Economical alumina precursors, derived from bauxite or nepheline ores, were calcined through a very short time process. Results showed that phases of the precursor are a key parameter on the flash calcination process. The number of structural water molecules is the dominant phenomenon. A porous and fine structure of activated alumina was developed as the hydroxyl groups are explosively driven off and establish a hydrothermal condition. The presence of gibbsite phase with larger particle size and most structural water molecules has better product properties in comparison of other alumina precursors phase with small particle size because water vapor pressure achieves a critical value for splitting particles and developing nucleation desirable phases. The changes rate of properties reaches to its maximum value for gibbsite phase at lower calcination temperature. FESEM images showed the precursor phase forms different morphologies of products like, nanodimensional, a coral-like co-continuous or loose particles surrounded with macropores.
Los precursores económicos de alúmina, derivados de minerales de bauxita o nefelina, se obtienen a través de un proceso de calcinación rápida. Los resultados mostraron que las fases del precursor son un parámetro clave en el proceso de calcinación rápida. El fenómeno dominante en este proceso es el número de moléculas estructurales que estén formando el agua. Cuando los grupos de hidroxilo se extraen de forma explosiva, elaboran un ambiente hidrotermal. El resultado genera alúmina activa con una estructura porosa y fina. A diferencia de otras etapas de los precursores de alúmina con el tamaño de partículas pequeñas, la gibbsite incluye el tamaño más grande de partículas con el número máximo de moléculas estructuradas de agua que producen mejores propiedades del producto, y que va a dividir partículas y a desarrollar las etapas de forma deseada cuando alcanza la presión del vapor del agua el punto crítico. En cambio, a baja temperatura se maximizará los cambios de gibbsite. Las imágenes de FESEM mostraron que la fase precursora forma diferentes morfologías de productos, como nanodimensionales, co-continuas como corales o partículas sueltas rodeadas de macroporos.
Alumina has favorable chemical and physical properties and is economically produced. Alumina has commonly used as a term for aluminum oxides and hydroxides, which exist as at least five thermodynamically stable phases and many more metastable transition forms [1]. Active aluminas are multifunctional materials. These aluminas are usually prepared through the dehydroxylation of various aluminum hydroxides. Transition aluminas appear during the thermal decomposition of aluminum trihydroxides and oxyhydroxides under several dehydroxylation sequences. The forms of transition aluminas are γ, δ, η, θ, χ, κ, ρ [2]. Four minerals of hydrated alumina are presently recognized. Aluminum trihydroxides with the formula Al(OH)3 that is designated as γ-Al(OH)3 (gibbsite) and α-Al(OH)3 (bayerite) with the monoclinic system. Aluminum oxyhydroxides with the formula AlOOH that is designated as γ-AlOOH (boehmite) with face-centered cubic system and α-AlOOH (diaspore) with the hexagonal close-packed system [1,2].
The structure of gibbsite makes up double layers of hydroxyl ion, and aluminium ion situates two-third of the octahedral site within the layers. The hydroxyls of neighboring layers are occupied instantly opposite each other. The layers are partly dislocated in the orientation of the a-axis and hexagonal symmetry is reduced to monoclinic. The boehmite lattice contains double layers that the oxygen ions array cubic packing. Hydroxyl ions of one double layer are located over the depression between OH ions the adjacent layer such that the double layers are connected by hydrogen bonds between hydroxyls in adjacent sheets [1].
There are different papers on the production of alumina types [3–9]. Generally, the alumina production was based on the processing of bauxite ore, alunite, and nepheline ores or clays from oil sand tailings. Bauxite is a source ore of aluminum hydroxide in major phases of gibbsite, boehmite, and diaspore. The bauxites with a high content of gibbsite are most easily processed by the Bayer process. Bayer process is the most important commercial method for the production of gibbsite. But the developing capacity of Bayer plants is limited by environmental constraints, especially in red mud disposal and odor. Alunite ((KNa)Al3(SO4)2(OH)3) is a chemical composition of aluminum, sodium, and potassium sulfate with an Al2O3 amount of 20–23%. Each tonne of alumina needs approximately 5 tonnes of nepheline and over 6 tonnes of limestone. The VAMI alunite process is a nearly energy intensive technique of alumina production. The major benefit of this technique is the perfect utilization of produced wastes and absence of any polluting the environment effect. Also, New Mexico has established a process to get the alumina in clays from oil and sand tailings. The alumina is recovered by washing the clay with sulfuric acid to form aluminum sulfate solution. This solution is filtered and then roasted to get alumina; the sulfur dioxide is recovered and recycled [10,11].
In the slow dehydration, aluminum hydroxides was raised to the reaction temperature at a slow rate and maintained there for a period ranging from minutes to hours. There are wide reports on the decomposition of different aluminum hydroxides at a slow rate and long time for various applications [12–18]. Delgado et al. [12] investigated the thermal conversion of boehmite into alumina at various temperatures ranged from 500 to 1500°C with heating rate of 20°C/min for 2, 7, and 12h. Boumaza et al. [13] considered the conversion of gibbsite powder in air at temperatures ranged between 25 and 1200°C with heating rate 10°C/min that hold for 4h at the calcination temperature. The transformations were determined using the X-ray diffraction (XRD), X-ray photoemission (XPS) spectra and 27Al magic angle spinning nuclear magnetic resonance spectroscopy (27Al MAS NMR). Lee et al. [14] found transformation rate and crystal size of the transition alumina can adjust by the electron beam dose. Lamouri et al. [15] evaluated the influence of the heating rate on the densification and microstructures of green bodies using γ-Al2O3 raw powder during sintering. Zhu et al. [16] studied the degree of gibbsite dehydration with a heating rate of 10°Cmin−1 at various temperatures. The kinetic factors of reactions such as: activation energy, the pre-exponential factor were calculated using the Kissinger equation and the derivative thermogravimetric method. Wang et al. [17] investigated temperature and time conditions with calcining bauxite for preparation of activated alumina. The best condition was taken at 500°C and 1h. Mamontov et al. [18] calcined aluminium trihydroxide at temperature range from 120 to 800°C for 2h. The effect of temperature presented on textural parameters of activated alumina.
