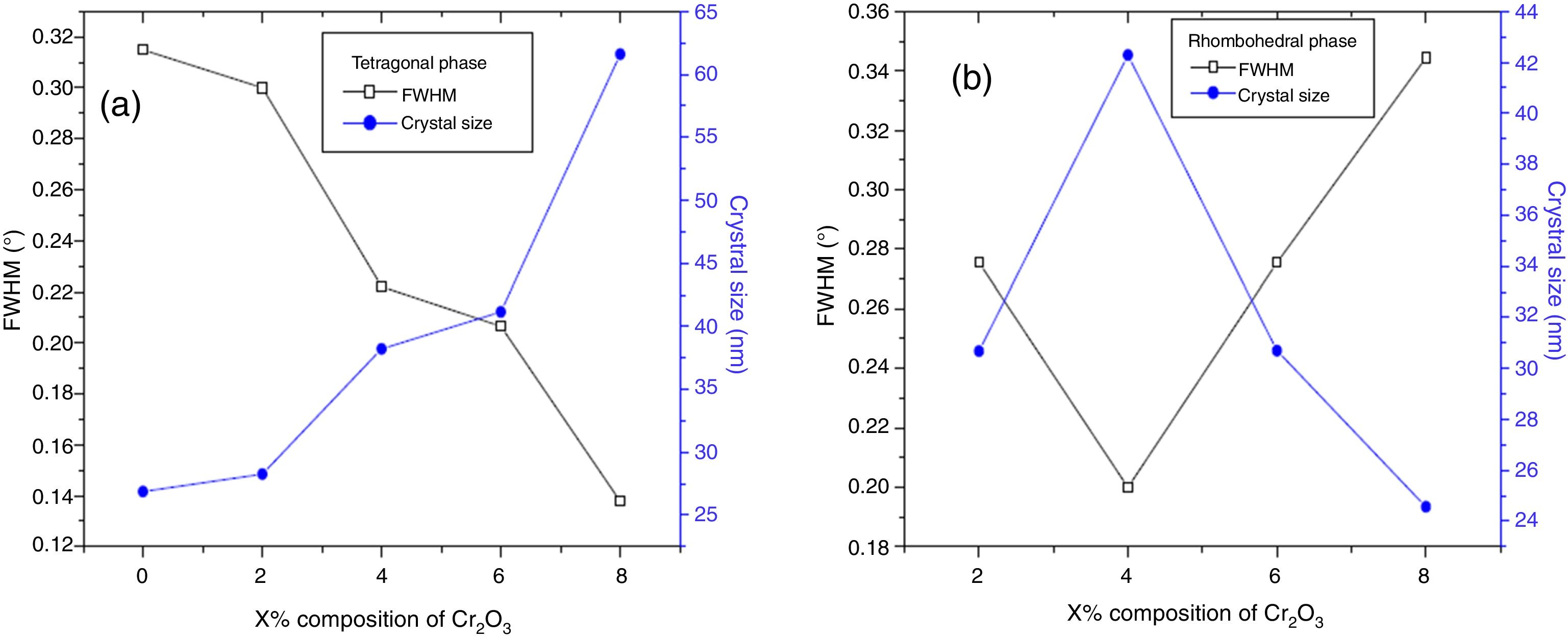
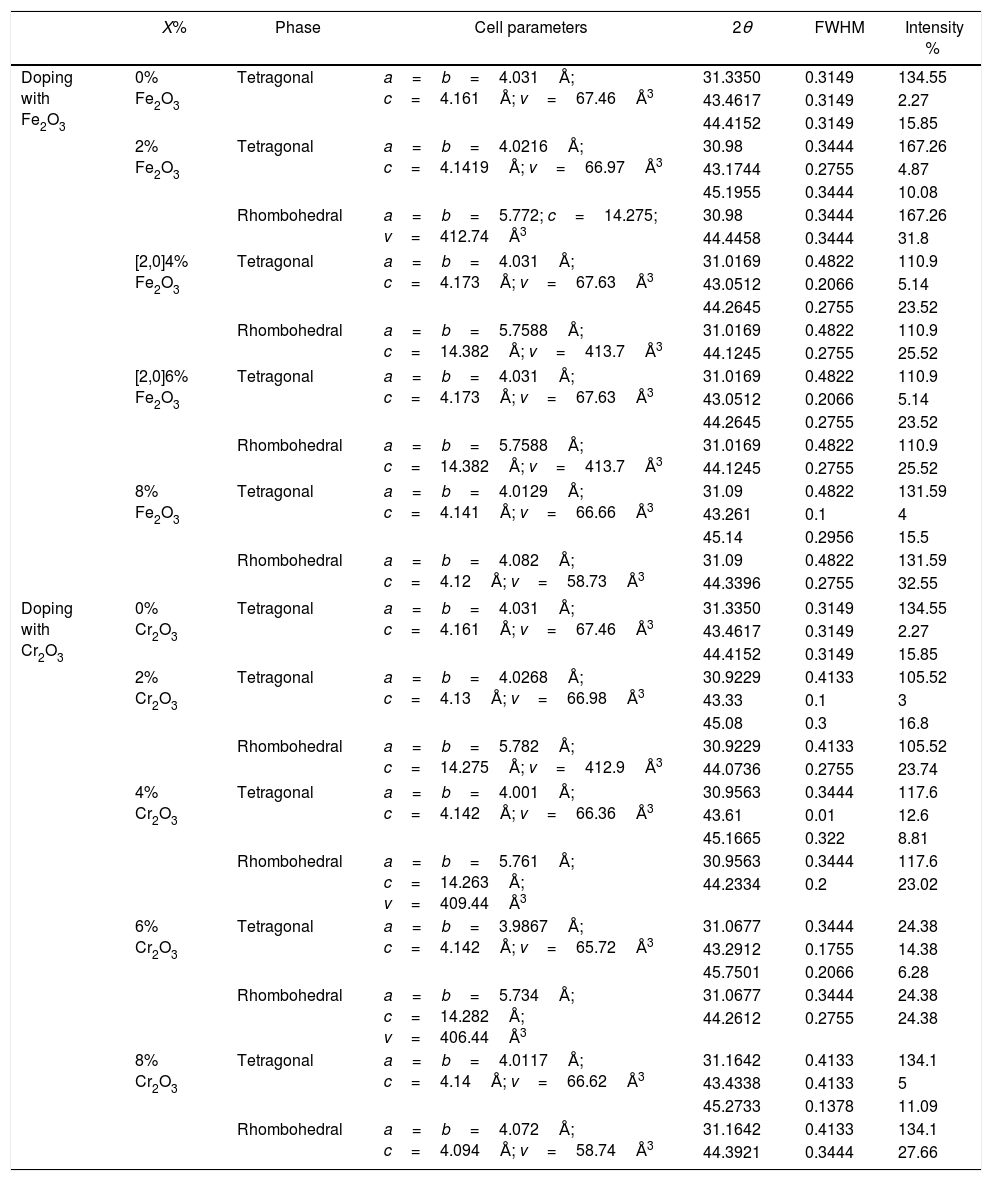
The increase of Fe2O3 content led to an increase in the crystal size for tetragonal and rhombohedral phases gradually, which may be due to the insertion of chrome atoms. However, the increasing Cr2O3 amount caused an increase in the crystal size for the tetragonal phase and a decrease in the size of the rhombohedral phase, This may be assessed to a substitution between the chromium ions with a smaller atomic radius and those of zirconium or titanium with a larger atomic radius. The phase structure and lattice parameters of (1−x) Pb (Zr0.48, Ti0.52) O3-x Cr2O3 and (1−x) Pb (Zr0.48, Ti0.52) O3-x Fe2O3 samples prepared with various contents are listed in Table 1.
