Novel (Cr,V)-ZrSiO4 jewel green pigments with near-infrared reflection performance were prepared at a low temperature of 900°C. Effects of Cr/Si mole ratio on phase composition, microstructure, Vis–NIR reflection performance, optical properties and thermal stability were studied. The results show that with the increase of Cr/Si mole ratio from 0 to 0.4, the color of the pigment changes from blue to jewel green and then to dark green. When the Cr/Si mole ratio is 0.2, the (Cr,V)-ZrSiO4 jewel green pigment shows the best comprehensive properties, which has rendering performance (L*=56.69, a*=−18.52, b*=−9.28), thermal stability, and near-infrared reflection performance. The properties especially excellent near-infrared reflection performance make it promising functional pigment in heat insulation.
Se prepararon nuevos pigmentos verde joya (Cr,V)-ZrSiO4 con rendimiento por reflejo en el infrarrojo cercano a una temperatura baja de 900 ̊C. Se estudiaron los efectos de la relación molar Cr/Si sobre la composición de fase, la microestructura, el rendimiento de reflexión Vis-NIR, las propiedades ópticas y la estabilidad térmica. Los resultados muestran que con el aumento de la relación molar Cr/Si de 0 a 0,4, el color del pigmento cambia de azul a verde joya y luego a verde oscuro. Cuando la relación molar Cr/Si es 0.2, el pigmento verde joya (Cr,V)-ZrSiO4 muestra las mejores propiedades integrales, que tiene rendimiento de renderizado (L*=56.69, a*=-18.52, b*=-9.28), estabilidad térmica y rendimiento de reflexión en el infrarrojo cercano. Las propiedades especialmente excelente rendimiento de reflexión en el infrarrojo cercano lo convierten en un pigmento funcional prometedor en el aislamiento térmico.
Inorganic pigments have been widely used in ceramic products and play an indispensable role in the decoration of ceramic products [1–3]. According to the differences in chromophore, the ceramic pigments can be divided into corundum-type [4], rutile-type [5], fluorite-type [6], spinel based [7] and zircon (ZrSiO4) based [8], etc. Zircon exhibits excellent physical and chemical properties due to its stable crystal structure, such as high melting point (2500°C), low thermal conductivity, small expansion coefficient, and strong corrosion resistance to molten glasses and acidic reagents, etc., which has received widespread attentions [9,10]. There are many researches on zircon (ZrSiO4) based ceramic pigments [11–14]. Solid solution zircon (ZrSiO4) based ceramic pigments belong to ion-substitution pigments, and the color obtained mainly depends on the oxidation state and coordination number of colored ions. In recent years, green zircon (ZrSiO4) based pigments have attracted increasingly more attentions. Yang et al. [12] successfully synthesis green submicronic Cr-ZrSiO4 ceramic pigments with 1wt% Li2CO3. Ocana et al. [13] used NaF as the mineralizer to synthesize Cr-doped zircon pigments at 1100°C by hydrolysis aerosol. Naga et al. [14] prepared Cr-ZrSiO4 pigment using CaF2, LiF and NaF as mineralizers by reacting around at 1200°C. However, extremely high-temperature leads to an increase in energy consumption, making it difficult for large-scale industrial production. Nowadays, with the improvement of quality of life, people not only put forward color requirements for pigments, but also hope to seek functional breakthroughs [15,16]. Chromium doped pigments have near-infrared properties [17,18], however, there are few studies on the near-infrared properties of Cr-doped zircon based pigments. In addition, most of the green ceramic pigments are colored by chromium and the hue of pigments is relatively monotonous. Jewel green is a symbol of nobility, and it is popular with people. The jewel green pigment becomes mainstream for its soft and bright color and velvety texture. The Cr–V co-doping reduces the synthesized temperature while obtaining pigment with good rendering performance, and also possesses near-infrared performance. In this work, we reported a novel (Cr,V)-ZrSiO4 jewel green pigment with near-infrared reflection performance at a low temperature of 900°C. The effect of Cr/Si mole ratio on the pigment performance was investigated.
