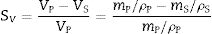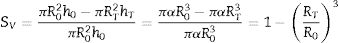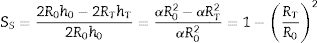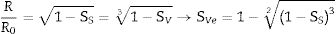Tin oxide is one of the most extensively studied semiconductor materials due to its broad field of applications. On the one hand, its high conductivity and its corrosion resistance are the most remarkable properties. Therefore, one of the most developed uses in the recent decades has been as ceramic electrode for electrooxidation process. On the other hand, its poor sinterability hinders a broader use. As a result, the use of advanced techniques or sintering aids for obtaining low-porosity specimens is necessary. So far, many additives have been studied, CaCO3, Co3O4, Nb2O5 or MnO2, among others. In the present work, the sintering behaviour of SnO2-based powder, containing Li2CO3 as a sintering aid, which generates a liquid phase, has been analysed, since it is one of the additives that has been studied to a lesser extent. The effect of the amount of sintering aid just like the thermal treatment parameters (maximum temperature, heating rate and soaking time) on volumetric contraction's evolution has been studied through a factorial experiment designs 2n. The results show that an amount of lithium carbonate greater than 1mol.% is unfavourable to densification. With regards to the thermal cycle's parameters, it is advisable to have thermal treatments at high temperatures (1300°C) with moderate soaking times (1h), as maximum temperatures have the biggest influence on the densification followed by soaking time while the heating rate has a lesser influence. Under these conditions, a microstructure of closed and rounded pores is obtained, in which a residual phase is enclosed, but the small proportion of which prevents its characterisation.
El óxido de estaño es uno de los materiales más estudiados, dado su extenso campo de aplicación. De entre sus propiedades, cabe destacar su alta conductividad eléctrica y su resistencia a la corrosión, de ahí que entre sus aplicaciones se encuentre la de electrodo cerámico. Sin embargo, su baja capacidad de densificación dificulta su uso. Como consecuencia, se requieren técnicas avanzadas o aditivos de sinterización que faciliten la obtención de piezas con baja porosidad. Hasta el momento han sido muchos los aditivos estudiados, CaCO3, Co3O4, Nb2O5 o MnO2, entre otros. En este trabajo se estudia el proceso de sinterización de piezas de SnO2 a las que se ha incorporado Li2CO3 como aditivo de sinterización, el cual genera una fase líquida, por ser uno de los aditivos en cuyo estudio se ha profundizado en menor medida. A través de diseños factoriales de experimentos 2n, se han determinado los efectos de los diferentes parámetros del ciclo de cocción (temperatura máxima, velocidad de calentamiento y tiempo de permanencia), así como la cantidad de aditivo de sinterización, sobre la evolución de la contracción volumétrica. Los resultados muestran que la incorporación del carbonato de litio, en porcentajes superiores al 1% molar, no es favorable para la densificación. Respecto a los parámetros de cocción, resultan recomendables tratamientos térmicos a elevada temperatura (1.300°C) con tiempos de permanencia moderados (1 hora), mientras que la velocidad de calentamiento ejerce una menor influencia. En estas condiciones se obtiene una microestructura de poros cerrados y redondeados, en la que queda encerrada una fase residual cuya reducida proporción impide su caracterización.
Tin oxide is a material whose remarkable physicochemical properties, including its high conductivity (n-type semiconductor) and resistance to corrosion, allow it to be used in a wide range of sectors. Traditionally, it has been used in the ceramic industry as a raw material to produce pigments [1] and as opacifier in glazes [2]. But nowadays its use has been extended to the field of electronic and chemical industries, becoming a widely used material in the production of gas sensors [3,4] or components that require high resistance to chemical corrosion [5]. In the latter case, the production of electrodes for the processing of aluminium by electrolysis is noteworthy [6,7].
However, tin oxide is also known to sinter without densifying [8], which makes it difficult to use and requires specific sintering techniques to obtain parts with low porosity, such as hot isostatic pressing [9] or activated sintering [10,11]. Another option is the addition of certain metal oxides that act as sintering promoters to increase densification. In the latter case, the usual mechanism consists of the formation of a eutectic liquid at a low temperature between SnO2 and the sintering additive, thus favouring a sintering in the presence of liquid phase. Several metal oxides have been studied in recent years for this purpose, including ZnO, CaCO3, Co3O4, Nb2O5 or MnO2, among others [12–17].
In a previous work, the sintering process of SnO2 electrodes to which Bi2O3 had been incorporated as a sintering additive was analysed [18]. In the present work, we study the influence of Li2CO3 as sintering aid over the sintering process of SnO2 with the aim to compare the results with the obtained ones in the aforementioned publication, since lithium oxide is an abundant material, not very expensive, with a high electrochemical potential, which does not contribute colour when introduced into a composition and which, despite having been mentioned, has not received as much attention as those mentioned above.
Respect to the sintering process, Li2O has been proposed as a dopant for SnO2[19], concretely for obtaining transparent conducting films by spray pyrolysis [20]. In addition, Li2O can also be proposed as sintering additive for SnO2, under the hypothesis that it generates a liquid phase during the heat treatment. However, this cannot be confirmed from a thermodynamic point of view as the phase diagram of the system Li2O–SnO2 has not been published (to our knowledge, the only diagram containing both oxides corresponds to Li2O–SnO2–SiO2 system, but it does not cover the SnO2-rich zone [21]). As additional data that support the hypothesis, the crystal structures of two mixed oxides in the SnO2–Li2O system, Li8SnO6 and Li2SnO3 have been described, compounds which have been synthesised by solid state reaction at around 950°C and 1000°C respectively [22,23], but their melting temperature has not been reported. Additionally, one study indicates that Li2SnO3 can be sintered at around 800°C [24]. With these data and considering the melting point of pure Li2O (1473°C) [25], it is likely that there is an eutectic in the Li2O–Li8SnO6 system capable of generating a liquid phase at moderate temperatures which helps to densify SnO2. In addition, their high melting point could reduce the losses of additive along sintering that are characteristic of other sintering aids as Bi2O3[18].