Another type of decomposition is flash calcination process that is highly economical, continuous and produced in a very short time (a few seconds) [10]. This process has various applications especially catalysts, carriers, absorbents, and raw materials for ceramics and refractories industry [19–21]. Kowalik et al. [19] demonstrated that the use of flash-calcination of CuZnAl-LDH precursor in catalyst synthesis is efficient method for preparation of high-activity LT-WGS catalysts because this method restricts undesirable sintering of material in comparison with traditional calcinations method. Antoniak et al. [20] showed that high surface area of flash-calcined alumina acts as a suitable support for highest activity Co–Mo catalysts in the water gas shift.
Mista [22] prepared active alumina by flash calcinations and evaluated thermokinetics of heat emission during their rehydration by isothermal calorimetry method. Also, Redaoui et al. [23] decomposition mechanism and kinetic parameters gibbsite obtained by thermogravimetric method. Novakovij et al. [24] established that gibbsite hydration follows first order reaction kinetics on the base of water amount. Also, the effect of temperature in the narrow range of 550–650°C, wide range of 350–1150°C and fine/coarse grain sizes have been studied on the properties of activated alumina produced by flash calcination [25–27].
The importance of precursor phases is not investigated on physicochemical properties of active alumina. This paper reports to study the effects of precursor phases derived from bauxite or nepheline ores as a function of flash calcination temperature.
Experimental procedureRaw materialsThree precursors were used with different gibbsite and boehmite percents. The first precursor (GB) was supplied by alunite and nepheline ores from Azerbaijan. The second precursor (G) was supplied by bauxite ore from India. Third precursor (B) was synthesized through high energy milling of the second precursor (G). These precursors were characterized by X-ray diffraction (XRD), thermogravimetry analysis (TGA), particle size analyzer (PSA), X-ray diffraction fluorescence (XRF) and N2 adsorption measurements.
InstrumentsThermogravimetry analysis was carried out on a TGA/SDTA 851 instrument (Mettler-Toledo). Samples were heated from 50 to 1000°C at 10°Cmin−1 in flowing air gas.
The particle sizes and particle size distributions were measured by a laser particle analyzer (LAB-300, HORBIA).
XRD patterns were performed on a Philips PW1840 diffractometer using Cu-Kα radiation (λ=0.154nm), operating at 40kV and 30mA and a graphite monochromator in the 2θ range of 10o–80o. The transition aluminas and aluminum hydroxides were characterized from ICCD files (θ-Al2O3 (ICCD 11-0517), δ-Al2O3 (ICCD 04-0877), γ-Al2O3 (ICCD 29-0063), χ-Al2O3 (ICCD 04-0880), and α-Al2O3 (ICCD 42-1668), Al(OH)3 (ICCD 33-0018) AlOOH (ICCD 21-1307).
The chemical composition was estimated by X-ray fluorescence spectroscopy (XRF) on a Philips PW1480 instrument.
The textural properties were analyzed by N2 adsorption at −196°C using an adsorption apparatus (Belsorp max, BEL Japan). BET surface area was determined by the linear part of the BET plot from nitrogen adsorption isotherms up to P/P0=0.3. The average pore diameter was calculated using the adsorption branch of the isotherm and the Barrett–Joyner–Halenda (BJH) model. The pore volume was estimated from the volume of N2 adsorbed at P/P0=0.995.
The external features and morphology were obtained using field emission scanning electron microscope (FE-SEM, EO Elektronen-Optik-Service GmbH) which was performed at 15kV. A small amount of powder was dispersed into ethanol by an ultrasound bath, and then a droplet was deposited on the stub and allowed to dry.
MethodThe different precursors were treated by flash calcination process at different temperatures (350–1200°C) under a hot air stream in a designed laboratory tubular furnace. The treatment time of precursors was up to 3s in the furnace. The rate of the mass feed is 0.5g/s through a quartz pipe of diameter 0.7cm and length 390cm in this designed tubular furnace. Product is collected by a cyclone [26]. Samples were designated by AT where A was the precursor type and T was the flash calcination temperature in °C.