Material and methodsAnalytical grade ZrOCl2·8H2O (Shanghai Aladdin Co., Ltd.) and Na2SiO3·9H2O (Shanghai Aladdin Co., Ltd.) were used as starting materials directly without further refinement. Na3VO4 (Sinopharm Chemical Reagent Co., Ltd.) and Cr2O3 (Sinopharm Chemical Reagent Co., Ltd.) were used without purification. In typical process for the pigments synthesis, 10mmol ZrOCl2·8H2O, 10mmol Na2SiO3·9H2O, 1mmol Na3VO4 and x mol% Cr (x=0, 2.5, 5, 10, 20 and 40, 2-fold isometric interval for doping samples) were mixed and homogenously ground in an agate mortar for 15min. In the mechanical grinding process, the solid reaction takes place between ZrOCl2·8H2O and Na2SiO3·9H2O. Then the mixture was transferred into a corundum crucible with a lid and calcined at desired temperature 900°C for 2h. After natural convection inside the furnace for cooling, the final products were obtained by washing with deionized water for three times, filtering and drying at 70°C for 6h.
The ceramic body and glaze were supplied by Peiyintang Ceramic Glaze Shop in Jingdezhen, China. The color of the ceramic body is white, and the glaze is applied by dipping on the ceramic body. The glazing process was performed by mixing the synthesized pigments with a transparent glaze (using mass ratio 5–95, respectively), followed by calcination in air at 1200°C for 30min.
The crystalline phases were identified by X-ray diffraction (XRD, D8 Advance, Bruker, Germany) analysis with Cu Kα radiation (λ=0.154nm) and the experimental data were collected in the 2θ=10–70° with step width and counting time of 0.02° (2θ/s) and 0.2s, respectively. The microstructure of the powders was observed by field-emission scanning electron microscopy (FE-SEM, SU-8010, JEOL, Japan) and transmission electron microscope (TEM, JEM-2010, JEOL, Japan). The particle size and distribution range of the powders were characterized by laser particle size analyzer (Mastersizer 3000, Malvern, UK), and the particle size distribution curve was given according to the results. The reflectance of visible light and near infrared of samples was characterized by UV–Vis–NIR spectrophotometer (PerkinElmer, Lambda 950). The colorimetric L*a*b* parameters of the