Phase structure and lattices parameters of (1−x)PZT-xCr2O3 and (1−x)PZT-xFe2O3.
| X% | Phase | Cell parameters | 2θ | FWHM | Intensity % | |
|---|---|---|---|---|---|---|
| Doping with Fe2O3 | 0% Fe2O3 | Tetragonal | a=b=4.031Å; c=4.161Å; v=67.46Å3 | 31.3350 | 0.3149 | 134.55 |
| 43.4617 | 0.3149 | 2.27 | ||||
| 44.4152 | 0.3149 | 15.85 | ||||
| 2% Fe2O3 | Tetragonal | a=b=4.0216Å; c=4.1419Å; v=66.97Å3 | 30.98 | 0.3444 | 167.26 | |
| 43.1744 | 0.2755 | 4.87 | ||||
| 45.1955 | 0.3444 | 10.08 | ||||
| Rhombohedral | a=b=5.772; c=14.275; v=412.74Å3 | 30.98 | 0.3444 | 167.26 | ||
| 44.4458 | 0.3444 | 31.8 | ||||
| [2,0]4% Fe2O3 | Tetragonal | a=b=4.031Å; c=4.173Å; v=67.63Å3 | 31.0169 | 0.4822 | 110.9 | |
| 43.0512 | 0.2066 | 5.14 | ||||
| 44.2645 | 0.2755 | 23.52 | ||||
| Rhombohedral | a=b=5.7588Å; c=14.382Å; v=413.7Å3 | 31.0169 | 0.4822 | 110.9 | ||
| 44.1245 | 0.2755 | 25.52 | ||||
| [2,0]6% Fe2O3 | Tetragonal | a=b=4.031Å; c=4.173Å; v=67.63Å3 | 31.0169 | 0.4822 | 110.9 | |
| 43.0512 | 0.2066 | 5.14 | ||||
| 44.2645 | 0.2755 | 23.52 | ||||
| Rhombohedral | a=b=5.7588Å; c=14.382Å; v=413.7Å3 | 31.0169 | 0.4822 | 110.9 | ||
| 44.1245 | 0.2755 | 25.52 | ||||
| 8% Fe2O3 | Tetragonal | a=b=4.0129Å; c=4.141Å; v=66.66Å3 | 31.09 | 0.4822 | 131.59 | |
| 43.261 | 0.1 | 4 | ||||
