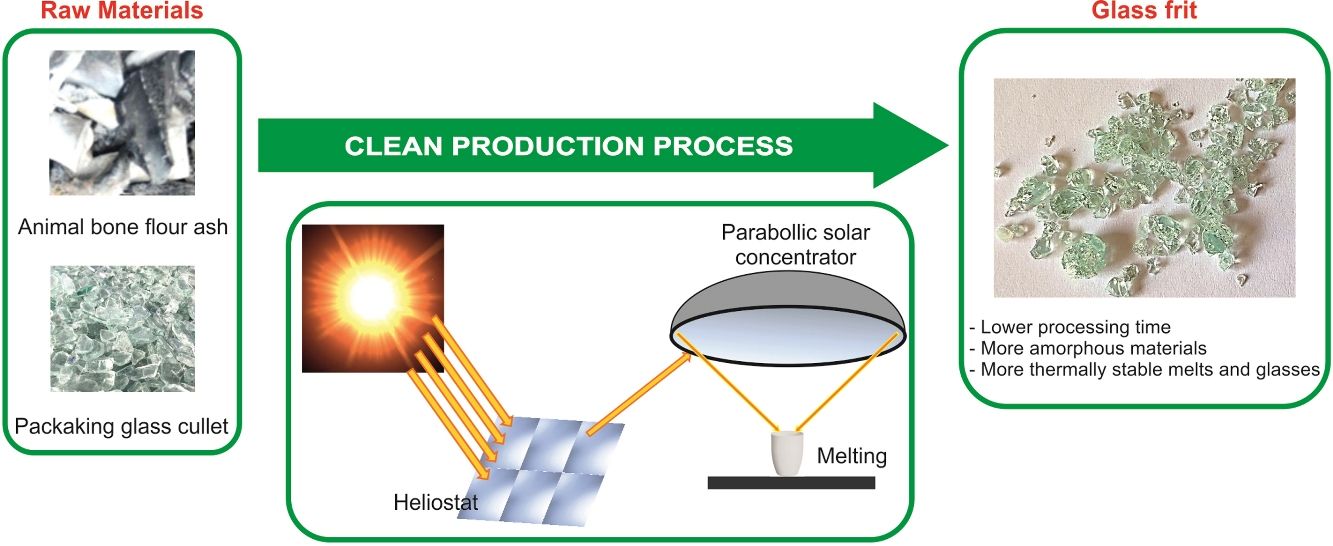
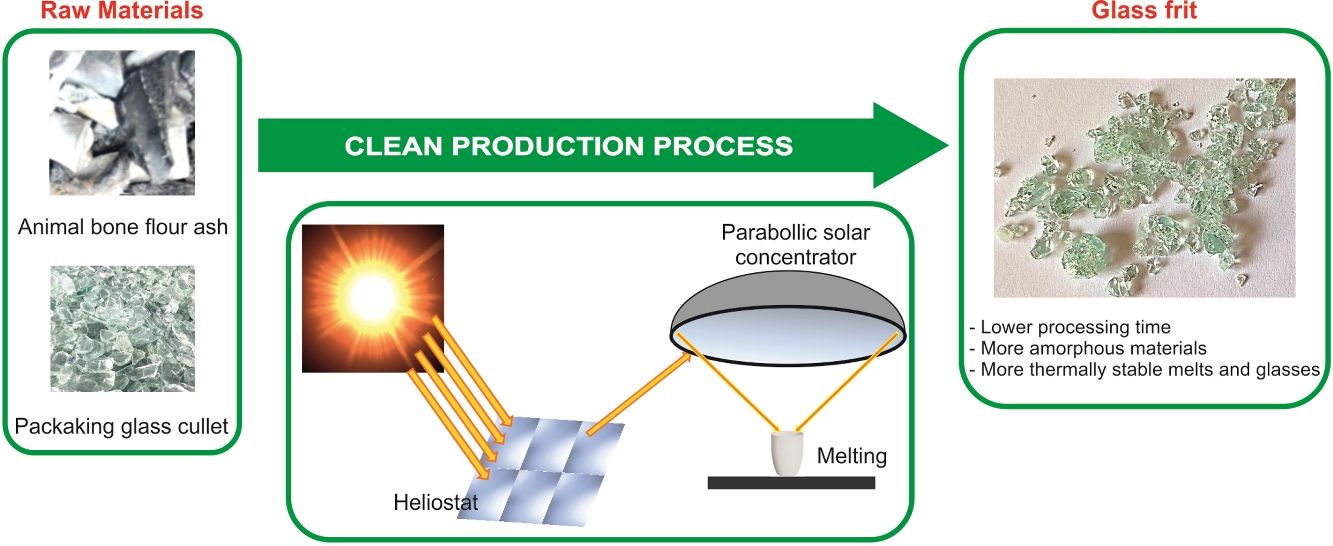
Sustainable glasses were prepared by green technology including the use of different wastes as raw materials and concentrated solar power (CSP) as renewable energy. The raw materials used to formulate the glasses were animal bone flour ash, which presents high contents of CaO, P2O5 and alkaline oxides; glassy sand from the waste of packaging glass submitted to a primary treatment, composed principally of SiO2; and potassium carbonate of reagent grade. Different exposure times to solar radiation were tested. For comparison, the same composition of glass was melted into a conventional electric furnace. The use of CSP to produce glasses reduces the melting time by approximately 90%, with consequent energy savings. The increased CSP processing time results in more amorphous and thermally stable glasses. The results showed the viability of producing ecofriendly glasses in the SiO2–P2O5–CaO–K2O system, which could be used as matrix-based fertilizers.
Se prepararon vidrios sostenibles mediante una tecnología alternativa que incluye el uso de diferentes residuos como materias primas y energía solar concentrada (ESC) como fuente de energía renovable. Las materias primas utilizadas para formular los vidrios fueron ceniza de harina de hueso animal, que presenta altos contenidos de CaO, P2O5 y óxidos alcalinos; arena vítrea procedente de residuos de envases de vidrio sometidos a un tratamiento primario, compuesta principalmente por SiO2, y carbonato potásico de grado reactivo. Se evaluó el efecto del tiempo de exposición a la radiación solar sobre las características de los vidrios obtenidos. Como material de referencia se empleó un vidrio de la misma composición obtenido en un horno eléctrico convencional. El uso de la ESC para producir vidrios reduce el tiempo de fusión en aproximadamente un 90%, con el consiguiente ahorro de energía. El aumento del tiempo de procesamiento da lugar a vidrios más amorfos y de mayor estabilidad térmica. Los resultados mostraron la viabilidad de la producción de vidrios sostenibles en el sistema SiO2-P2O5-CaO-K2O, que podrían utilizarse como fertilizantes.
The increase in population and industrial activities, while producing goods, services and materials that favor economic growth and contribute to our modern living standards, can nevertheless negatively impact our environment and well-being. When addressing waste management, a quantitative increase in their generation and important management problems related to their heterogeneity is observed. Regulatory policies have been introduced within the European Union to control and minimize the related impacts and develop a model that can also lead to economic benefits while protecting the environment and human health. The circular economy is the answer to the “take-make-dispose” concept of the linear economy, and it aims to reduce the generation of waste through biological, technical or commercial cycles. Moreover, the use of waste or byproducts as raw materials within open-loop recycling is a good practice recommended by the European Community in the legislative field. In this regard, considerable research has been carried out aimed to the re-use of waste in the manufacture of different materials such as, glass and ceramic materials [1–9], mortars [10,11], geopolymers [12], gypsum [13] or lime [14], among others.