pigments was tested by an automatic WSD-3C whiteness meter (produced by Beijing Kangguang Instrument Co., Ltd.). The standard illuminant is standard D65 light source, the standard observer is 10° field of view, and the measuring diameter is Φ18mm. In this system, L* is the color lightness (from black L*=0 to white L*=100), a* is the green (−a*)/red (+a*) axis, and b* is the blue (−b*)/yellow (+b*) axis, respectively.
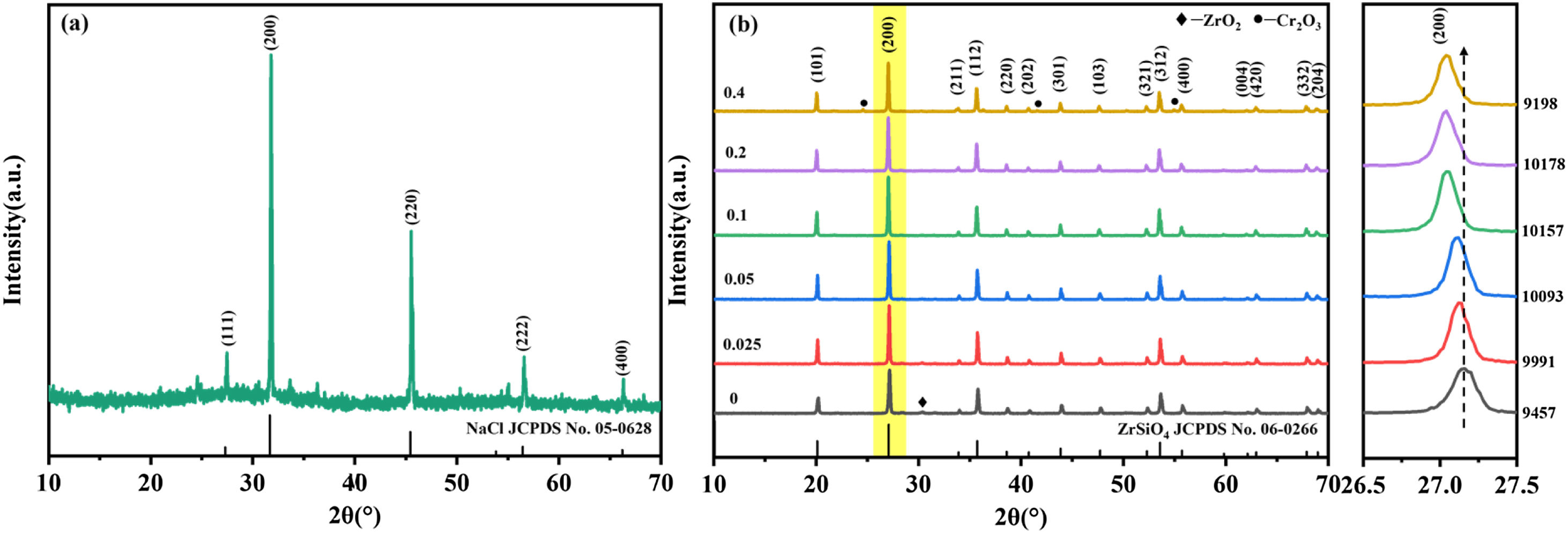
Results and discussionFig. 1(a) shows the XRD patterns of precursor mixture with Cr/Si mole ratio of 0.2 after mechanical grinding, as can be seen that the NaCl (JCPDS No. 05-0628) phase is shown in the precursor mixture after mechanical grinding, indicating that a solid-state reaction does indeed occur during the grinding process. Fig. 1(b) shows the XRD patterns of samples prepared with different Cr/Si mole ratios, in which Cr/Si mole ratios are 0.025, 0.05, 0.1, 0.2 and 0.4 respectively. For the sake of comparison, the XRD pattern of the sample containing without Cr is also given in Fig. 1(b). As can be seen from the figure, the primary crystalline phase of the samples is ZrSiO4. The intensities of diffraction peaks of zircon phase (JCPDS No. 06-0266) in samples with Cr–V co-doping are significantly higher than that of the sample without Cr. When the mole ratio of Cr/Si increases to 0.4, it shows the unexpected Cr2O3 heterophase. These results indicate that an appropriate amount of Cr–V co-doping can effectively promote the growth and development of ZrSiO4 crystal, while the excessive Cr-doping is easy to cause heterophase and affect the synthesis of ZrSiO4.
Additionally, it can be found from the local magnification of the (200) crystal plane of zircon phase (JCPDS No. 06-0266) that the diffraction peaks of samples showed significant shift in comparison with the sample without Cr-doping, indicating that Cr ions can be successfully doped into the lattice. The figures of (200) zircon reflection area are also given the value increases first and then decreases as the Cr/Si ratio increases from 0 to 0.4. Recent studies have shown that V4+ exists in the interstitial site (16g) in the ZrSiO4 lattice [11,19]. Therefore, as the Cr/Si molar ratio increases, the diffraction peaks of samples shift to lower angle can be attributed to the mutual promotion of Cr–V solid solutions in the lattice [20]. However, the displacement near (200) peak is no longer shifted to a large angle as the Cr/Si mole ratio is 0.4, indicating that the amount reaches the maximum.