| 45.14 | 0.2956 | 15.5 | ||||
| Rhombohedral | a=b=4.082Å; c=4.12Å; v=58.73Å3 | 31.09 | 0.4822 | 131.59 | ||
| 44.3396 | 0.2755 | 32.55 | ||||
| Doping with Cr2O3 | 0% Cr2O3 | Tetragonal | a=b=4.031Å; c=4.161Å; v=67.46Å3 | 31.3350 | 0.3149 | 134.55 |
| 43.4617 | 0.3149 | 2.27 | ||||
| 44.4152 | 0.3149 | 15.85 | ||||
| 2% Cr2O3 | Tetragonal | a=b=4.0268Å; c=4.13Å; v=66.98Å3 | 30.9229 | 0.4133 | 105.52 | |
| 43.33 | 0.1 | 3 | ||||
| 45.08 | 0.3 | 16.8 | ||||
| Rhombohedral | a=b=5.782Å; c=14.275Å; v=412.9Å3 | 30.9229 | 0.4133 | 105.52 | ||
| 44.0736 | 0.2755 | 23.74 | ||||
| 4% Cr2O3 | Tetragonal | a=b=4.001Å; c=4.142Å; v=66.36Å3 | 30.9563 | 0.3444 | 117.6 | |
| 43.61 | 0.01 | 12.6 | ||||
| 45.1665 | 0.322 | 8.81 | ||||
| Rhombohedral | a=b=5.761Å; c=14.263Å; v=409.44Å3 | 30.9563 | 0.3444 | 117.6 | ||
| 44.2334 | 0.2 | 23.02 | ||||
| 6% Cr2O3 | Tetragonal | a=b=3.9867Å; c=4.142Å; v=65.72Å3 | 31.0677 | 0.3444 | 24.38 | |
| 43.2912 | 0.1755 | 14.38 | ||||
| 45.7501 | 0.2066 | 6.28 | ||||
| Rhombohedral | a=b=5.734Å; c=14.282Å; v=406.44Å3 | 31.0677 | 0.3444 | 24.38 | ||
| 44.2612 | 0.2755 | 24.38 | ||||
| 8% Cr2O3 | Tetragonal | a=b=4.0117Å; c=4.14Å; v=66.62Å3 | 31.1642 | 0.4133 | 134.1 | |
| 43.4338 | 0.4133 | 5 | ||||
| 45.2733 | 0.1378 | 11.09 | ||||