Since lithium oxide reacts violently with water, lithium carbonate was used as an additive in this work. This salt has a melting point of 732°C and decomposes completely at around 1300°C. However, Li2CO3 decomposition is a complex multistage process, which can start at temperatures of the same order as melting point, depending on the composition of the surrounding atmosphere [26]. Thus, the liquid phase generated by the Li2CO3 melt, although its exact nature depends on the temperature and the surrounding atmosphere, could initiate densification at temperatures lower than those required for Li2O to form a eutectic, especially if sintering is performed at moderate temperatures. If sintering is performed at temperatures close to the decomposition temperature of Li2CO3, a eutectic liquid could already be formed, corresponding to the Li2O–Li8SnO6 system, which in this case would also facilitate densification. In this sense, the use of lithium oxide as a sintering aid has been described for various materials such as garnets [27,28], samarium-doped ceria [29], or yttrium-doped ceria [30], as well as lithium carbonate for the sintering of calcium carbonate [31]. In the case of doped ceria, which would be the closest system to SnO2, the results suggest that the key role is played by Li2O from the decomposition of carbonate, but there is no agreement about the mechanism of action.
In this work, the behaviour of lithium carbonate as a sintering additive for tin oxide has been analysed, together with the effects of heat treatment parameters, by means of 2n factorial designs of experiments [32]. In a first design, the effects of Li2CO3 ratio and heating rate on the sintering evolution during the heating stage have been studied. In a second design, in addition to the aforementioned variables, the effects of maximum temperature and soaking time have been analysed. From the results obtained, it has been possible to define the variables with the greatest effect on the sintering of SnO2 in the presence of Li2CO3.
Experimental procedureThe raw materials used were SnO2, (Quimialmel S.A., purity 99.85%, Spain) and Li2CO3 (Panreac S.A., purity 98%, Spain). Polyvinyl alcohol (PVA, Mowiol 4-88, Clariant Iberica S.A., Spain) was used as binder for the green specimens. Two compositions A and B were designed, with Li2CO3/SnO2 molar ratios of 1/99 and 2/98 respectively (to compare with a previous work, above mentioned, where the molar ratios for the composition of Bi2O3/SnO2 were the same [18]). The PVA was added in a proportion of 0.8g per 100g of oxides. The raw materials were weighted and then wet homogenised in a planetary mill (Pulverisette 5, Fritsch GmbH, Germany) at 230rpm for one hour using water as a fluid. The obtained suspension was dried in a laboratory oven at 110°C for 24h, after which the dried material was disaggregated in an agate mortar until it passed through a 600μm mesh. The powder was then moistened to a water content of 5wt.% (dry basis). A fraction of each mixture was used for experiments on a heating microscope (Misura 3, Expert Systems Solutions Srl, Italy). The rest of the mixture was shaped as cylinders (20mm in diameter and about 5mm thick) in a laboratory uniaxial press (Robima, Spain), at a pressure of 450kgcm−2. After drying in a laboratory oven, the specimens were subjected to the selected heat treatments for sintering in an electric laboratory furnace (RHF1600, Carbolite Furnaces Ltd., UK).
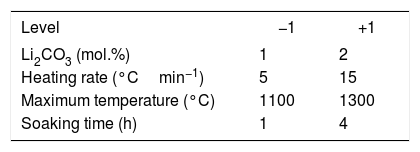
The experiments with the heating microscope constituted a 22 design in which the factors evaluated were the Li2CO3 proportion and the heating rate (keeping the maximum temperature of the test at 1300°C and taking images every 5°C). The sintering experiments in the furnace constituted a 24 design in which the factors evaluated, in addition to those already mentioned, were the maximum temperature and the soaking time (they correspond to the first three rows of Table 1 and to the whole table respectively).
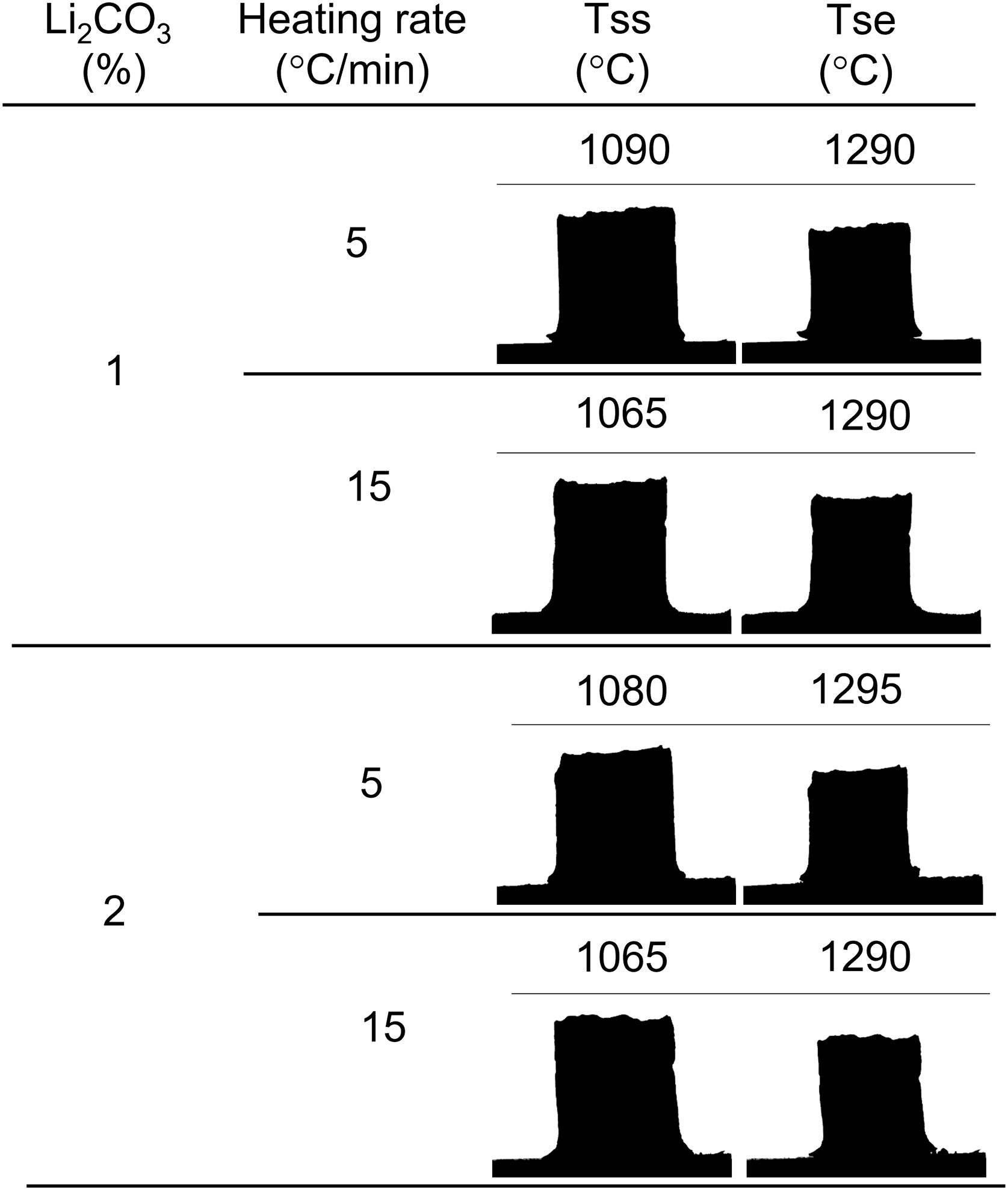
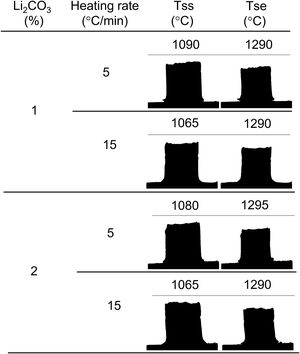
In this work, the use of heating microscopy is proposed to deduce the behaviour of the samples during heating, since this equipment makes it possible to evaluate the shrinkage with respect to the initial section of the specimen by means of image analysis (hereinafter sectional shrinkage, SS), which makes it possible to measure the temperatures when shrinkage starts (Tss, corresponding to a 1% shrinkage) and the shrinkage ends (Tse, when the specimen stops shrinking). In addition, it has been used to measure the shrinkage corresponding to the point at which the maximum temperature of the sintering experiments in the furnace was reached. This data allowed to estimate the value of the volumetric shrinkage (SVe) to which these values corresponded using the model described in the annex, and thus to compare them with the volumetric shrinkage experienced by the sintered specimens. To obtain this parameter, the bulk density of both dry and sintered specimens was determined using the mercury immersion method (based on Archimedes’ principle). From these values, the volumetric shrinkage after each thermal cycle (SV) was calculated. Additionally, these values allow the calculation of relative density and densification (the change in bulk density due to sintering divided by the change required to obtain a porosity-free material).