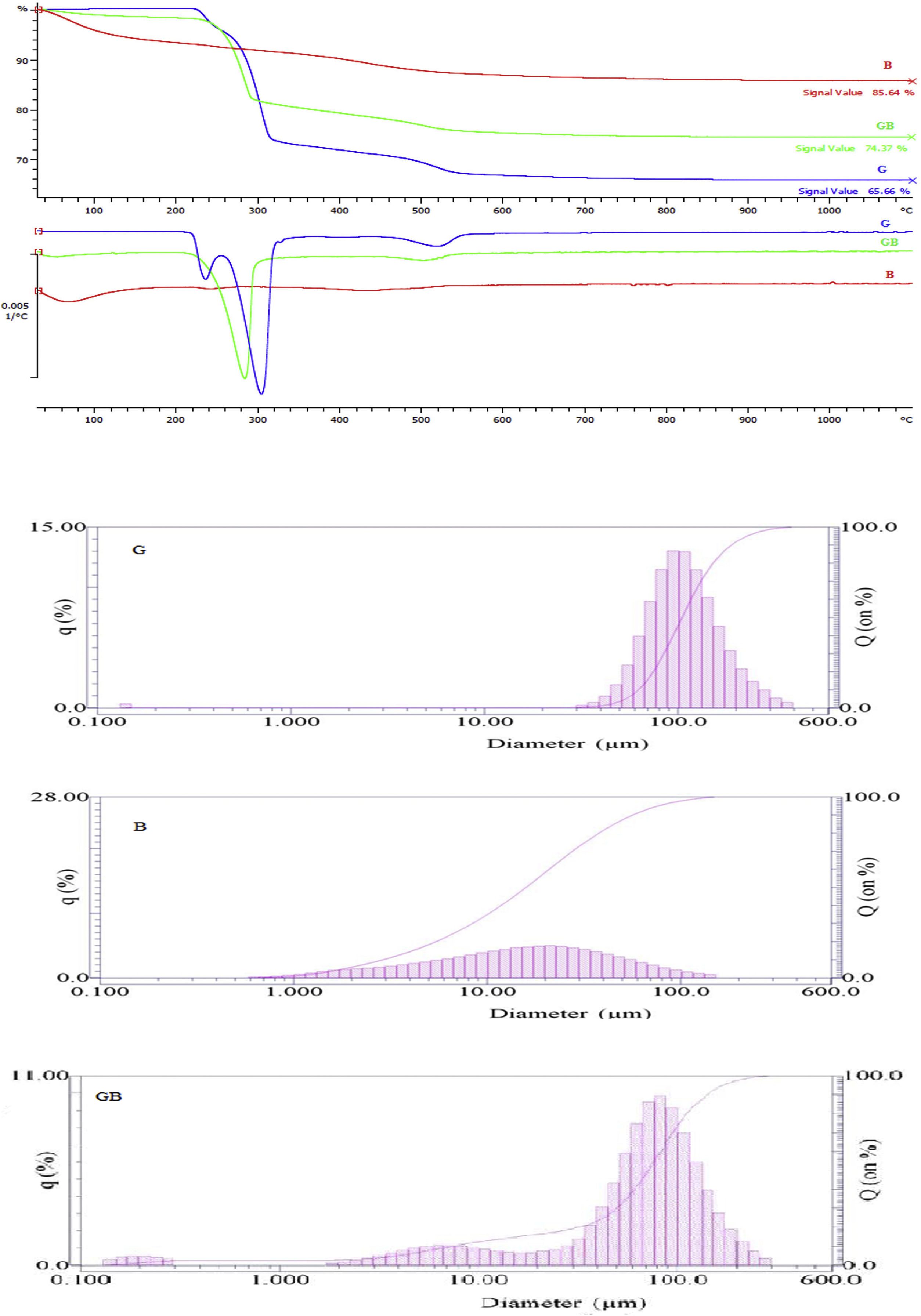
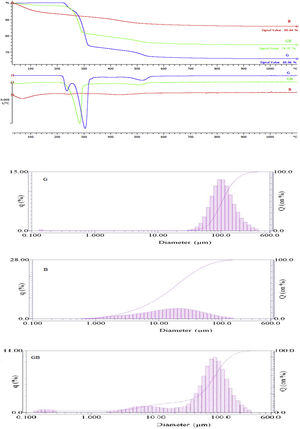
Results and discussionFig. 1 shows thermogravimetry curves and particle size distribution (PSD) of three precursors (G, B, and GB). According to TG data, the thermolysis of precursors correlates to their phase. It shows a weight loss in the aluminum hydroxide up to 500°C, because of the removal of water during the formation of the oxide phase. The water removal percents of G, GB, and B were 34.34, 25.63, and 14.36%, respectively. Maximum water removal percent related to G precursor that had gibbsite phase with 3mol of H2O per mol Al2O3 (corresponding to a stoichiometry Al2O3:3H2O). But after its milling (B precursor), the resulting aluminum hydroxide had the least water content. This water quantity is related to boehmite, 550°C. There are three peaks nearly at 240, 310, 525°C on DTG curve of G precursor. The first two peaks are associated with the loss of water during the transformation from gibbsite to boehmite, and the latter with a further transition from boehmite to γ-Al2O3. The weight loss in the first peak is due to the partial transformation of gibbsite to boehmite, the second peak relates to decomposition of gibbsite to boehmite. The TG curves between 550 and 1100°C are flat due to overall removal of OH groups up to 550°C. Therefore, Transformation of alumina phases have no loss weight after 550°C. In the DTG curves of precursors, these endothermic peaks are present sharply in G precursor, but their number and intensity decrease with increasing boehmite percentage and removal OH groups. The DTG curve of B precursor shows no peak, indicating that there is the least amount dehydroxylation with respect to others precursors (GB and G).
Table 1 shows the chemical composition of G and GB precursors. As shown in Table 1, the precursors have high purity. The purity of B and G precursors are same because the B precursor was synthesized through high energy milling the G precursor.