On the other hand, the alarming increase in CO2 emissions to the atmosphere encourages urgent changes toward less contaminant systems to produce energy. Such a high concentration of global CO2 of 413ppm was recorded by the American National Oceanic and Atmospheric Administration for September 2021 [15]. This is a worrying situation, and accordingly, changes in our production systems must be highly considered.
Both the use of waste and the reduction of CO2 emissions have been taken into account in the Sustainable Development Goals for 2030 [16], which are the blueprint to achieve a better and more sustainable future. Thus, targets of SDGs are substantially increase the reuse of waste (Goal 12 – Responsible consumption and production) and reducing CO2 levels and other greenhouse gases in the atmosphere (Goal 13 – Climate action).
Sustainable agriculture is also a target of the SDGs, which aim to ensure sustainable food production systems that progressively improve land and soil quality. Given this scenario, research on developing new controlled-release fertilizers (CRFs) has been encouraged worldwide in the last years. The use of CRFs can help to mitigate the problem of soil contamination from the abusive use of traditional mineral fertilizers (ammonium nitrate or urea, ammonium phosphate, potassium chloride, etc.), due to the need to use large quantities to have a good yield harvest [17]. Fertilizing glasses are a type of matrix-based fertilizer, in which nutrients (N, P, K, Ca or Mg) are included in the glassy network and they are gradually solubilized [18]. Some research has reported the manufacture of fertilizing glasses containing the main nutrients P and K prepared from pure reagents [19,20].
In recent decades, the use of renewable energy sources instead of traditional ones for high energy demanding processes has received great attention. In this sense, the use of concentrated solar radiation for high-temperature processes has been most investigated. Among its advantages, it is almost limitless energy and does not produce greenhouse gases, and concentration technologies have quickly developed for high-temperature industrial processes. Research studies conducted to obtain glasses by using concentrated solar power (CSP) have scarcely been performed. In this sense, the authors have recently studied the use of CSP to produce glass frits with excellent results related to the characteristics of the obtained materials in comparison to those obtained by conventional processes [21,22].
In previous papers, the authors demonstrated the possibility of obtaining fertilizing glasses in the SiO2–P2O5–CaO–K2O system by using alternative raw materials and low-cost products from the perspective of the circular economy [23–25]. Such active glasses were obtained through an energy-intensive conventional melting process, which leads to high CO2 emissions due to the use of fossil fuels [26].
With the aim of contributing to the conservation of natural resources, the re-use of waste and, also to the decrease of the CO2 emission derived to the use of non-renewable energy, this research focuses on the production of glasses in the SiO2–P2O5–CaO–K2O system from waste as raw materials (animal bone flour ash and packaging glassy sand from municipal solid waste) by providing the energy required to melt the glass through concentrated solar radiation. To our knowledge, this is the first time that such a study has been undertaken.
Materials and methodsRaw materialsThe raw materials used to prepare glasses in the SiO2–P2O5–CaO–K2O system were animal bone flour ash, glassy sand and K2CO3 (reagent grade). Animal bone flour ash presents high contents of CaO (42.1wt.%) and P2O5 (29.2wt.%), as well as other oxides such as Na2O (3.9wt.%), SiO2 (1.7wt.%), K2O (2.7wt.%) and MgO (1.4wt.%). It consists principally of inorganic and nontoxic compounds such as Ca5(PO4)3OH (hydroxyapatite), CaCO3 (calcite) and Ca9MgK(PO4)7. Glassy sand is an end of waste coming from the secondary treatment of the waste (19 12 05 EWC code) of the primary treatment of packaging glass. This raw material is produced by an authorized plant in northern Italy and consists of sodium-calcium silicate glass (SiO2, 71.3wt.%; CaO, 10.0wt.%; Na2O, 12.7wt.%; MgO, 2.2wt.%; Al2O3, 2.0wt.% and other minor oxides). The characterization of raw materials has been previously reported [23].
Glass preparationA mixture of animal bone flour ash (40wt.%), glassy sand (42wt.%) and K2CO3 (18wt.%) were formulated to get a glass with the theoretical composition shown in Table 1. This glass composition was previously tested as fertilizing glass [23]. The mixture of the raw materials was homogenized in a ball mill for 15min. The melting of the batches was performed using concentrated solar power (CSP) under different process parameters in a Medium Size Solar Furnace (MSSF) located in the CNRS-PROMESS Solar facilities (Font Romeu-Odeillo, France). The solar furnace of 0.9kW equipped with a vertical axis parabolic reflector of 1.5m diameter produces a focal spot, approx. 15mm in diameter, with a very high power density (1000Wm−2). To control the incident solar radiation, a shutter is positioned between the parabolic concentrator (placed on a 6th floor level) and the heliostat (placed on a first floor level). This vertical configuration is the only configuration that allows the heat treatment of powdered materials. A picture showing the configuration of the CSP system has been previously reported [21,22].
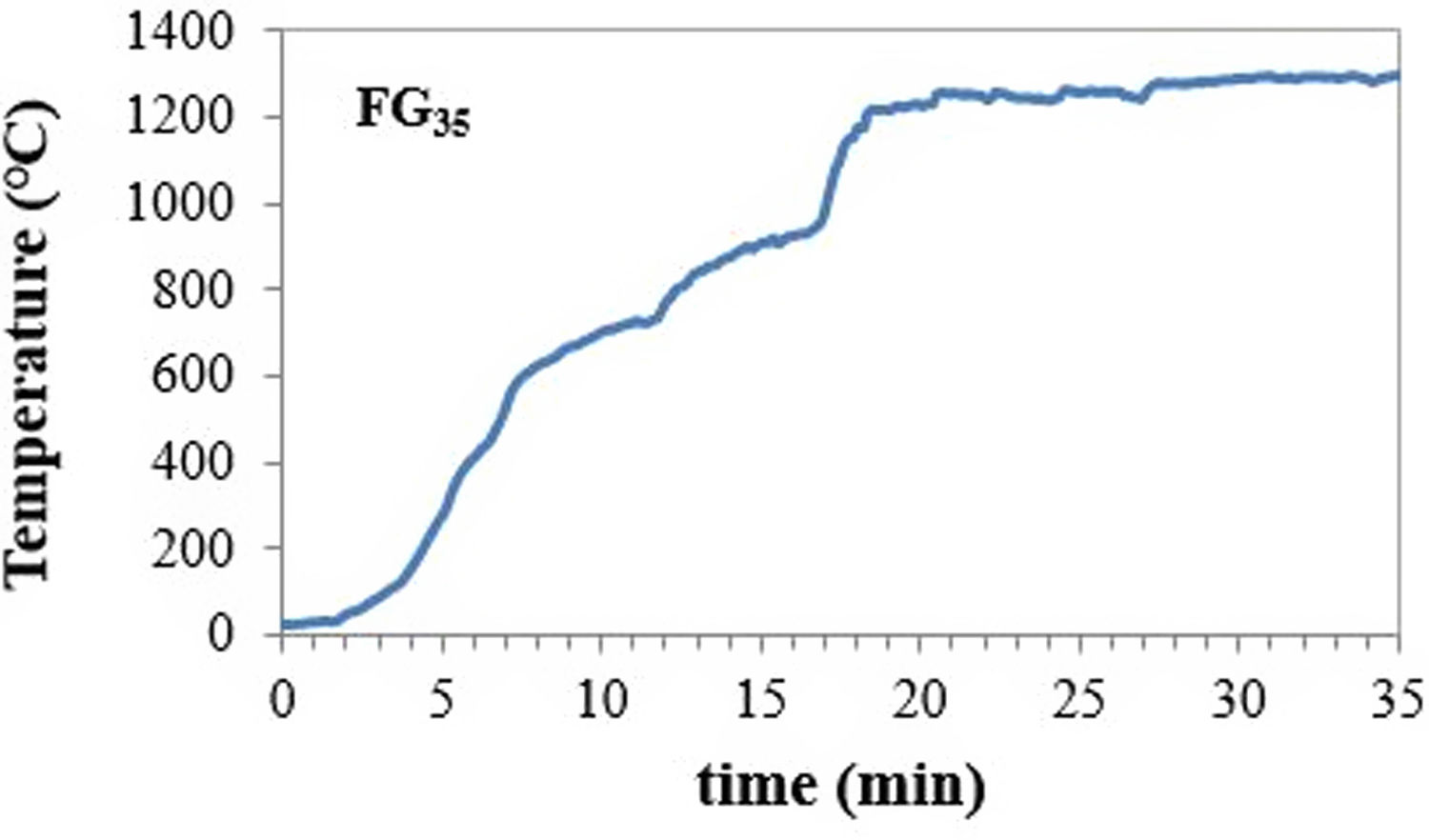
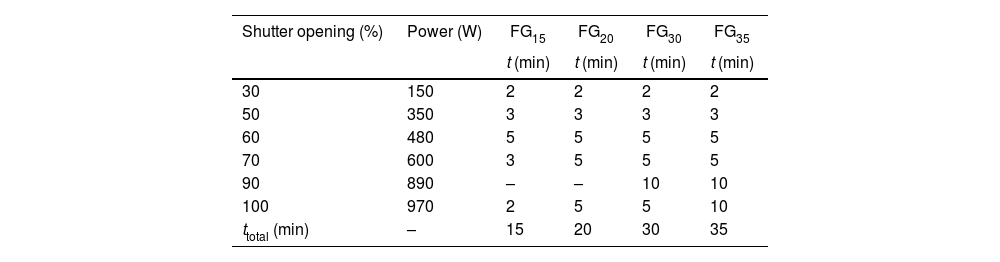
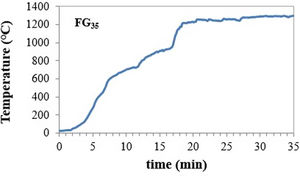