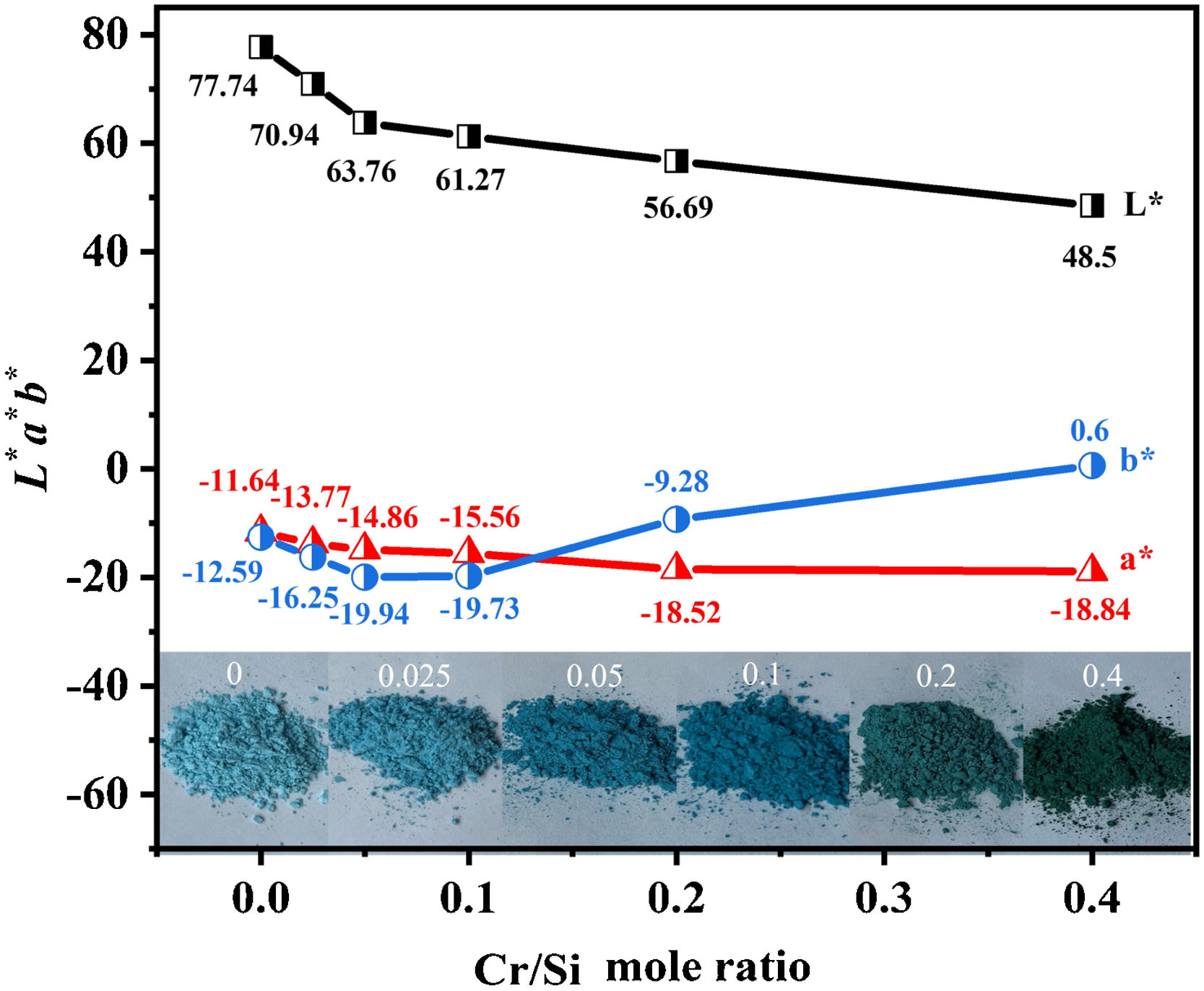
Fig. 2 exhibits chromatic values of samples with different Cr/Si mole ratios, and the inner inserted illustrations are the physical graphs of the samples. The color of the pigments changes from blue to jewel green and then to dark green, with the increase of Cr/Si mole ratio from 0 to 0.4. The sample without Cr-doping shows in sky blue due to the reduction of V5+ to V4+[21], and it dissolves in the ZrSiO4 lattice to form V-ZrSiO4. The introduction of Cr-doping promotes the formation of (Cr,V)-ZrSiO4 solid solution, due to the presence of (V)-ZrSiO4 in which (V) plays the roles of both mineralizer and dopant. The extrinsic electron configuration of Cr (1s22s22p63s23p63d54s1) is similar to that of V (1s22s22p63s23p63d34s2). After Cr-doping, the whole energy band is shifted, and the Fermi energy level enters the conduction band. The band gap narrows with the formation of an electron transition ladder, which is conducive to the visible light absorption [22]. More Cr ions enter into the lattice of ZrSiO4 with the increase of Cr/Si mole ratio. According to the data shown in Fig. 2, the sample without Cr-doping appears in sky blue. The L* and a* values decrease gradually, and the value of b* decreases first and then increases with the increase of Cr-doping content. This indicates that the increase of Cr/Si mole ratio can increase the greenness values of the pigments. However, Cr2O3 heterophase appears in the sample with the Cr/Si mole ratio further increases to 0.4, and the change of greenness value is not obvious. Cr2O3 heterophase gives the pigment an overall black hue. Therefore, the optimized Cr/Si mole ratio is 0.2 in the investigation Cr-doping content, at which the (Cr,V)-ZrSiO4 pigments possess optimized performance. This pigment shows in a typical jewel green with L*=56.69, a*=−18.52, and b*=−9.28, respectively.