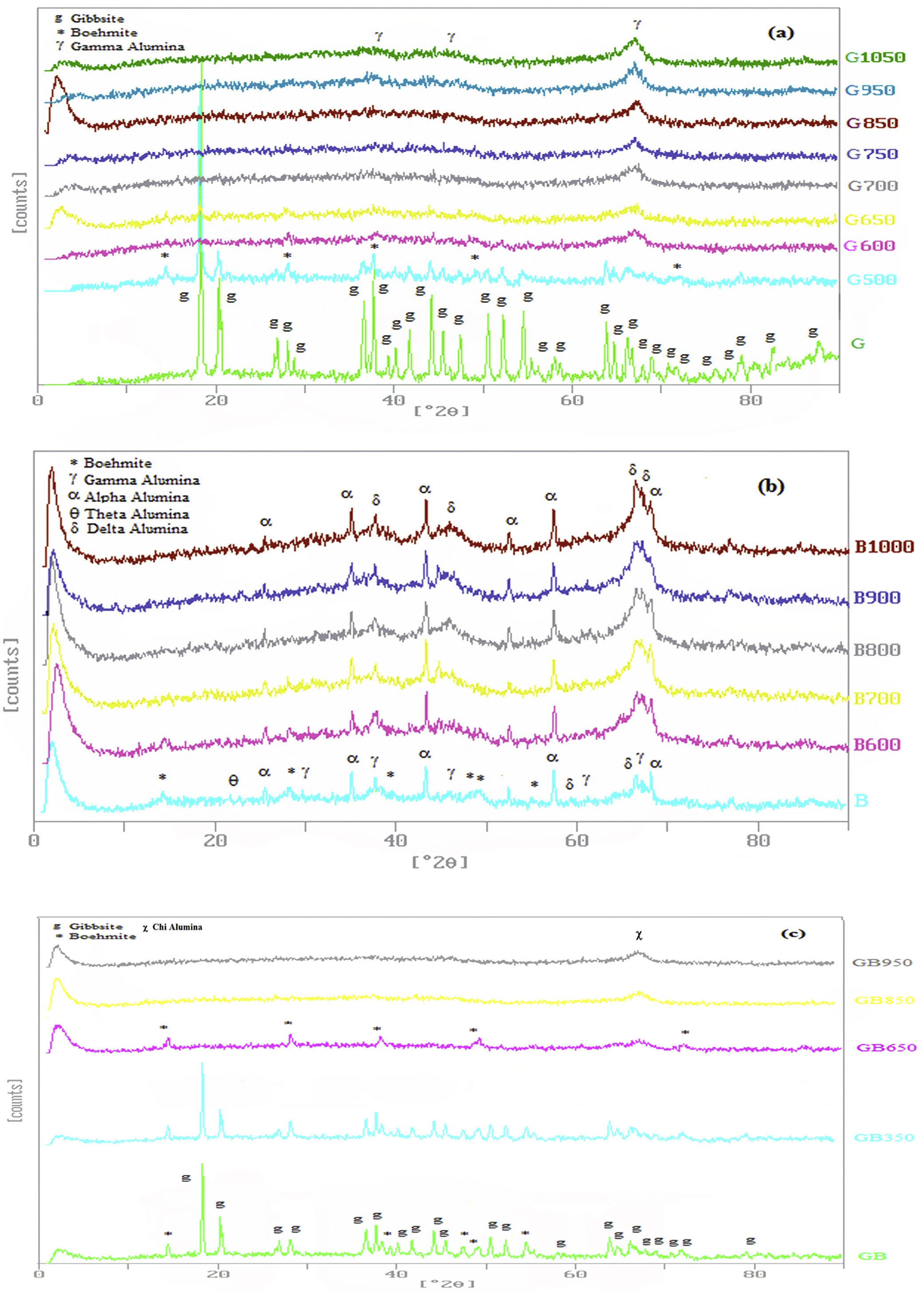
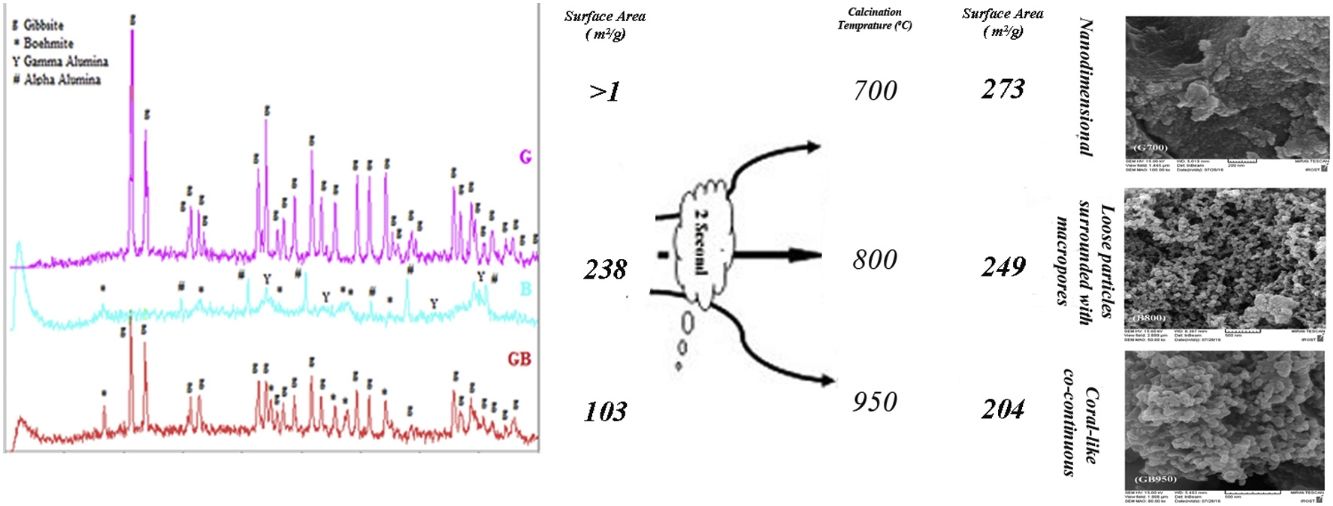
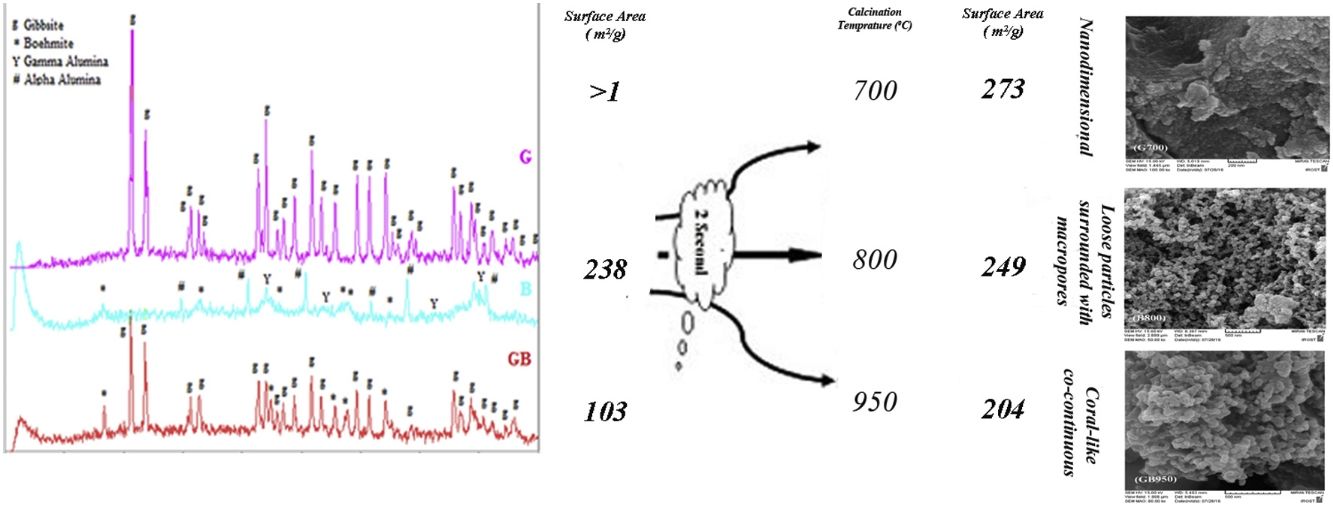
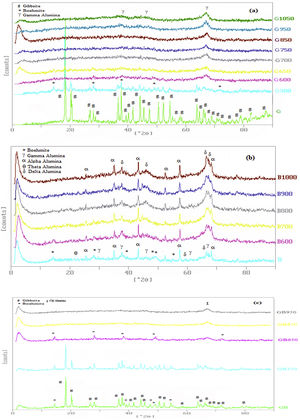
Fig. 2 indicates XRD patterns of precursors. As shown in Fig. 2, the characterized phases of the G, B and GB precursors contain gibbsite (100%), boehmite (∼82%) accompanying with aluminas (gamma, delta, theta, and alpha phases) and the mixture of boehmite (∼50%) and gibbsite (∼50%) phases, respectively. Alpha alumina phase is not contamination during milling. α-Alumina is produced from transformation gibbsite and boehmite by milling [28,29]. The PSD results show that the mean particle sizes of GB, G, and B precursors are 76.22μm, 117.67μm, and 24μm, respectively. As shown in Fig. 1, the particle size distribution of GB precursor is bimodal, because of the presence of boehmite phase with accompanying gibbsite phase. The particle size distribution