During the experiments, solar normal direct irradiation was quasi-steady, and values between 837 and 895Wm−2 were registered. Batches were placed in cylindrical (22mm diameter and 25mm high) silicon-aluminous crucibles. The temperature was measured by a type K thermocouple, which was positioned into a hole located at half the height of the crucible wall. The shutter was partially opened (30%) at the beginning of the experiments to avoid an abrupt increase in temperature on the sample; immediately after, the shutter was progressively opened until the temperature was approximately 1300°C, and the mixture was completely melted. Nevertheless, the temperature inside the bulk melt is supposed to be much higher, but its direct measurement was not able due to work limitations. Table 2 collects the experimental conditions related to the shutter opening percentage and the time of exposure to concentrated solar radiation of different samples. Hereafter, the samples melted with CSP are named FGx, where x is the time of exposure to solar radiation (15, 20, 30, and 35min).
An example of the temperature-time register followed for the experiment to produce glass frits with CSP is shown in Fig. 1. The inflection points in the curve correspond to the successive opening of the shutter (see Table 2).
To compare the properties of glasses obtained by CSP and traditional melting, a glass referred as FGT was prepared in a conventional electric furnace. The batch was heated at 20°Cmin−1 to 900°C, where an isotherm was maintained for 60min for raw material degasification. Subsequently, the batch was heated at 10°Cmin−1 to 1450°C and held for 120min.
After thermal schedule, melts obtained in both the electric furnace and by CSP were quenched in cold water and subsequently dried and characterized.
Glass characterizationGlasses were characterized to determine whether the type of energy, power density, and thermal schedule used in their preparation led to materials with similar physical-chemical characteristics. The chemical analysis of samples was determined by X-ray fluorescence (XRF) using a Bruker S8 Tiger spectrometer. The analysis was performed on pressed pellets of powder glass samples (< 63μm). The evaluation of the amorphous or crystalline nature of samples after melting was performed by X-ray diffraction (XRD) using Bruker D8 Advance equipment with Ni-filtered Cu Kα radiation operating at 30mA and 40kV. Data were recorded in the 5–60° 2θ range (step size 0.019732° and 0.5s counting time for each step). The relative crystallinity of the samples was determined by the DIFFRAC EVA v4.2 program.
The thermal stability of glasses was analyzed by differential thermal analysis (DTA) up to 1400°C at a heating rate of 50°Cmin−1 under flowing air in a Setaram Labsys Thermal Analyzer. Samples of 40mg were placed in platinum crucibles, and calcined Al2O3 was used as the reference material. The DTA curves were normalized with respect to the sample weight.
The microstructure and occurrence of phase separation in the glasses were studied by field emission scanning electron microscopy (FESEM) with a HITACHI S-4800P microscope using an acceleration voltage of 20kV. Fresh fracture surfaces were etched for 10s in a solution of 5% HF, ultrasonically washed with distilled water and ethylic alcohol, dried, and coated with Au-Pd in a Balzers SCD 050 sputter.
Finally, the characterization of samples was completed by Fourier transform infrared (FTIR) spectroscopy in Nicolet Nexus 670-870 equipment. FTIR spectra were recorded in the range of 400–4000cm−1 by means of transmission measurements from samples diluted in KBr.
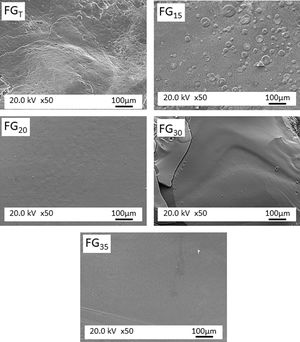
Results and discussionThe macroscopic appearance of all materials obtained by CSP was similar to those obtained by the traditional furnace. In Fig. 2, the glassy aspect of one of the FGs obtained is depicted.
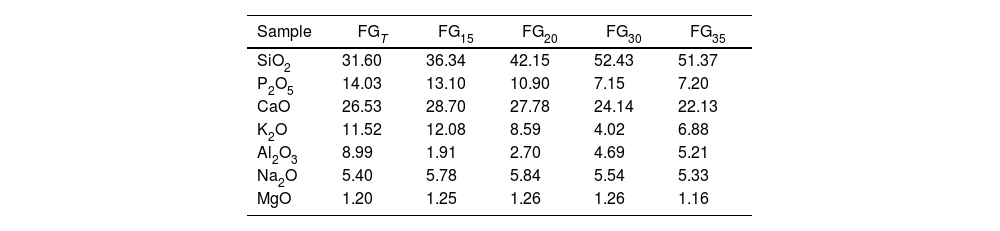
Chemical compositionThe chemical composition of the materials determined by XRF is shown in Table 3 (major) and Table 4 (minor components). With respect to the theoretical composition (Table 1), the FGT glass, prepared in an electric furnace, shows the characteristic variation observed when batches of raw materials are melted in silicon-aluminous crucibles, namely, a considerable increase in the alumina content due to corrosion of the crucible walls by the molten glass. The chemical composition of FG15 glass prepared with CSP and with the shortest process time (15min) is similar to that of the FGT glass except for the lower alumina content, which indicates lighter corrosion of the crucible. This result is in agreement with that reported by Romero et al. in a study on the melting of ceramic frits with different compositions using CSP [22]. By increasing the CSP processing time from 15 to 35min, the composition of the obtained glass undergoes important modifications, the most important being the decrease in P2O5 and K2O due to their volatilization during melting. Bourgel et al. [27] studied the gasification of sewage sludge containing 21wt.% P2O5 and highlighted the volatilization of phosphorus in the form of PO and PO2 (above 1300°C) and P2O5 (above 1400°C). Moreover, according to Beerkens [28], the kinetics of volatilization of phosphorus from glass melts increase considerably with temperature, so that at 1500°C, the reaction kinetics are at least two orders of magnitude higher than those at 1000°C. This behavior has also been observed in the chemical composition of the glass obtained by conventional procedures; in fact, the percentage of phosphorus oxide drops from 17.47wt.% (theoretical value) to 14.03wt.% (experimental value). During the melting of the FG15 and FG35 glasses, 25wt.% and approximately 60wt.% of the initial phosphorus is released, respectively. This result indicates that a longer processing time could lead to a higher melting temperature and that the core of the melt probably reached a temperature of over 1500°C. This finding would also explain the decrease in potassium content as the process time increases, releasing on average almost 50% of the initial potassium content in the glass composition. According to Bourgel et al. [27], the volatilization of potassium as KOH starts in the 1000–1100°C interval and increases considerably with temperature. KOH evaporation is consequence of the reaction of potassium oxide contained in the glass melt with water vapor at the surface of the melt. The evaporation rate is highly dependent on the composition of the gas atmosphere above the melt [28].