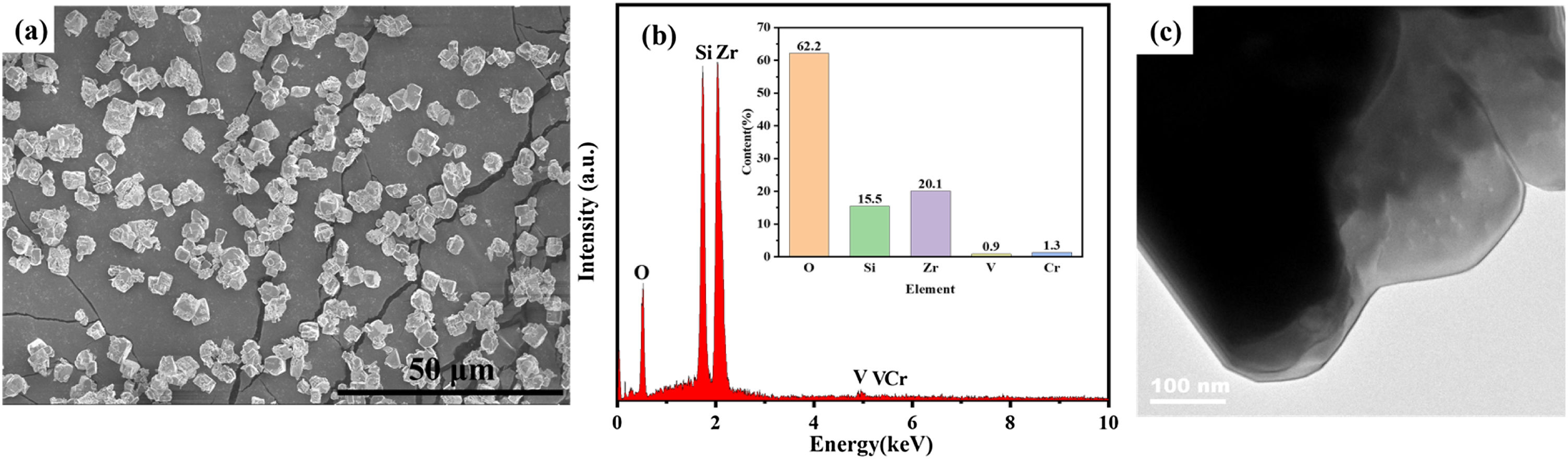
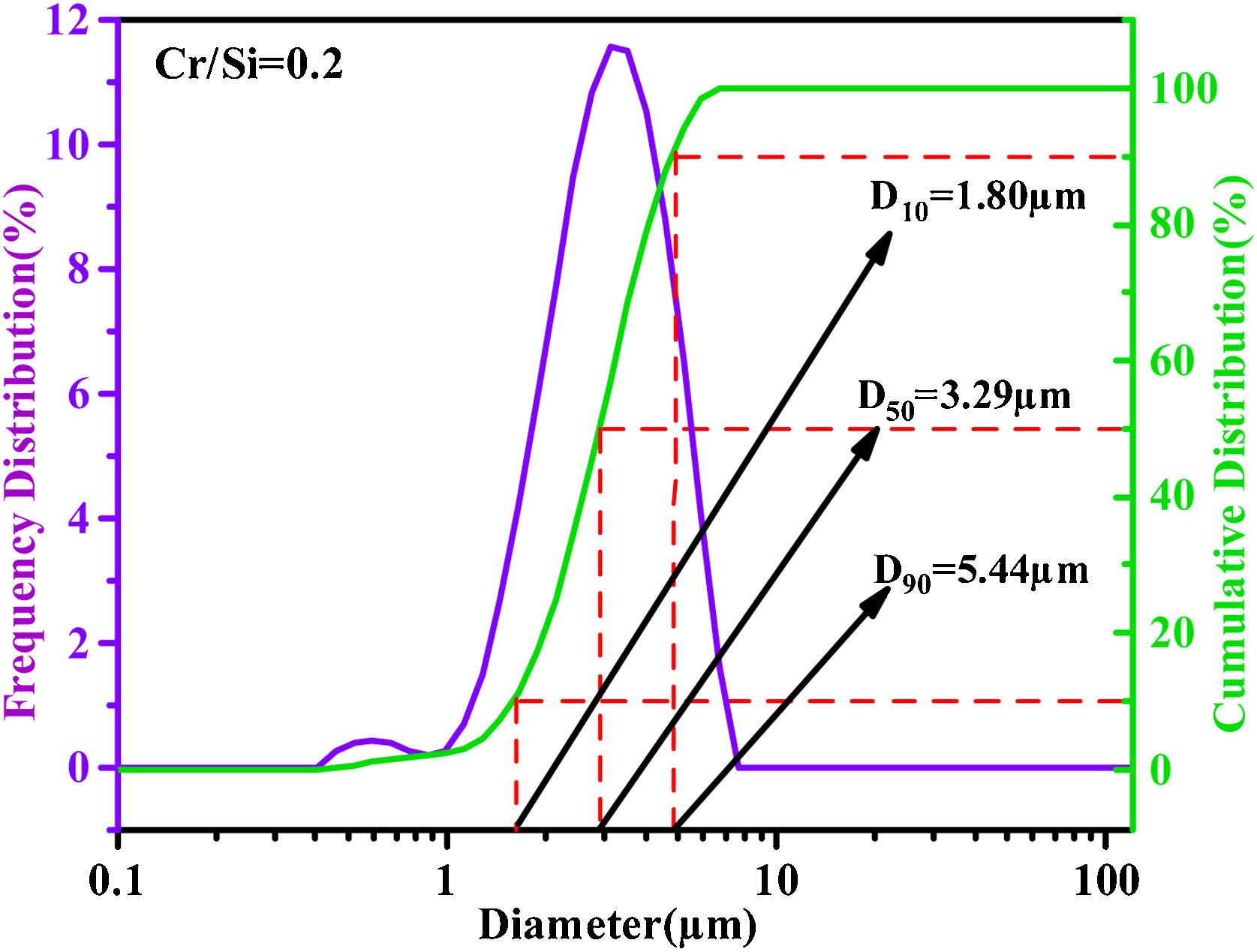
Fig. 3 shows the SEM image (a), EDS spectrum (b) and TEM (c) images of the sample with Cr/Si mole ratio of 0.2. As can be seen in Fig. 3, the particle distribution of the pigments is relatively uniform. It can be found from the EDS spectrum that the sample contained Zr–Si–O–Cr–V elements. The surface of the pigment is dense with distinctive edges and crystal faces, and the average particle size is about 2–6μm. Fig. 4 shows the particle size distribution of the sample with Cr/Si mole ratio of 0.2. The D50 of the pigment is 3.29μm. In addition, the particle size distribution of the pigment is narrow, which is consistent with the particle size observed in the SEM image shown in Fig. 3(a). These results indicate the optimized (Cr,V)-ZrSiO4 pigment has appropriate and relatively uniform particle sizes for glazing.
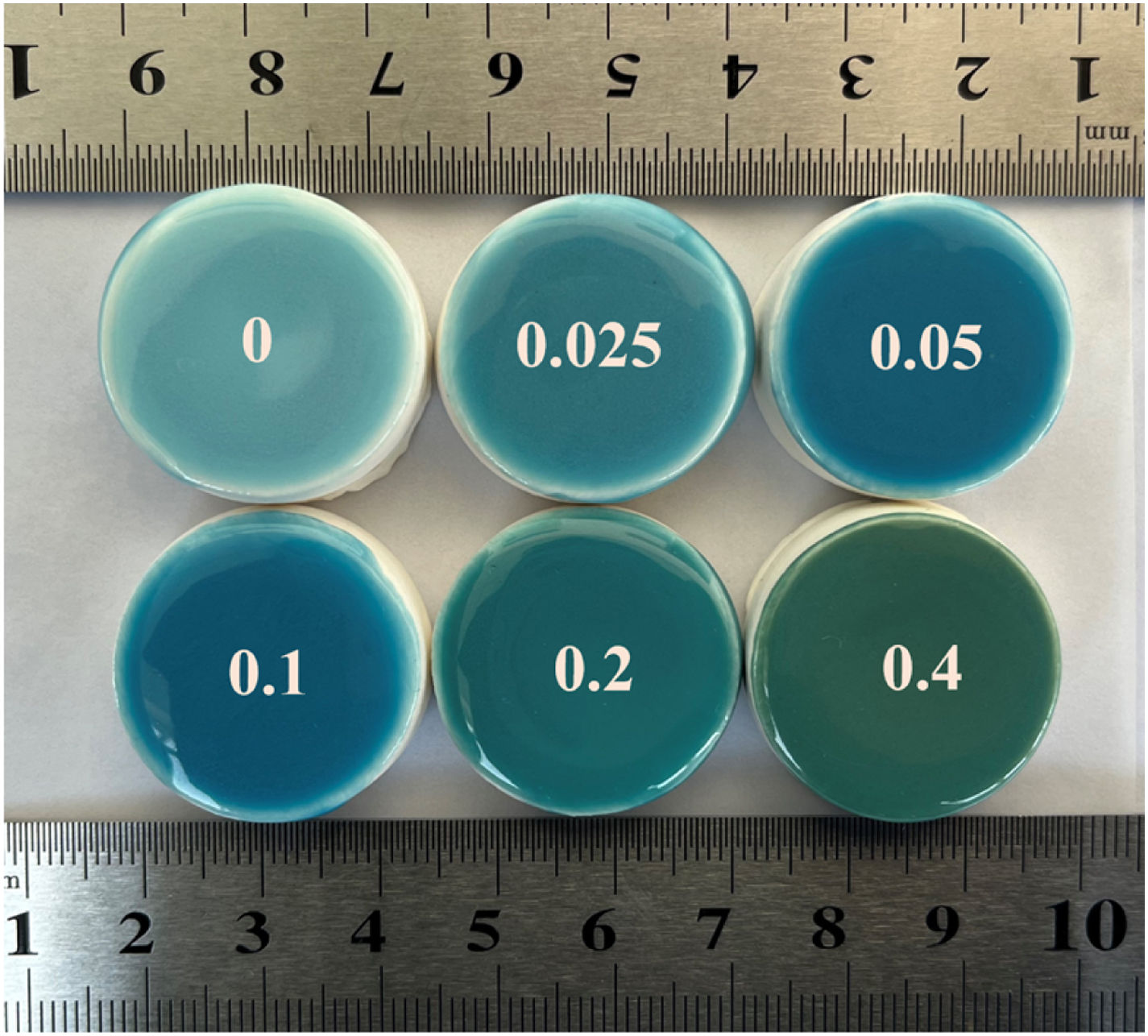