of G precursor revealed monomodal curve. Also, the particle size distribution of B precursor had a nearly monomodal curve but it was wider and finer due to milling treatment.
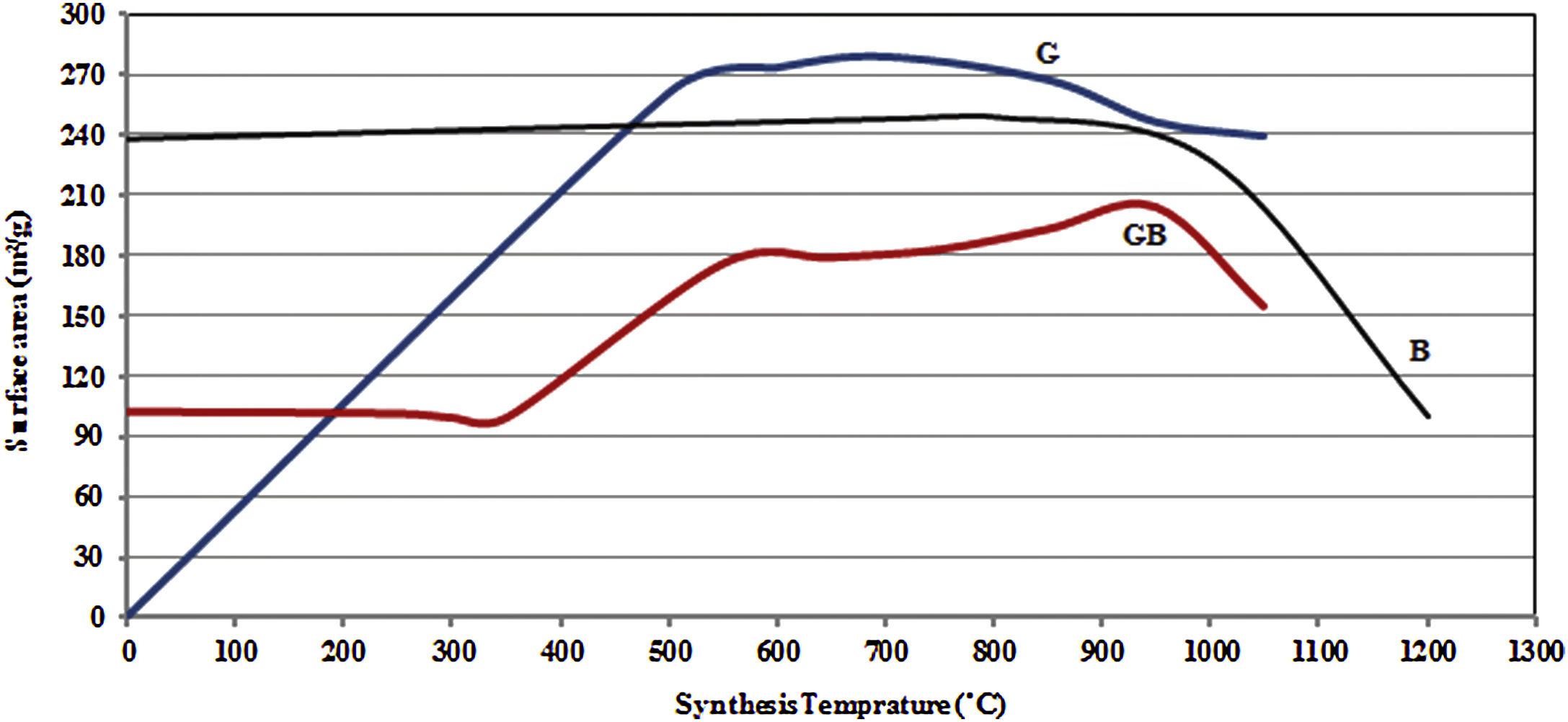
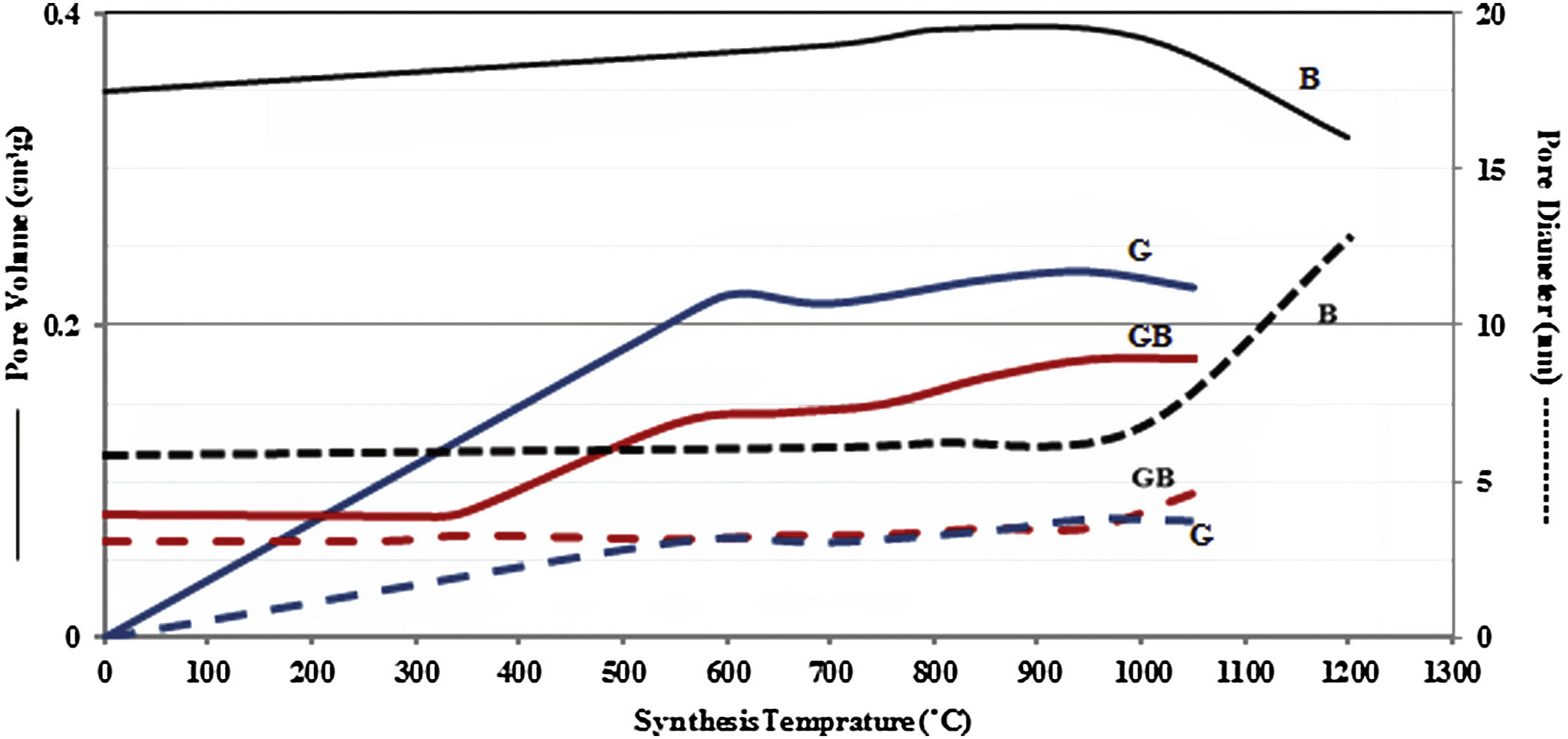
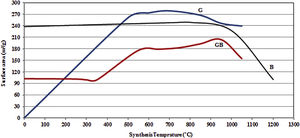
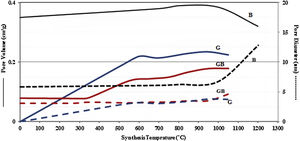
Figs. 3 and 4, show the changes of specific surface area, pore volume and mean pore diameter for G, GB and B precursors at the different temperatures of flash calcination. As shown in Fig. 3, the highest specific surface area (278m2/g) of product and its changes have been obtained for G precursor that has a low specific surface area (<1m2/g). While least changes of surface area (249m2/g) of product have been obtained for B precursor that has a high specific surface area (100m2/g). As shown in Fig. 4, the highest pore volumes and mean pore diameters of products were obtained to B precursor at the different temperatures of flash calcination. The pore diameter of products from G and GB precursors are the same at temperatures above 500°C, but pore volume of products derived from G precursor are higher than GB precursor. Therefore, products originated from G precursor have higher specific surface area in comparison with GB precursor. Table 2 shows the textural parameters of precursors and the best products. The significant step is the adjustment of surface area in customizing alumina products as a catalyst or absorbent. The specific surface area is one of the important parameters in the establishment of activity. As shown in Table 2, when the gibbsite phase is the highest amount in the precursor, the properties of activated alumina will be better in the flash calcinations process. Therefore, the maximum specific surface area will get at the lower flash calcination temperature.