Chemical composition (expressed as oxide wt.%) of glasses obtained by XRF (major components).
| Sample | FGT | FG15 | FG20 | FG30 | FG35 |
|---|---|---|---|---|---|
| SiO2 | 31.60 | 36.34 | 42.15 | 52.43 | 51.37 |
| P2O5 | 14.03 | 13.10 | 10.90 | 7.15 | 7.20 |
| CaO | 26.53 | 28.70 | 27.78 | 24.14 | 22.13 |
| K2O | 11.52 | 12.08 | 8.59 | 4.02 | 6.88 |
| Al2O3 | 8.99 | 1.91 | 2.70 | 4.69 | 5.21 |
| Na2O | 5.40 | 5.78 | 5.84 | 5.54 | 5.33 |
| MgO | 1.20 | 1.25 | 1.26 | 1.26 | 1.16 |
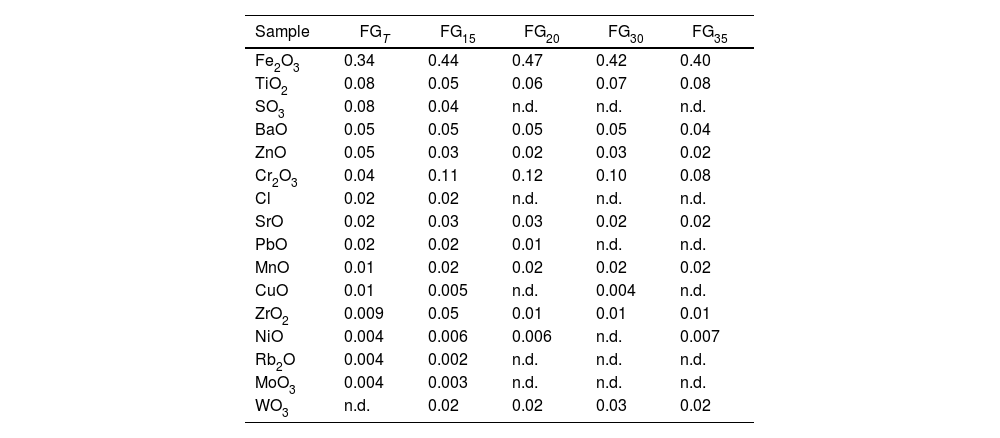
Chemical composition (expressed as oxide wt.%) of glasses obtained by XRF (minor components).
| Sample | FGT | FG15 | FG20 | FG30 | FG35 |
|---|---|---|---|---|---|
| Fe2O3 | 0.34 | 0.44 | 0.47 | 0.42 | 0.40 |
| TiO2 | 0.08 | 0.05 | 0.06 | 0.07 | 0.08 |
| SO3 | 0.08 | 0.04 | n.d. | n.d. | n.d. |
| BaO | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 |
| ZnO | 0.05 | 0.03 | 0.02 | 0.03 | 0.02 |
| Cr2O3 | 0.04 | 0.11 | 0.12 | 0.10 | 0.08 |
| Cl | 0.02 | 0.02 | n.d. | n.d. | n.d. |
| SrO | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 |
| PbO | 0.02 | 0.02 | 0.01 | n.d. | n.d. |
| MnO | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| CuO | 0.01 | 0.005 | n.d. | 0.004 | n.d. |
| ZrO2 | 0.009 | 0.05 | 0.01 | 0.01 | 0.01 |
| NiO | 0.004 | 0.006 | 0.006 | n.d. | 0.007 |
| Rb2O | 0.004 | 0.002 | n.d. | n.d. | n.d. |
| MoO3 | 0.004 | 0.003 | n.d. | n.d. | n.d. |
| WO3 | n.d. | 0.02 | 0.02 | 0.03 | 0.02 |
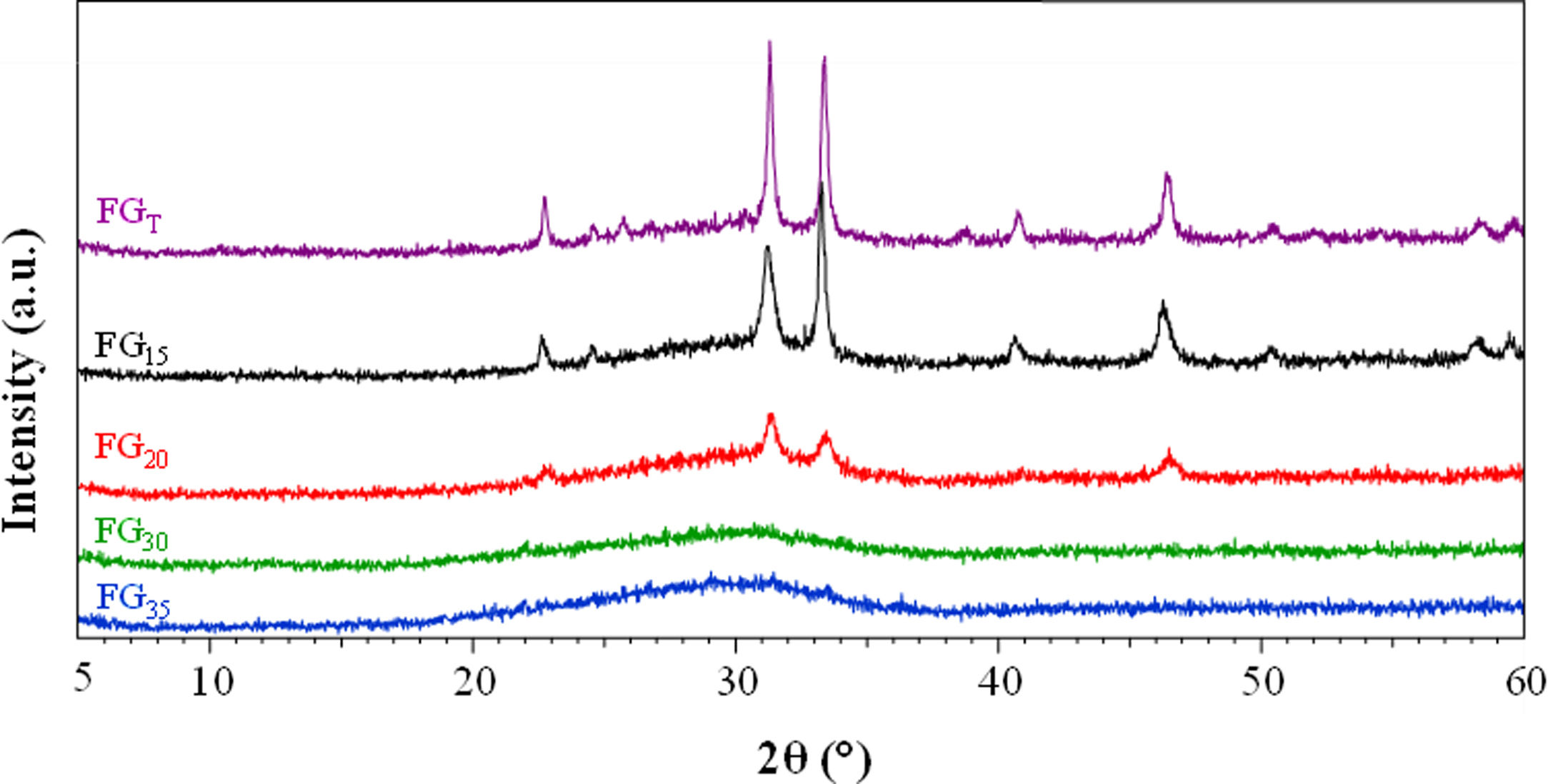
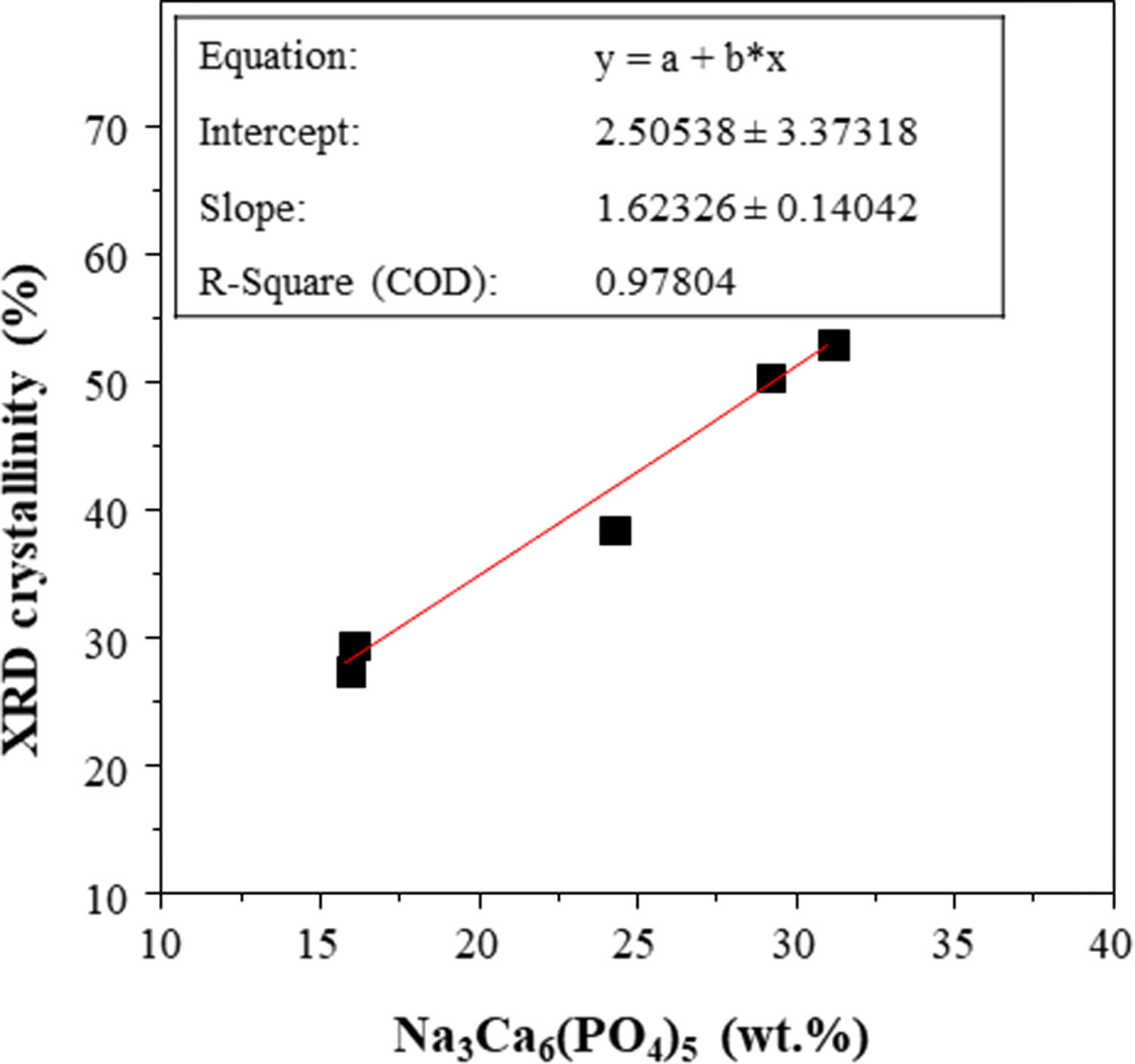
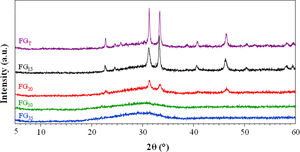
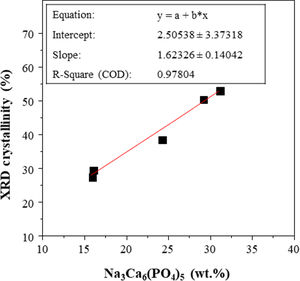