| Rhombohedral | a=b=4.072Å; c=4.094Å; v=58.74Å3 | 31.1642 | 0.4133 | 134.1 | ||
| 44.3921 | 0.3444 | 27.66 | ||||
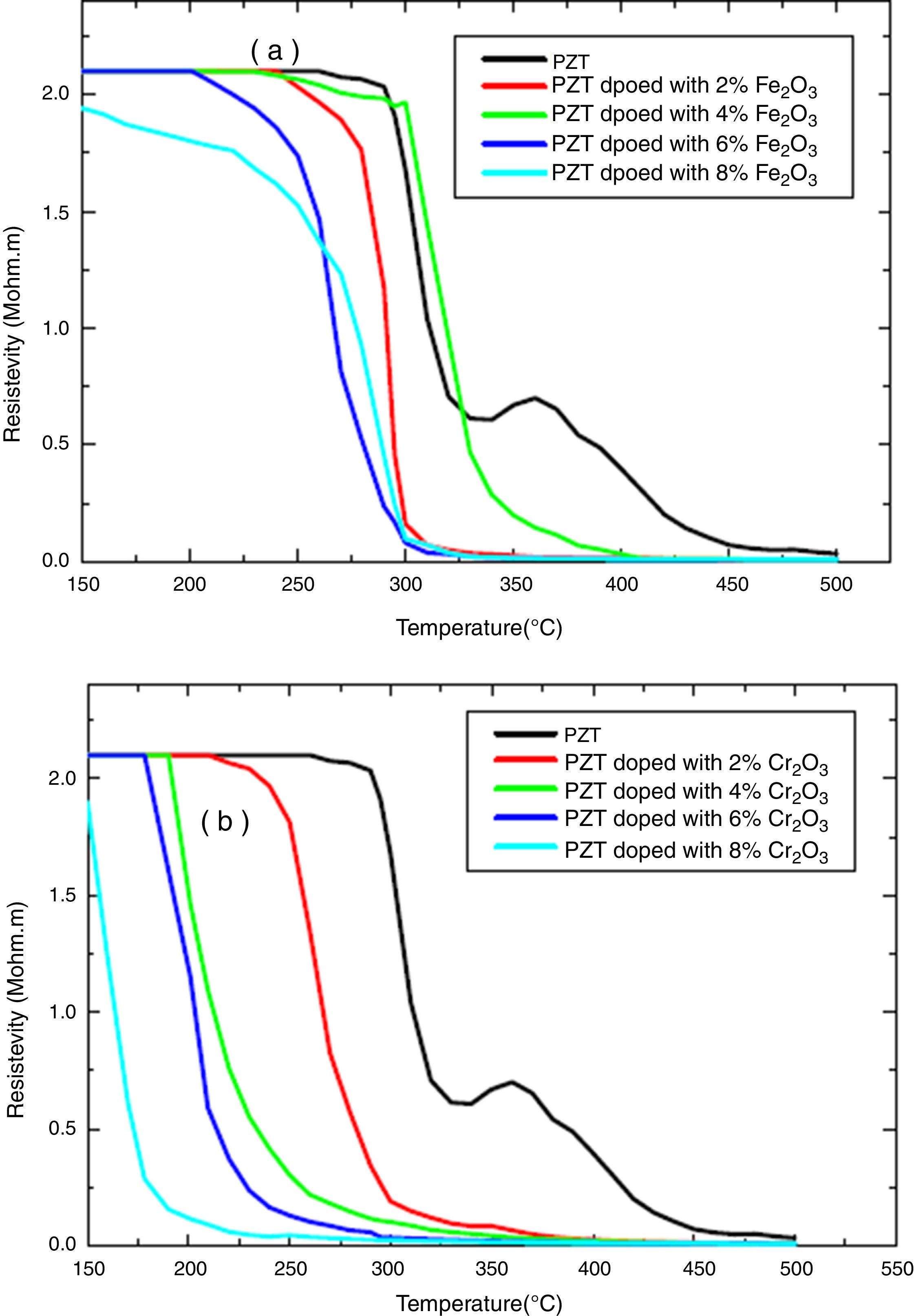
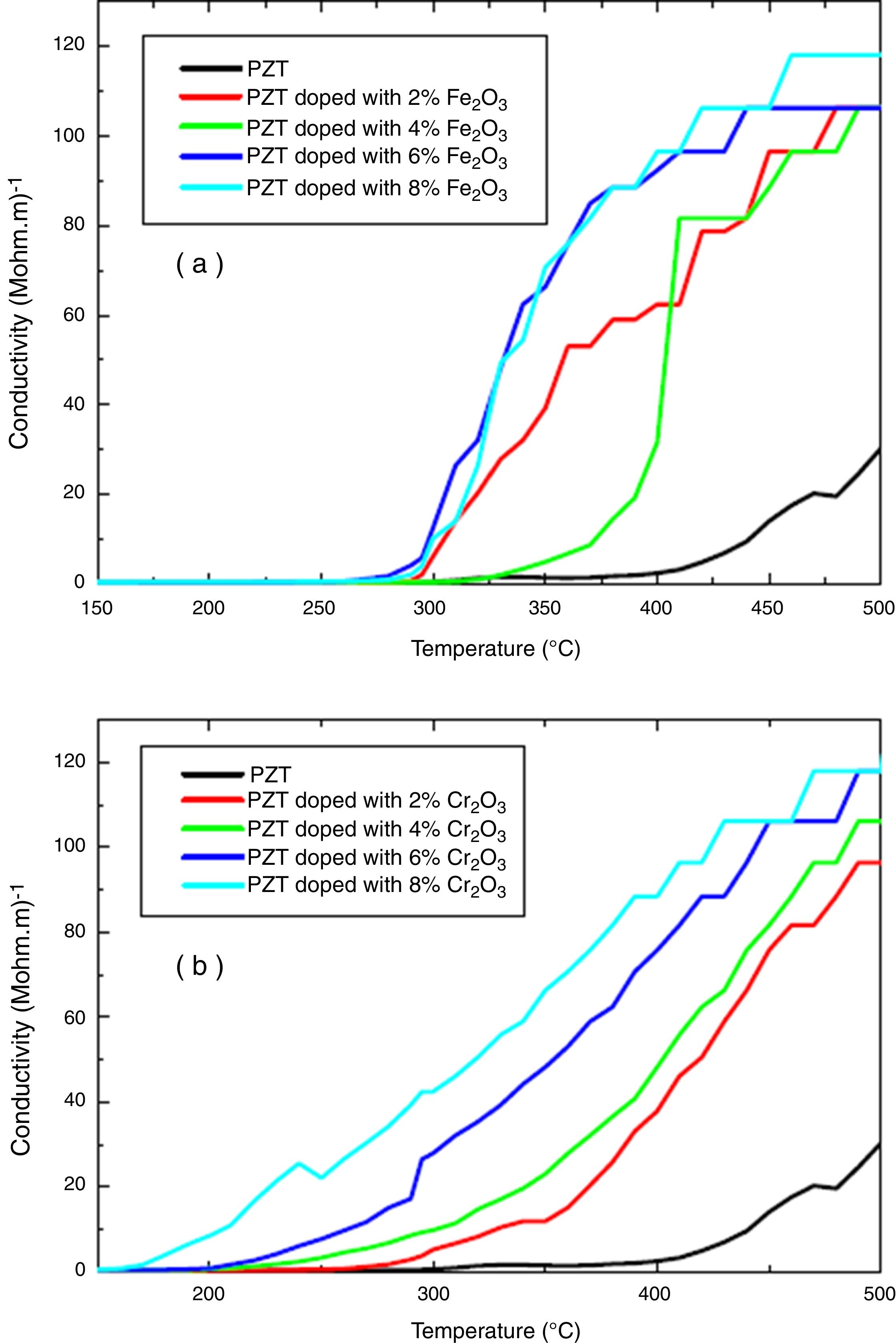
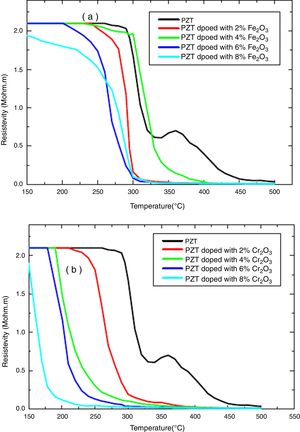
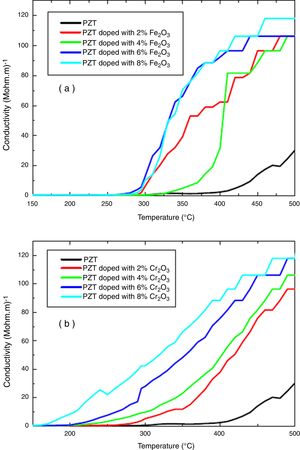
The electrical properties of (1−x) PZT-x Fe2O3 and (1−x) PZT-x Cr2O3 showed evidence of a phase transformation. Figs. 6(a, b) and 7(a, b) plot the resistivity and conductivity as functions of temperature for various amounts of Cr2O3 and Fe2O3 doping. As can be seen in these figures, doping with Cr2O3 leads to a progressive decrease in the resistivity and increase in the conductivity of samples. This can be assessed to the charge carrier mobility due to the presence of chrome, which therefore makes the sample electrically more conductive. On the other hand, addition of iron oxide results in a decrease in the electrical resistivity of un-polarized samples, in an alternating and non-progressive manner compared to chromium oxide. This may be attributed to the difference in thermal conductivity between chromium (0.937w/cmk) and iron (0.802w/cmk) [21]. Consequently, chromium being thermally more conductive, it insures a rapid heat transfer to the charge carriers so that they are thermally excited and mobilized. This trims the un-polarized material electrically less resistant and more conductive, and this is the reverse case of iron.
In this paper, X-ray diffraction analysis and electric properties measurement of non-polarized PZT ceramics were implemented to investigate the effect of chrome oxide and iron oxide on the coexistence of rhombohedral tetragonal phases, microstructure and electrical properties. The results show that:
- 1.
The increasing Cr2O3 and Fe2O3 composition caused a change in the lattice parameters for tetragonal and rhombohedral phases. In general, the increase in the composition of the chromium and iron oxides favors tetragonal moieties (until x=0.06), while it disadvantages the rhombohedral shape toward a cubic structure.
- 2.
The increase of Fe2O3 composition results in an increase of the crystal size for tetragonal and rhombohedral phases progressively.
- 3.
Increasing Cr2O3 content increases the crystal size of the tetragonal phase and decreases the size of the rhombohedral phase.
- 4.
Addition of Cr2O3 decreases progressively the resistivity and increases the conductivity of samples.
- 5.
Addition of Fe2O3 causes a decrease in the electrical resistivity of the un-polarized sample in an alternating and non-progressive manner compared to Cr2O3 doping.