Fig. 5 shows the photographs of glazing samples. The glazing samples are obtained by adding 5wt% (mass percentage of transparent glaze) pigment into the transparent glaze, applying it to the blank body and then calcined at 1200°C for 30min. It can be seen from this figure that the glazing exhibits with a smooth and glassy surface. In addition, the hue of glazing samples matches well with the hue of the pigments, which suggests that the pigments have excellent chemical and thermal stability.
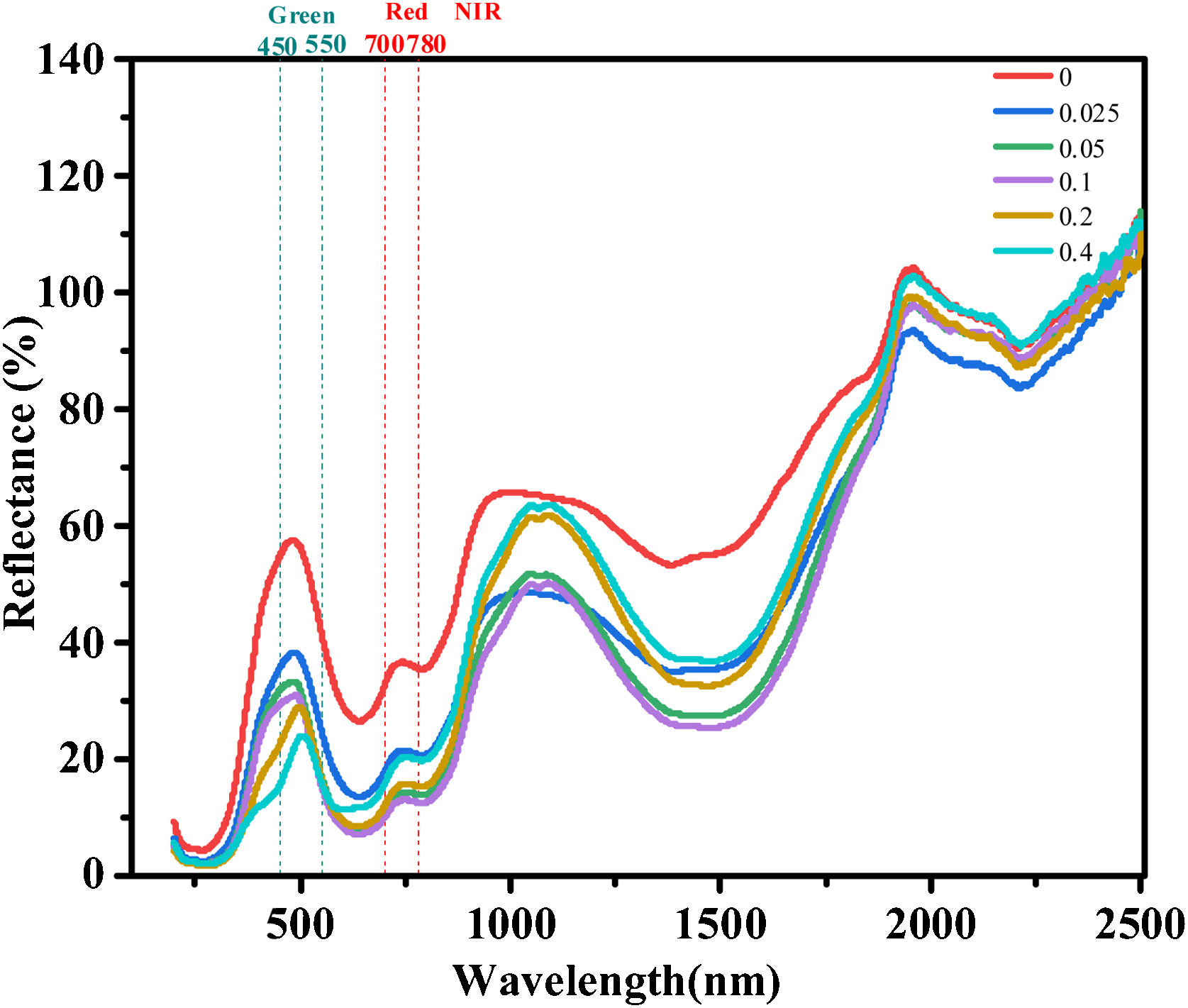
The glazing samples are applied to the visible and near-infrared reflection performance test. Fig. 6 shows the reflectivity curves of glazing sample with different Cr/Si mole ratios. As can be seen from Fig. 6 that the glaze with (Cr,V)-ZrSiO4 pigment have high reflection characteristics at 400–2500nm. The visible light band in the range of 450–550nm corresponds to a typical jewel green, indicating a good rendering performance of the sample. The wavelength range of 700–780nm corresponds