The X-ray patterns of the glasses prepared by both CSP and conventional melting are shown in Fig. 3. Concerning the samples melted by CSP, it can be observed that the glassy nature increases as the solar radiation exposure time increases. Thus, for shorter exposure times (15 and 20min), the materials have a partially glassy nature because a crystalline phase is developed. This phase was identified as sodium and calcium phosphate, Na3Ca6(PO4)5, according to the reference file (ICFF PDF 00-011-0236). The XRD pattern of FGT exhibits the same XRD profile as that of FG15. This result agrees with the XRF analysis: the glass sample obtained in the electric furnace is similar to that prepared by CSP, although in this case, the reaction time is 90% shorter than the time used in the electric furnace. The decrease in the crystalline nature of the samples as CSP processing time increases is likely related to the reduced availability of groups (PO4)3− required to develop the crystalline phase during melt cooling since, as mentioned above, an increase in processing time leads to higher volatilization of phosphorus. In fact, Fig. 4 depicts the percentage of Na3Ca6(PO4)5 that could be formed in each sample, considering that all the phosphorus in the glass composition is forming the crystalline phase, versus the relative crystallinity determined from the X-ray diffractograms. A good correlation between both parameters is obtained. Therefore, the results indicate that in the melting tests carried out with CSP, the increase in the amorphous nature of the samples is due to a decrease in the P2O5/CaO and P2O5/Na2O ratios with respect to the stoichiometric ratios necessary for the formation of the Na3Ca6(PO4)5 phase.