The crystalline phases present in the sintered specimens were identified by X-ray diffraction to determine whether any mixed oxide of the SnO2–Li2O system had formed. For this purpose, the specimens were reduced to powder and analysed with a Theta-Theta D8 Advance (Bruker, Germany), with CuK radiation (λ=1.54183Å), operating at 45kV and 40mA, using a VANTEC-1 detector in a 2θ range between 5° and 90° (step length of 0.015° at 1.2s/step). Scanning electron microscopy (FEG-SEM QUANTA200F, FEI Co., USA) was also used to analyse the microstructure in polished sections of the sintered samples, without additional treatments.
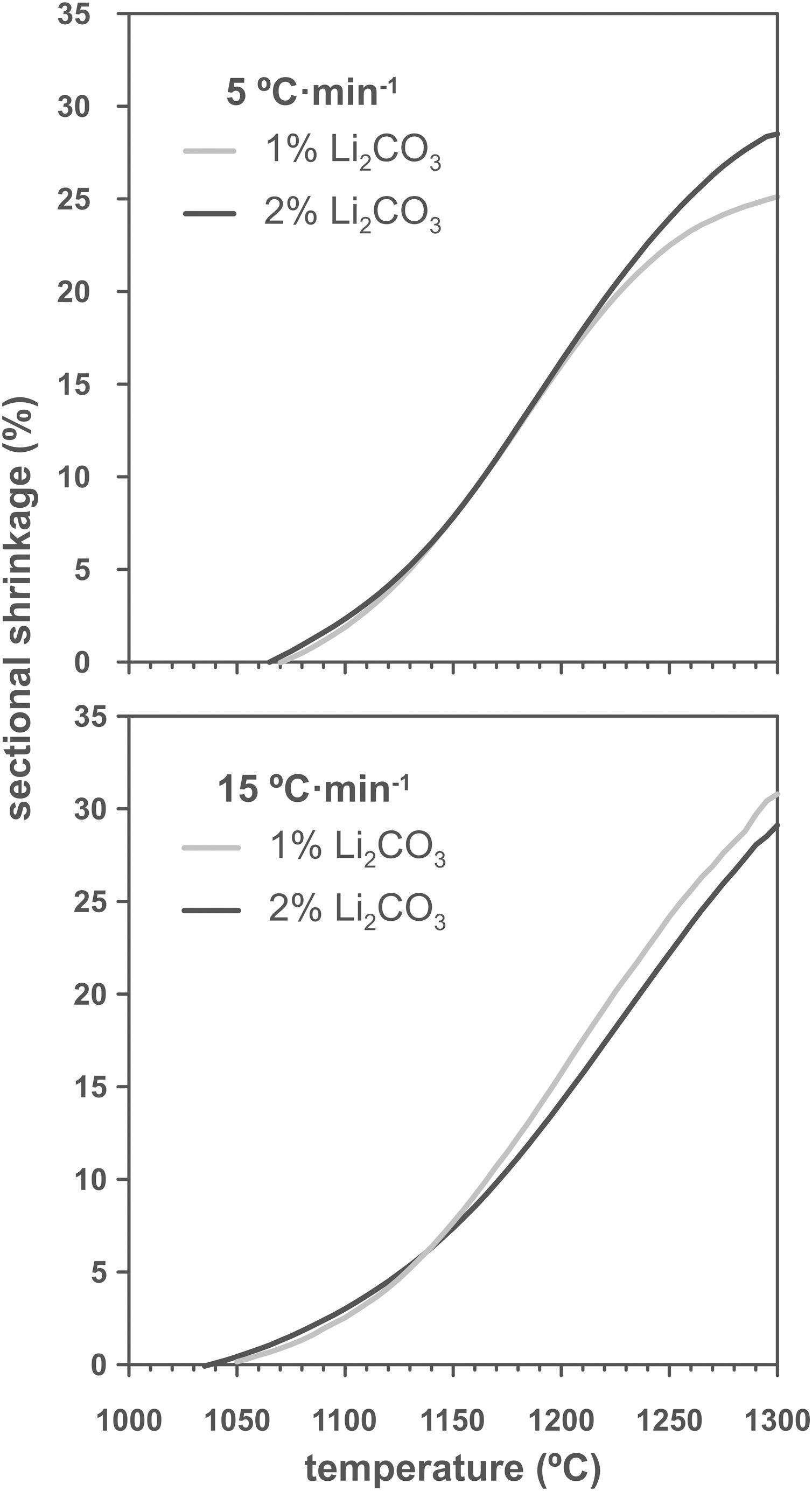
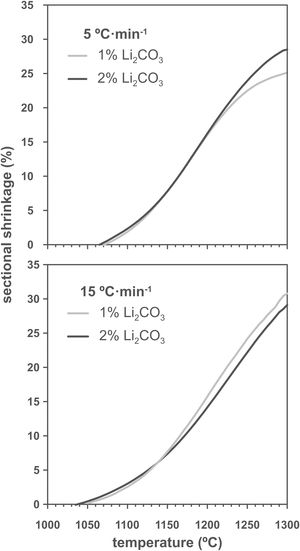
Results and discussionThe shrinkage–temperature curves obtained in the heating microscope (Fig. 1), as well as the shrinkage initiation temperatures (Fig. 2) allowed estimating the effects of the Li2CO3 ratio and the heating rate. Firstly, it was found that the chosen additive allowed SnO2 to start sintering during the heating section of the thermal cycle, as under the same conditions a pure tin oxide specimen did not show any shrinkage (the curve of pure SnO2 is not included in Fig. 1 because at 1300°C it has not yet started to shrink). Secondly, the heating rate slightly affects the position and shape of the curves. By increasing the heating rate from 5 to 15°Cmin−1 the shrinkage onset temperature decreases by about 20°C and the initial slope of the curve decreases. Thirdly, all four curves show an increase in slope around 1150°C but the final evolution is different. Heating at 5°Cmin−1 shows a progressive reduction in the slope from about 1250°C, while heating at 15°Cmin−1 the slope remains stable almost until 1300°C. In no case the end of sintering was reached in a definite way, which suggests that the samples would continue to shrink as the temperature increases (or maintain the final temperature of the test for a certain time). No clear effect of the Li2CO3 proportion was seen on the curves, except in the case of the 1mol.% at the lowest heating rate and in the higher temperature range, where a further decrease in the slope of the curve is seen, leading to a lower final shrinkage. In contrast, for the higher heating rate, the differences between the two curves are much smaller. Consequently, it follows that there is an important interaction between the lithium carbonate proportion and the heating rate during this part of the heat treatment.