Textural parameters of precursors and products.
| Precursor | Product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code | Phase (%) | Surface area (m2/g) | Pore volume (cm3/g) | Pore diameter (nm) | Max. surface area (m2/g) | Pore volume (cm3/g) | Pore diameter (nm) | Temperature (°C) | Phase | |
| Boehmite | Gibbsite | |||||||||
| GB | 50 | 50 | 103 | 0.08 | 3.04 | 204 | 0.18 | 3.48 | 950 | Chi |
| G | 0 | 100 | <1 | 0 | 0 | 278 | 0.22 | 3.10 | 700 | Chi |
| B | 82a | 0 | 238 | 0.35 | 5.88 | 249 | 0.39 | 6.28 | 800 | Gamma, delta, and alpha |
In Fig. 2, XRD patterns have been presented for three precursors at different temperatures of flash calcination. XRD patterns clarify well transformations of precursors. These changes can also be confirmed with XRD peaks. When the flash calcination temperature was increased, the peaks intensity of aluminum hydroxides declined and these phases were converted to the other alumina phases.
For G precursor (Fig. 2a), rays of gibbsite still exist up to 500°C, afterward undergo a partial dehydroxylation that transformed to boehmite phase. The transformation of gibbsite to boehmite phase is completed at 600°C due to the dehydration of gibbsite. It can be seen that none of boehmite peaks remains at 700°C. So, boehmite phase completely transformed into rho and chi phases. Chi phase formation causes maximum surface area (278m2/g) because it has the smallest size crystal. According to Table 2, the surface area of G precursor was less than 1m2/g. After the flash calcination at 700°C, it is observed that its surface area increased from 1 to 278m2/g. The change rate of the specific surface area reaches its maximum value (nearly 280 times). The percent of these phases was reduced and transformed to the crystalline phase of gamma alumina on the flash calcination above 700°C. So that, the peaks of gamma phase extended at 1050°C. Because the skeletal structure becomes denser transforming to an increased arrangement of the crystalline lattice and collapsed the small pores during the progression from chi to gamma phase. Therefore, this condition decreased specific of surface area to 239m2/g at 1050°C.
According to Table 2, with the presence of boehmite phase and decreasing the amount of gibbsite phase in GB precursor, the specific surface area of precursor increases to 100m2/g. As shown in Fig. 2c, boehmite phase exists up to 850°C for GB precursor. Boehmite phase completely transformed to chi phase at 950°C temperature at which the maximum surface area is generated. This maximum surface area (204m2/g) is approximately doubled in comparison of GB precursor. At 1050°C, the surface area of GB precursor decreased to 155m2/g.
According to Table 2, B precursor (with 82% boehmite phase) has a specific surface area of 238m2/g before the flash calcinations process. The increasing calcination temperature does not noticeably effect on the increased surface area.
As shown in Fig. 2b, the amount of boehmite phase decreases when the calcination temperature increases. Boehmite phase eliminated at 800°C but others phases still exist at this temperature. The maximum surface area of product was obtained 249m2/g at 800°C. This maximum surface area approximately equivalent with the surface area of G precursor (239m2/g) at 1050°C. Thus, G precursor has a superior surface area in the comparison of B and GB precursors at the different flash calcination temperatures. For B precursor, when the calcination temperature is increased, gamma phase was eliminated and the delta and alpha phases grow up. This situation accompanies with decreasing its surface area that this decreased drastically to 99.6m2/g at 1200°C.
Although B precursor has the finest mean particle size but this precursor has the lowest changes in surface area of product than the other precursors. Therefore, the phase type is more effective than the mean particle size in the precursors. The breaking and removal of the structural water from the precursor generate pressurized water vapor during the flash calcination process. This pressure diffuses between the layers of crystalline precursors and cracks grow up all over the particles and cause an explosive state inside the precursors. This situation, also, establishes hydrothermal and self-steaming conditions inside the particles due to water cannot rapidly evaporate. Therefore, the presence of water vapor changes the transformation consequence and the morphology of alumina. So that the formation rate of Chi phase and other transition of alumina phases accelerate. As a result, the water vapor pressure has a considerable effect on the surface area and porosity in the active alumina. This situation is intensified with increasing the number of these molecules in alumina hydroxide phases since the internal water vapor pressure reaches a critical value in gibbsite containing three water structure molecule. As a result, more particles were split and assisted to nucleate and grow the phases (χ-Al2O3 and some rho alumina). This increases 280 times its surface area in comparison with the gibbsite precursor while the boehmite phase with one molecule of water structure presents less effect.