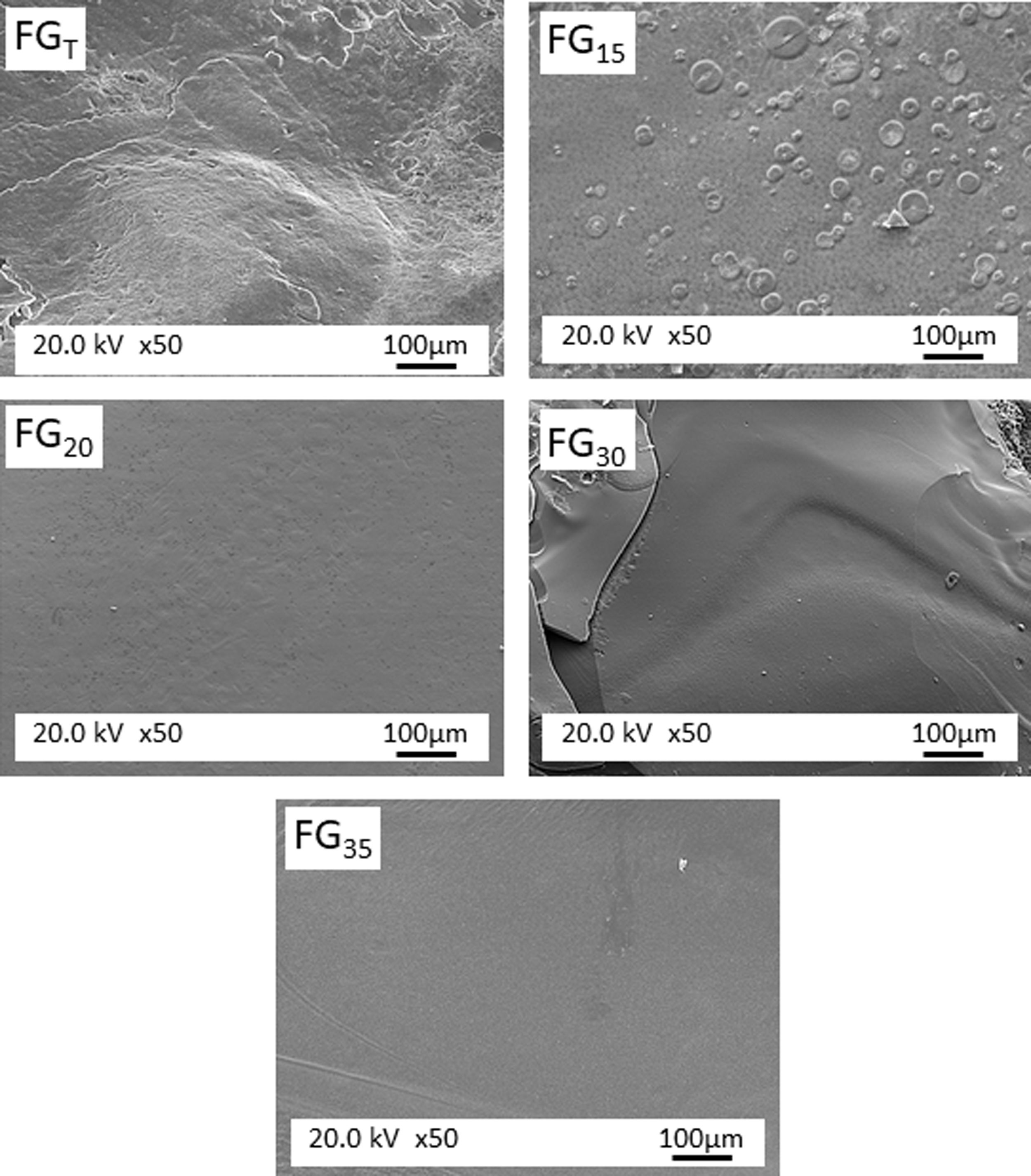
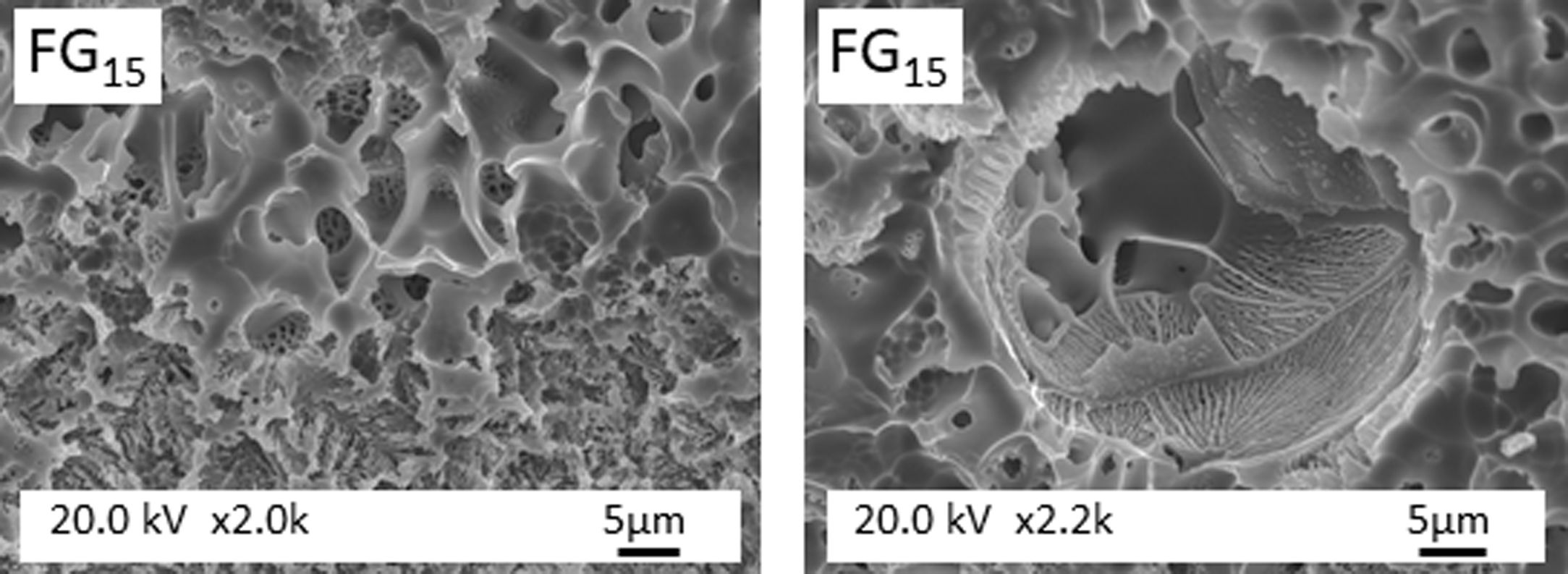
Fig. 5 shows the microstructure observed by FESEM on the fresh fracture surface of glasses. The FGT and FG15 glasses show a rough surface due to the precipitation of some Na3Ca6(PO4) crystals, as detected in the XRD spectra (Fig. 3). These crystallizations are visible in glass FG15 in the form of spherulites of variable size in the 10–60μm range. The FG20, FG30, and FG35 glasses appear smoother due to the lower content or even lack of crystalline phases and their higher amorphous character (Fig. 3).