As increasing the heating rate usually delays the onset of sintering in the heating microscopy test, the detected anomaly can be assigned to the melting and decomposition process of Li2CO3 and the subsequent interaction of the generated liquid with the SnO2 particles, which could generate the eutectic liquid of the Li2O–Li2SnO3 system as a secondary effect. The appearance of a liquid phase causes a rearrangement of the particles under capillary forces if no rigid bridges have previously formed in the structure [33]. However, the significant difference between the melting temperature of lithium carbonate (732°C) and the onset of shrinkage (from 1.065°C) seems to indicate that after Li2CO3 melting, the liquid phase is either too viscous or does not reach enough proportion to cause particle rearrangement, and the temperature needs to be raised to initiate rearrangement. Considering that a heating microscope test of pure Li2CO3 confirmed the melting point of the bibliography and showed that the liquid has a low viscosity, the second option was the case. This would imply a further advance in the decomposition of the carbonate, and therefore the presence of a higher proportion of Li2O, which would be the real sintering-promoting agent, if the mechanism is like the case of doped ceria [29,30]. For the lower heating rate, more time would be available for interaction between Li2O and SnO2, which could mean a lower proportion of effective liquid phase involved in sintering, thus delaying shrinkage. Conversely, with the higher heating rate, the time available for Li2O–SnO2 interaction would be shorter, so there could be a higher proportion of effective liquid phase and therefore shrinkage could be initiated at slightly lower temperatures. The lack of a defined effect of the Li2CO3 ratio on Tss would be because this temperature is defined for a fixed shrinkage of 1%, which would depend more on the viscosity of the liquid phase, which in turn depends more on the temperature, than on its ratio.
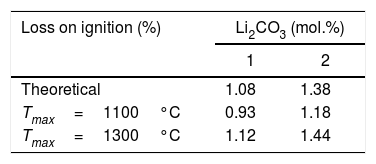