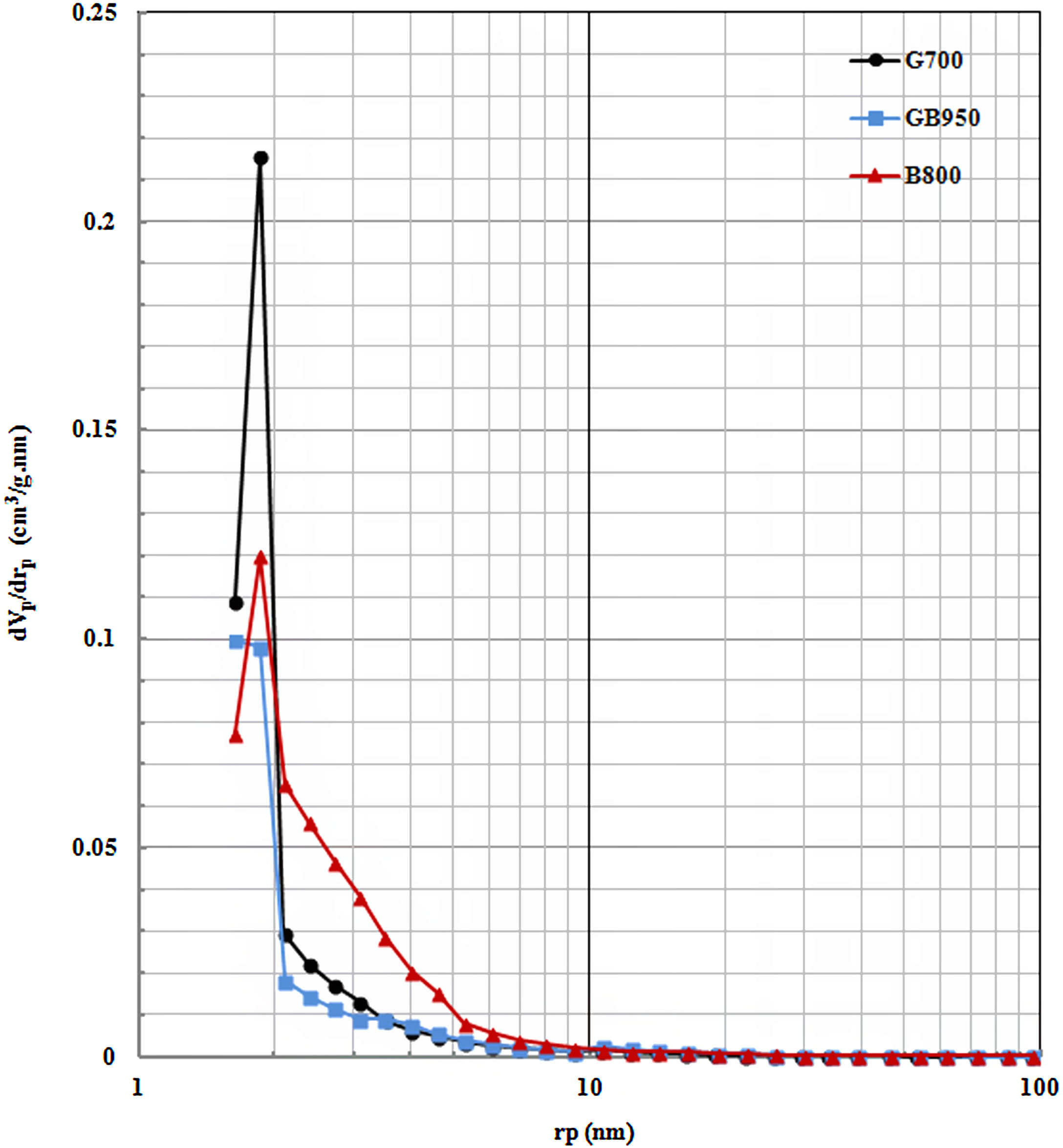
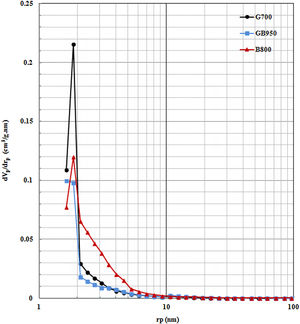
Fig. 5 shows the pore size distributions of active powders (G700, GB950, and B800) that have the maximum surface area. The eliminating OH-groups will cause the pore size's development. As shown in Fig. 5, the peak of pores existed between 1 and 2nm for these active powders. The volumes of these small pores provide to their maximum surface area. G700 has most pore volume in this limit that causes the largest surface area in the comparison of other activated alumina powders. G700 exhibited a uniformly porous structure. Although B800 has the highest total pore volume, this pore volume belongs to the limit of larger pores (3–10nm) and has a wider pore size distribution. That its surface area is lower than G700, and its average pore size of B800 is twice average pore size than GB950 and G700, according to Table 2. GB950 has the lowest volume of small pores that causes the least surface area than the G700 and B800. Thus, the mesopore distribution is more impressive on the amount of surface area.
Fig. 6 shows FESEM images of B800, G700, and GB950. As shown in Fig. 6, there are various morphologies in these images. G700 has nanostructure and particle growth in nanodimensional. These nanoparticles cause a high surface area. GB950 forms a coral-like co-continuous morphology and B800 has loose particles surrounded with macropores.
ConclusionsThe main conclusions from this study are as follows:
- 1.
The precursor phases affect on the textural properties of activated alumina powders in the flash calcination process.
- 2.
The quantity of structural water molecules is the prominent phenomenon compared to the particle size of precursor.
- 3.
Releasing OH-groups establish hydrothermal conditions for nucleation and growth the desirable phases during the flash calcination process.
- 4.
The presence of gibbsite phase with larger particle size has better productivity condition and product properties compared to the boehmite phase with small particle size.
- 5.
Surface area of product increases nearly 280 times for gibbsite precursor that has the least surface area and larger mean particle size.
- 6.
Others alumina phases like boehmite with fine mean particle size presents less effect on the trend of specific surface area variations due to comparatively low water loss.
- 7.
The phase of precursor forms different morphologies of products like, nanodimensional, and a coral-like co-continuous or loose particles surrounded with macropores.
The author is grateful to the Iranian Research Institute of Petroleum Industry (RIPI) and Research, Development and Technology Directorate of National Iranian Gas Company for financial support, which enabled this work to be undertaken.