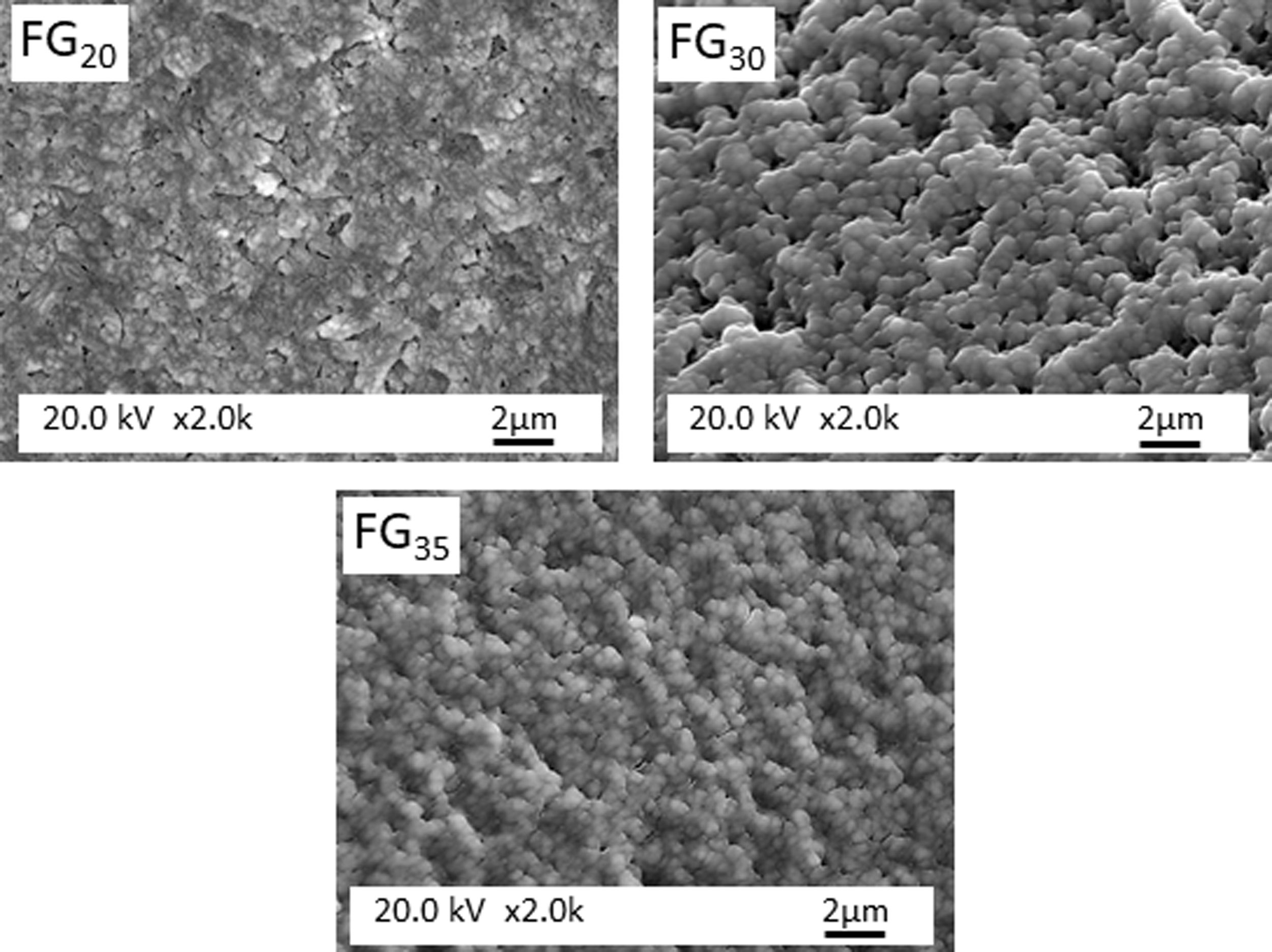
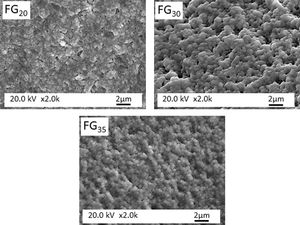
Fig. 6 presents FESEM observations of FG15 glass at high magnifications. It can be observed that the sample shows a high degree of devitrification and that crystals with a fibroradiated habit have developed during the cooling process. In addition, the intercrystalline matrix shows a high degree of porosity, possibly because of its solubilization during the etching stage preceding FESEM observation. This porous microstructure could also be due to the existence of a residual glass due to a phase separation, caused by a spinodal decomposition mechanism [29], which results in strongly interconnected phases separated by diffuse interfaces. Fig. 7 shows FESEM observations of the FG20, FG30, and FG35 glasses, where the absence of crystallization regions allows for better visualization of the microstructure of the glassy phase and the morphology of the spinodal decomposition, which is composed of small globules with sizes smaller than 0.5μm.
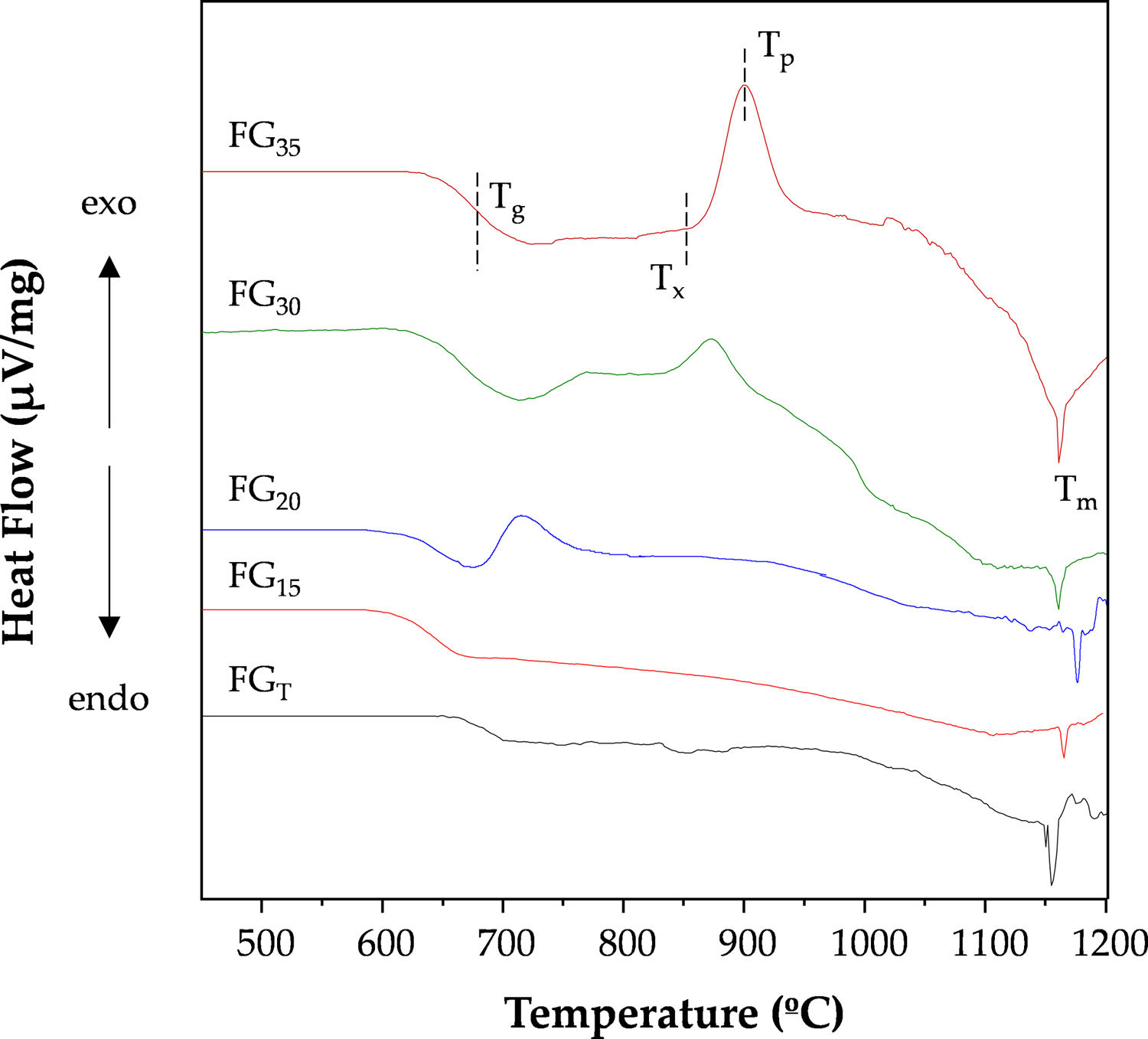
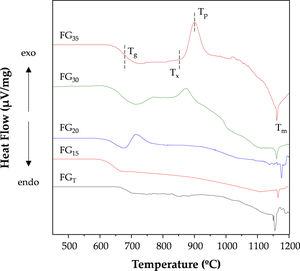
Thermal analysisFig. 8 depicts the DTA curves of the investigated glasses. Some differences in their profiles can be observed, mainly related to the different amorphous/crystalline characters, as shown in Fig. 3. Thus, samples FGT and FG15 are thermally stable, and their DTA curves only show endothermic events corresponding to glass transition temperatures (Tg) of approximately 670 and 630°C and liquid phase formation with melting temperatures (Tm) of 1155 and 1165°C, respectively. For the rest of the samples, their DTA curves show an exothermic effect, indicating that the glasses are unstable and that subsequent heat treatments result in a devitrification process and the consequent development of crystalline phases. This result is consistent with the increased amorphous nature of these samples, as shown in Fig. 3. The FG35 glass shows the most intense crystallization peak, possibly because crystal development could be favored by the finer morphology of the phase separation.