The proposed interpretation is supported by the average calcination loss data of the sintered specimens at each temperature, compared to the theoretical values obtained by assuming complete decomposition of the lithium carbonate and combustion of the PVA (Table 2). The specimens sintered at 1100°C do not reach the theoretical loss, suggesting that the lithium carbonate has not completely decomposed at that temperature, and if it has not done so after treatments of one or four hours, it can be considered that at the end of the heating ramp the proportion of undecomposed lithium carbonate will be even higher. On the contrary, after treatments at 1300°C the losses are of the same order or slightly higher than the theoretical ones, which points to the complete decomposition of the lithium carbonate. The fact that the losses are slightly higher than the theoretical ones may be due to a slight volatilisation of SnO2, which is a consequence of its sintering's mechanism in absence of additives, by surface diffusion at moderate temperatures or transfer of matter through the gas phase at high temperatures [34]. However, a partial volatilisation of Li2O at 1300°C cannot be excluded.
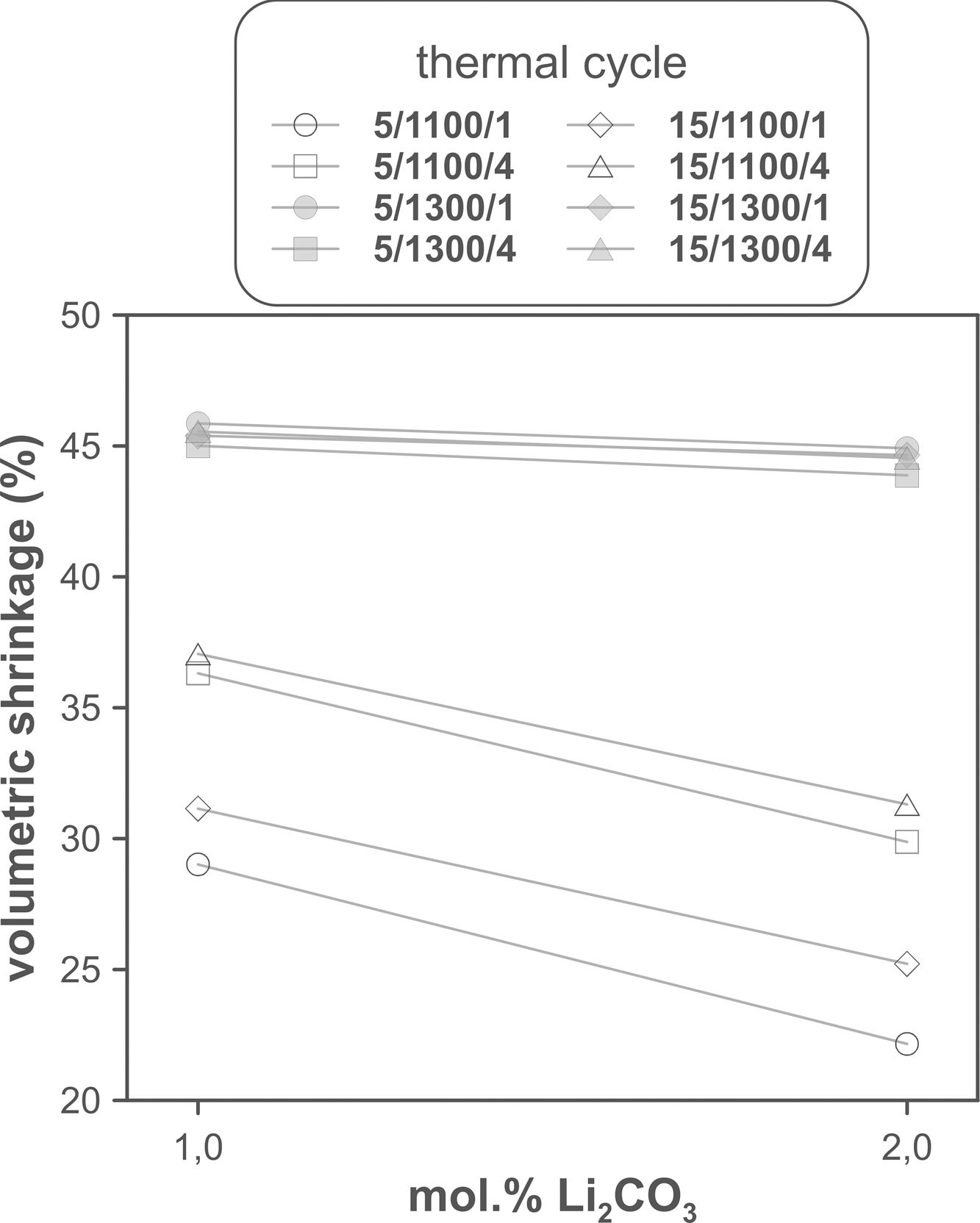
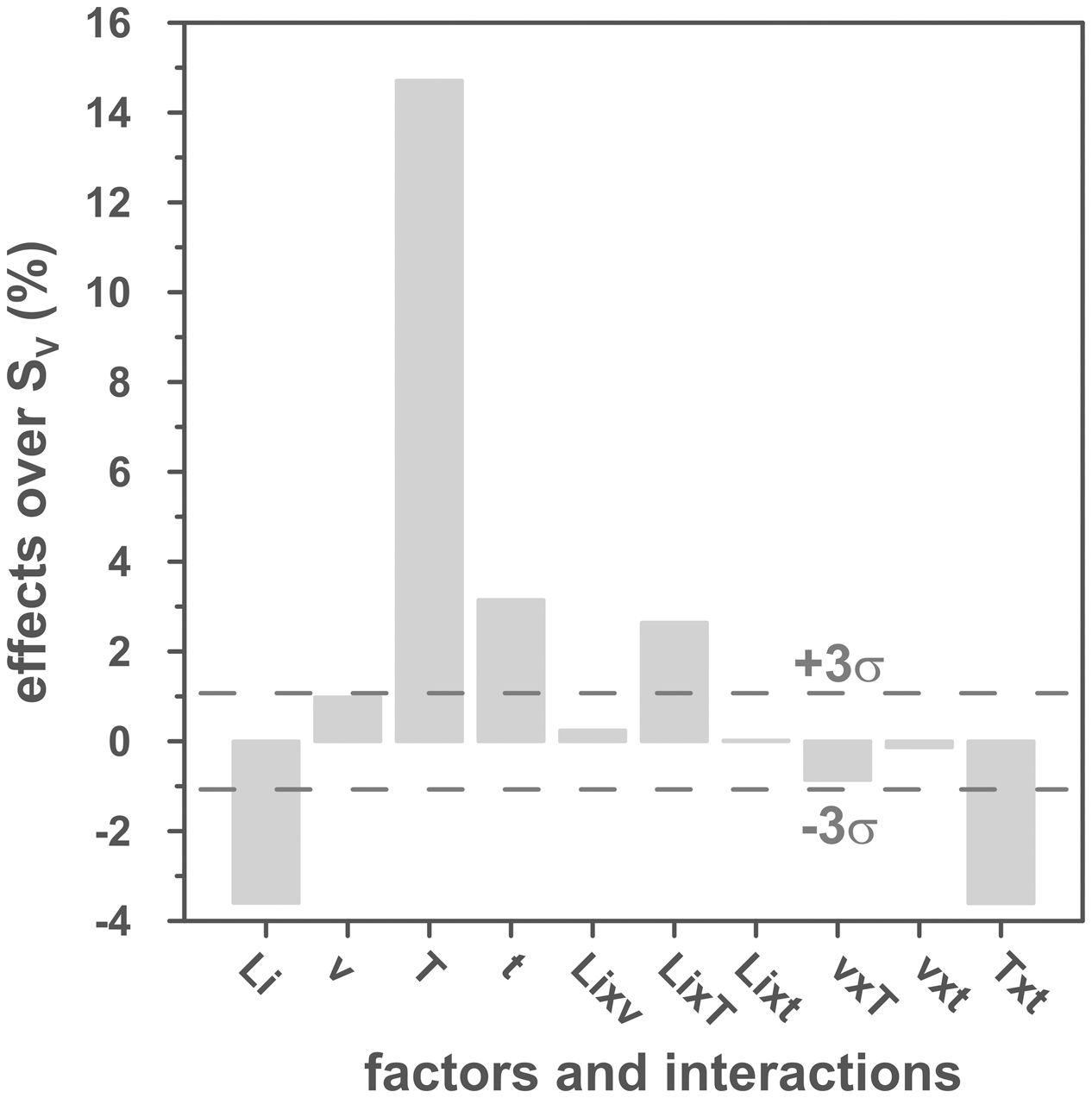
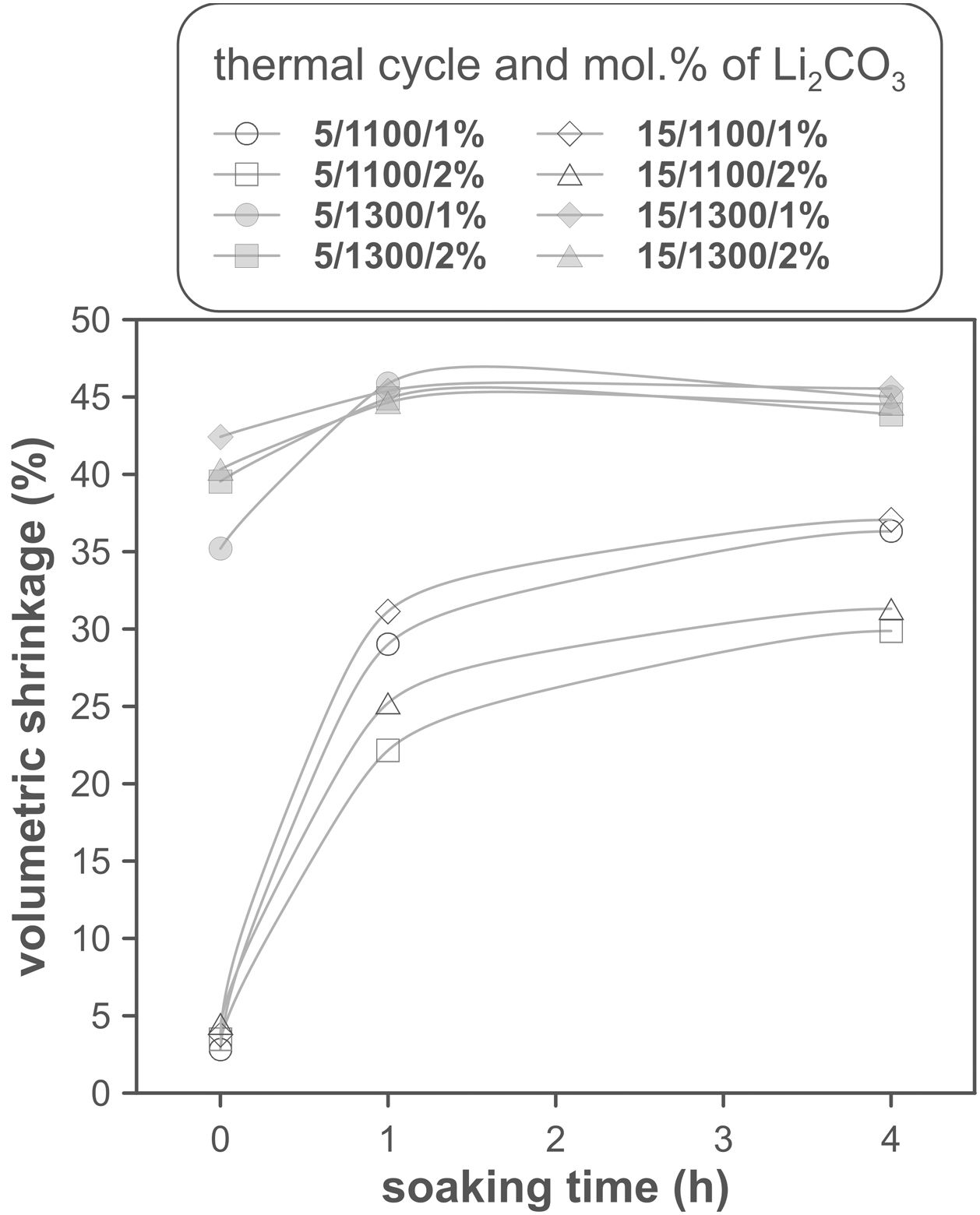
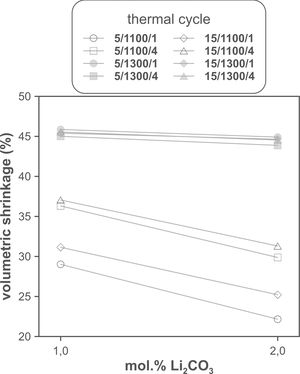
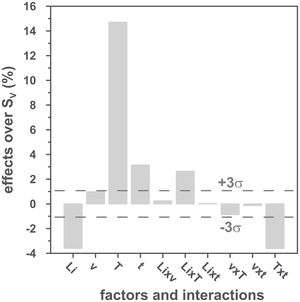
The volumetric shrinkage data obtained with the eight thermal treatments showed a very wide range of values, between 22% and 46% approximately (Fig. 3), which corresponds to densifications in the range of 29% to 86%. In a first approximation, there are two main groups of experiments, differentiated by the maximum temperature reached during the firing cycle. A dominant effect of this variable in the sintering process is thus revealed, with the highest SV values being obtained in the experiments carried out at a maximum temperature of 1300°C. To evaluate the influence of the rest of the parameters investigated, it is necessary, starting from these data, to calculate the effect of each one of them, as well as the value of their interactions as described by Box et al. [32]. Fig. 4 shows the main effects and their interactions, highlighting those that an ANOVA analysis has identified as significant. Of the four parameters studied, only the heating rate is not significant. In addition, among the second-order interactions, the Li2CO3 proportion with temperature (Li×T) and soaking time with temperature (t×T) are significant, while none of the higher-order interactions are significant.