to the red band, which is caused by Cr–V Co-doping. Furthermore, the reflection band at >780nm corresponds to the near-infrared band. It can be seen that when the Cr/Si mole ratio is 0.2, the average reflectivity in the wavelength range of 780–2500nm reaches 65%, indicating a typical near-infrared reflection. The reflectivity is still increasing with the increase of wavelength, indicating the (Cr,V)-ZrSiO4 pigments possess appreciable near-infrared reflection performance. Since near-infrared accounts 52% in total energy of sun light over 780–2500nm. Thus, the (Cr,V)-ZrSiO4 pigment can be used as cold pigments in the fields of architectural paints, vehicle paints, exterior wall brick and tile glazes for energy saving and health.
ConclusionsNovel (Cr,V)-ZrSiO4 jewel green pigments are synthesized. The results show that with the increase of Cr/Si mole ratio from 0 to 0.4, the color of the pigment changes from blue to jewel green and then to dark green. The appropriate amount of Cr–V co-doping can effectively promote the growth and development of ZrSiO4 crystal, while the excessive Cr-doping is easy to cause heterophase and affect the synthesis of ZrSiO4. The optimized Cr/Si of 0.2, results in a typical jewel green with L*=56.69, a*=−18.52, and b*=−9.28. The pigment is proved to possess excellent thermal and glazing stability, and good near-infrared reflection performance.
This work was supported by the National Natural Science Foundation of China [grant numbers 52072162, 51962014, 52262003]; Jiangxi Provincial Natural Science Foundation [grant numbers 20202ACBL214008, 2020ZDI03004, 20202BABL214013, 2020GYZD013-09]; the Science Foundation of Jiangxi Provincial Department of Education [grant numbers GJJ201345, GJJ211308]; the Jingdezhen Science and Technology plan project [grant numbers 2021ZDGG001, 20202GYZD013-14, 20224GY008-07]; National College Student Innovation and Entrepreneurship Training Program [grant numbers 202210408009].