The statistical calculation suggests that the maximum temperature is the parameter with the greatest influence on the sintering process of tin oxide in the presence of lithium carbonate. Additionally, the proportion of Li2CO3 and the soaking time, and their interactions with the temperature would be the rest of the effects to be considered to define sintering conditions. In other words, considering the values of the effects, the best combination of parameters would be to use the high level of temperature and the lower level of Li2CO3, while the level of time is almost indifferent, since the effect of the T×t interaction practically compensates the main effect of time. This result indicates, on the one hand, that the addition of Li2CO3 is limited to around 1mol.%, and that, on the other hand, the residence time can be shortened considerably by operating at 1300°C, but not at 1100°C.
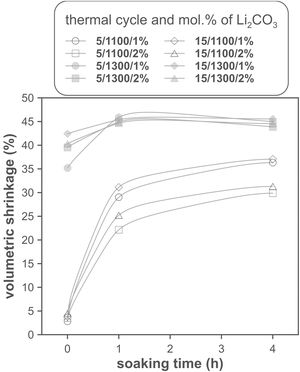
The effect of time is much more evident when comparing the volumetric shrinkage achieved for each set of conditions as a function of dwell time (having considered the shrinkage at the end of the heating ramp as the SVe from heating microscope test, Fig. 5). The data indicate that when 1300°C is reached, the shrinkage of the specimens is very close to the maximum shrinkage. This maximum shrinkage seems to be reached within one hour, and if soaking time is prolonged for much longer, a certain tendency towards expansion is observed, which would be an indication of over-sintering. On the other hand, increasing the heating rate influences the shrinkage reached at the end of the ramp when a Li2CO3 ratio of 1mol.% is used, but not when the ratio is 2mol.%. In the case of the specimens sintered at 1100°C, the differences in the shrinkage reached at the end of the heating stage are not relevant, but there is a clear effect of soaking time. Under these conditions, it is clearly favourable to use the lower Li2CO3 ratio and the higher heating rate, although it is obviously not possible to reach such high values of volumetric shrinkage as those obtained at the highest temperature, in the range of dwell times explored. These results suggest that the mechanisms involved in densification are highly temperature dependent.
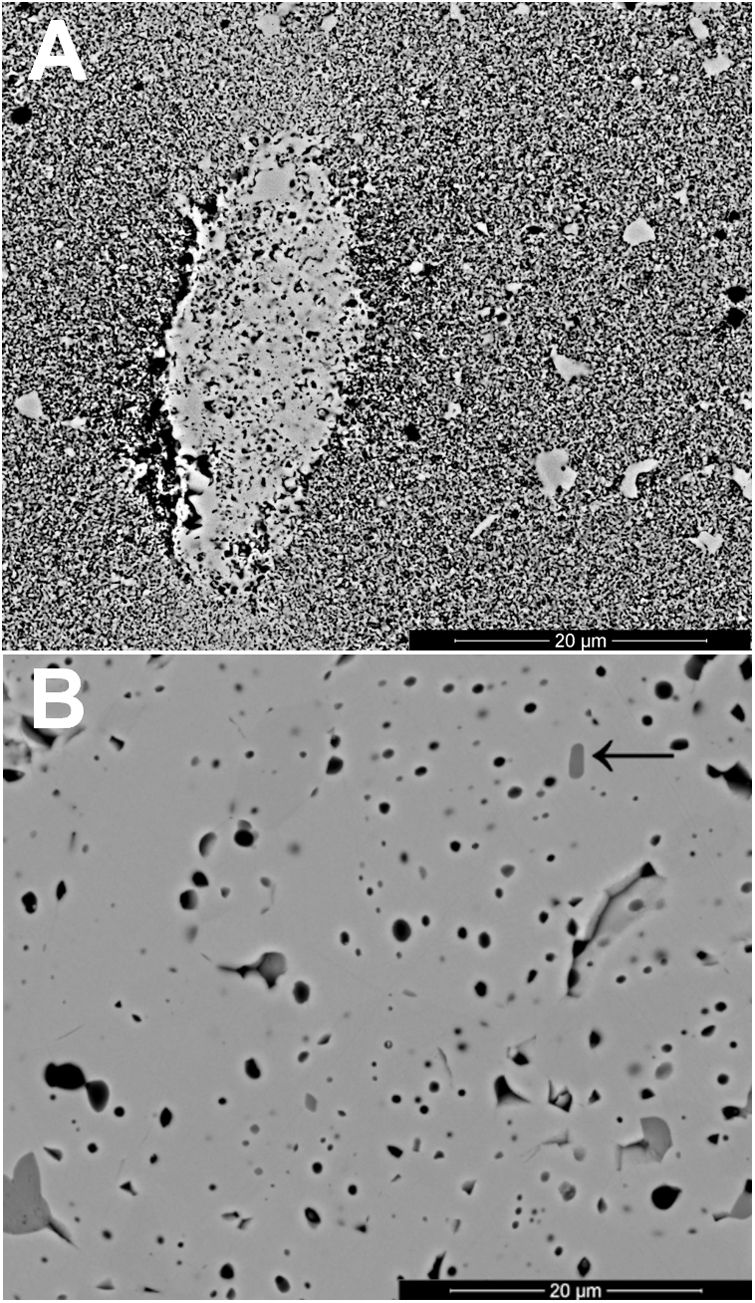
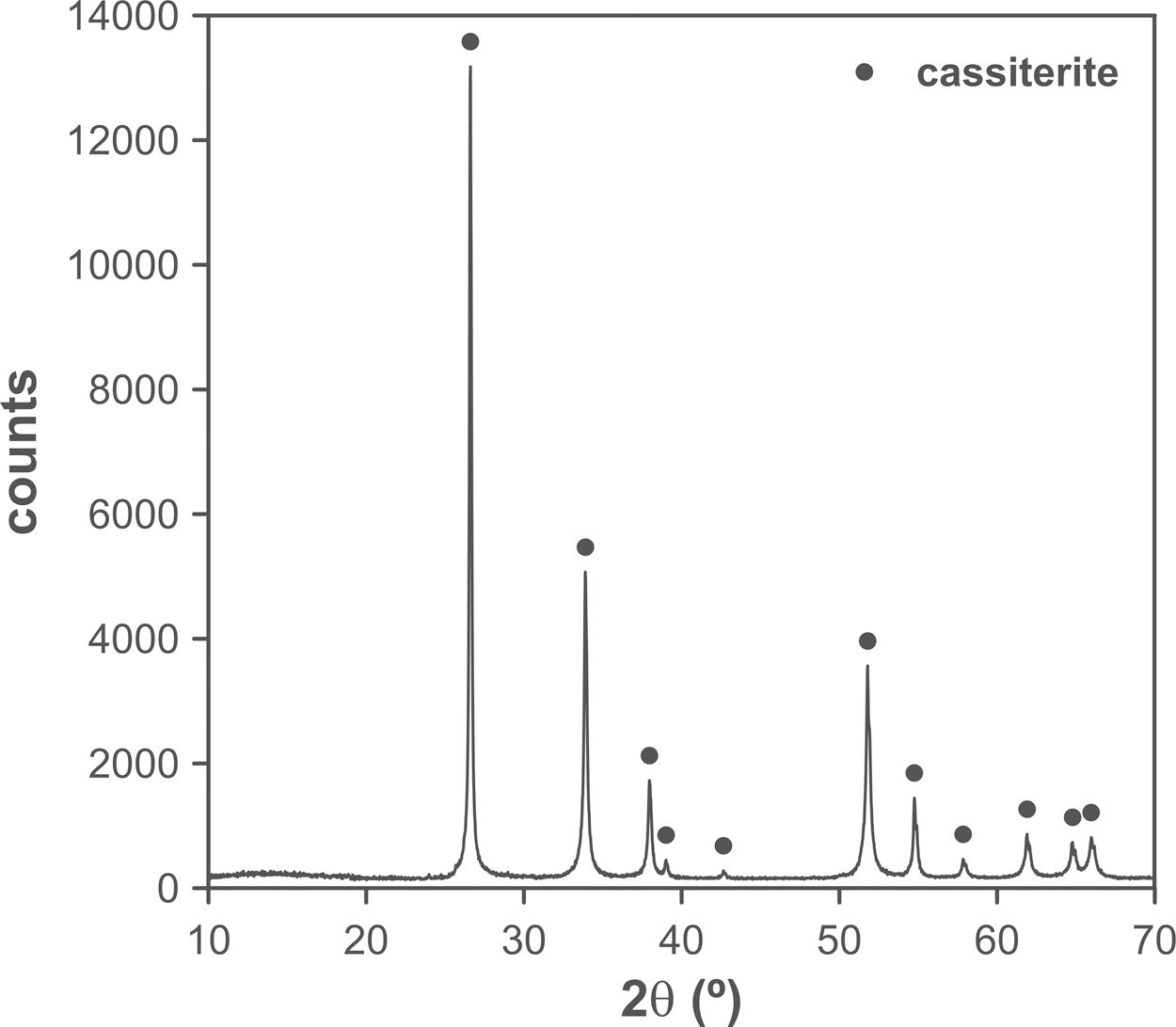
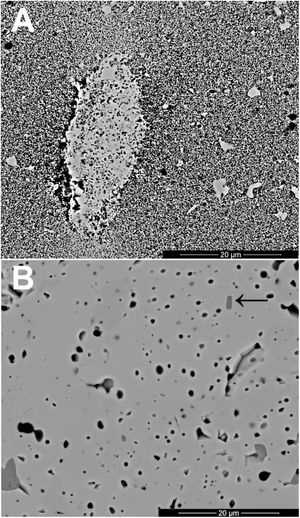
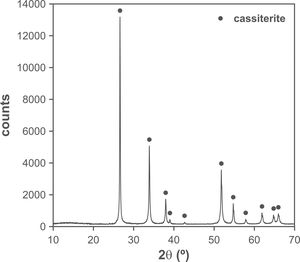
The changes in volumetric shrinkage are clearly reflected in the microstructure of the specimens. The samples sintered at 1100°C show a microstructure in which the submicronic particles of the SnO2 used as raw material are still visible (Fig. 6A), a sign that the initial stage of sintering has not been overcome. These specimens show the presence of voids that could be assigned to lithium carbonate particles that have melted, decomposed, and interacted with the surrounding SnO2 particles, generating a more densified zone. The darker grey level of the material surrounding the pore is consistent with the lower atomic weight of Li, but this could not be confirmed because this element cannot be detected by EDX. By contrast, the specimens sintered at 1300°C show a microstructure with grains much larger than the original SnO2 particles and rounded closed pores (Fig. 6B), characteristic of sintering's final stage. Darker areas can be detected in these specimens, which can be assigned to high Li concentrations, indicating that during sintering there was a liquid phase which facilitated densification, and which has remained embedded in the microstructure, possibly in a glassy form, since no crystalline phase containing lithium has been detected by XRD, even in the composition richest in Li2CO3 sintered under the most energetic conditions (Fig. 7). However, given the low proportion of Li2O in the compositions (below 1wt.%), the presence of lithium-containing crystalline phases cannot be completely ruled out as they would possibly be below the detection limit of this technique.
Results indicate that the effectiveness of lithium carbonate as a sintering agent is possibly due to the formation of a liquid phase, which is more active at higher temperatures, which is probably related to a higher progress of Li2CO3 decomposition reaction. This reaction generates Li2O, which would be the real sintering agent, as described in the case of (Sm,Ce)O2[29]. In addition, the high volatility of Li2O would facilitate its diffusion throughout the volume of the specimens at the higher operating temperature [35]. As the SEM images show, the presence of rounded pores, as well as the presence of areas with darker shades, are consistent with sintering in the presence of a liquid phase, part of which has been encapsulated between the SnO2 grains. Whether this liquid phase has generated any compounds of the SnO2–Li2O system or has remained as an amorphous liquid could not be discerned with the employed instrumental techniques due to their small proportion.
In general terms, lithium carbonate is an additive that facilitates the sintering of tin oxide, reaching values close to total densification, specifically volumetric contractions in the region of 46% in the most favourable conditions, which correspond to densifications of 86% and relative densities of 93%. Comparing these results with those obtained in a previous publication, better densification values were obtained with lithium oxide as a dopant than with bismuth oxide [18], with which the best results were around 45% densification, due to the high degree of volatilisation of the latter during the sintering process. Obviously, this study does not to reach relative densities around 99% as obtained with a 0.5mol.% of CoO [15] but the sintering cycle could be improved using the obtained information, to approximate these values. On the other hand, comparison with zinc oxide showed that for the same molar proportion of doping as used in this work, it was necessary sintering temperatures higher than 1400°C to obtain relative densities around 97–98% [36]. Consequently, the use of ZnO as sintering aid at lower temperatures could give relative densities not far from the ones obtained with lithium carbonate. From the economic point of view, using CoO as sintering aid is more expensive than Li2CO3, whereas ZnO is cheaper. As a consequence, lithium carbonate could be an economic alternative to CoO when high densifications are not necessary, but the election between lithium carbonate and zinc oxide would need a detailed study.
ConclusionsLithium carbonate promotes the densification of tin oxide, although the result depends on its proportion and the parameters of the heat treatment (heating rate, maximum temperature, and soaking time). The experimental designs carried out have made it possible to evaluate the degree of influence of each of the variables studied on the sintering process, determining that the most important factor to consider when designing a heat treatment to obtain high densification is the maximum temperature, followed by the proportion of Li2CO3, the residence time at Tmax and the interaction between the proportion of additive and the Tmax. Specifically, relative density values of 93% can be achieved with an additive percentage of 1mol.%, using a maximum temperature of 1300°C and short soaking times (1h). In addition, relative densities close to the maximum values can be obtained only with the heating section. This results in a microstructure of closed, rounded pores, in which a residual phase is enclosed, most likely including part of the lithium introduced, but whose exact nature has not been determined.
AnnexThe volumetric shrinkage of the fired specimens has been related to the sectional shrinkage recorded under the heating microscope by the following assumptions.
Given the mass and bulk density of both pressed and sintered specimens, the volumetric shrinkage (SV) is obtained with the following equation.
where VP and VS are the volume of the pressed and sintered specimen respectively, mP and mS their masses and ρP and ρS their bulk densities.The heating microscope, on the other hand, measures the variation in the cross-section of the specimen. As the specimen is cylindrical in shape, its side section is a rectangle of height h and base equal to twice the radius R. Assuming that during sintering the height/radius ratio remains constant and equal to α, the volumetric and surface shrinkage of the specimen is given by the following expressions:
where R0 and h0 are the initial radius and height of the specimen, and RT and hT are the radius and height when a given temperature T is reached.With this reasoning the two measurements of shrinkage can be related.
Consequently, it is possible to estimate the volumetric shrinkage of the specimens that would correspond to a given value of sectional shrinkage measured with the heating microscope.
The authors of this paper would like to thank the Ministry of Economy and Competitiveness and the European Regional Development Fund for supporting this research [Plan Nacional de I+D, project Ref. CTQ2015-65202-C2-2-R (MINECO/FEDER)].