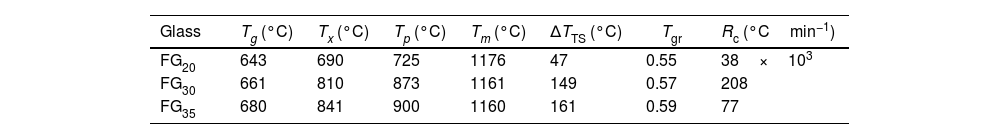
Table 5 lists the characteristic temperatures for the FG20, FG30, and FG35 glasses, namely, Tg, the onset of crystallization temperature (Tx), the temperature of the crystallization peak (Tp), and Tm. For better appreciation, these temperatures were indicated on the DTA curve of the FG35 glass (Fig. 8). From these temperatures, different parameters indicative of the stability of the glasses can be determined. Thus, the working range of the glass is an indicator of its thermal stability and is determined by ΔTTS=Tx−Tg[30]. The narrower this temperature range is, the more complex it is to prevent the occurrence of partial crystallization during the working steps. The thermal stability of FG20 glass is very low, probably because this material is not completely amorphous (Fig. 3) and presents a specific structural order that favors the beginning of the development of crystalline phases. On the other hand, longer exposure times to concentrated solar radiation lead to glasses with a working range close to or above 150°C; therefore, it is easy to avoid devitrification during thermal manipulation, e.g., sintering. Another useful parameter is the reduced glass transition temperature, Tgr=Tg/Tm, which is an indicator of the prevailing crystallization mechanism in the devitrification process. Thus, a Tgr value below 0.58 implies the predominance of bulk crystallization, whereas if Tgr is above 0.60, crystals develop mainly on the surface of the glass particles. A Tgr value in the range 0.58–0.60 denotes no distinctly predominant mechanism. In the FG20, FG30, and FG35 glasses, the crystallization mechanism in volume plays a predominant role, but surface crystallization also becomes important in the glasses manufactured with the highest exposure times to concentrated solar energy.
On the other hand, the glass-forming ability (GFA) is the capability of a melt to become glass during cooling, and it can be assessed by the critical cooling rate, Rc, which is the slowest rate from melting to Tg, to avoid crystallization. Rc can be determined as log10Rc=17.7−34.6KLL, where KLL is the Lu-Liu parameter and KLL=Tx/(Tg+Tm) [31,32]. The Rc values in Table 5 indicate that FG20 presents a high tendency to crystallize during cooling, and therefore, a very fast cooling rate would be necessary to achieve a completely amorphous material. This result is in agreement with the mineralogical study (Fig. 3), which revealed the semi crystalline character of this glass, even though the melt was quenched by pouring in cold water. As the exposure time to concentrated solar power increases, the melts become more stable, and the critical cooling rate decreases considerably so that glasses obtained with 35min of exposure can be cooled to a rate lower than 100°Cmin−1 without causing the atomic rearrangement necessary for the development of a crystalline phase.
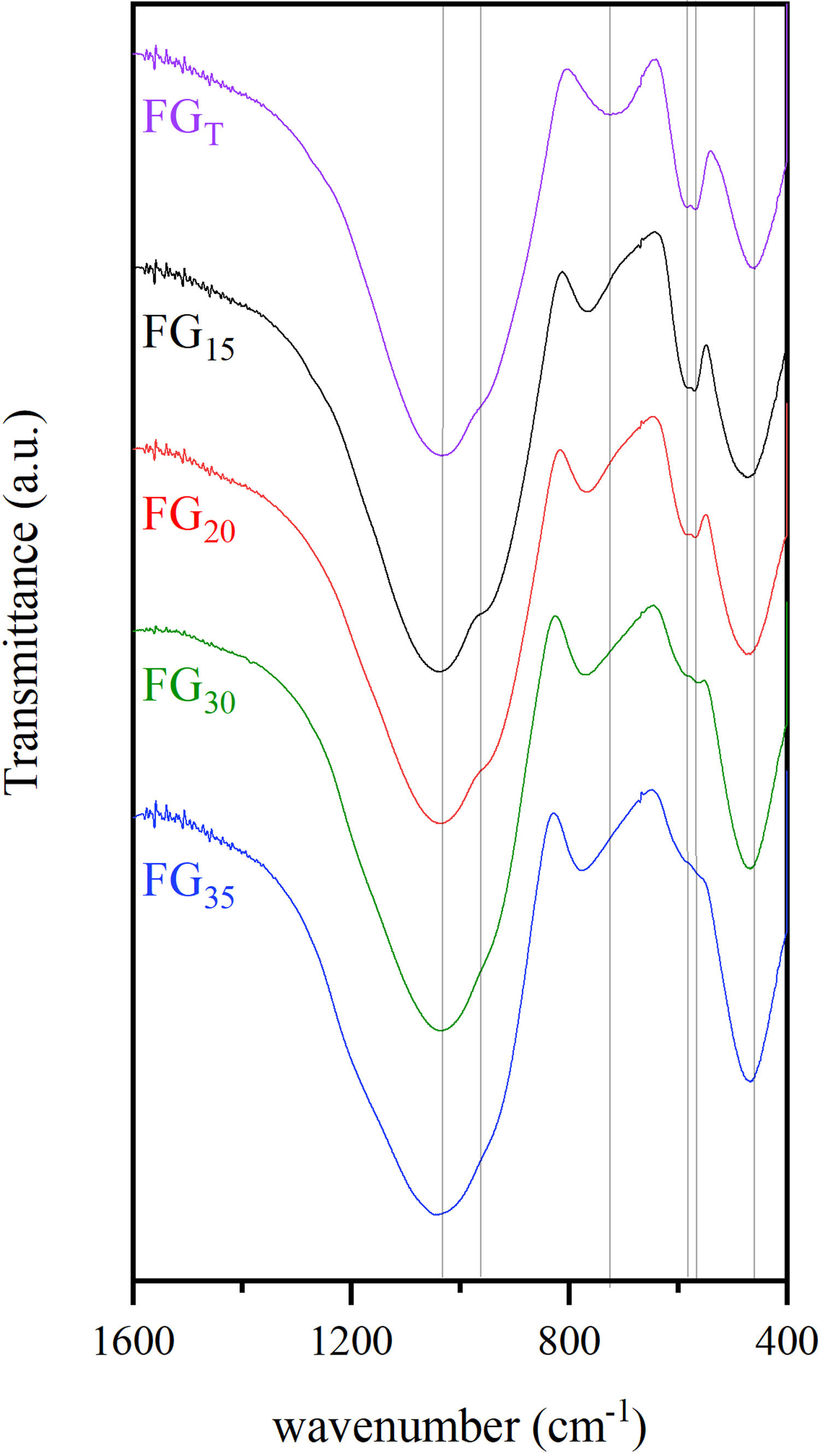
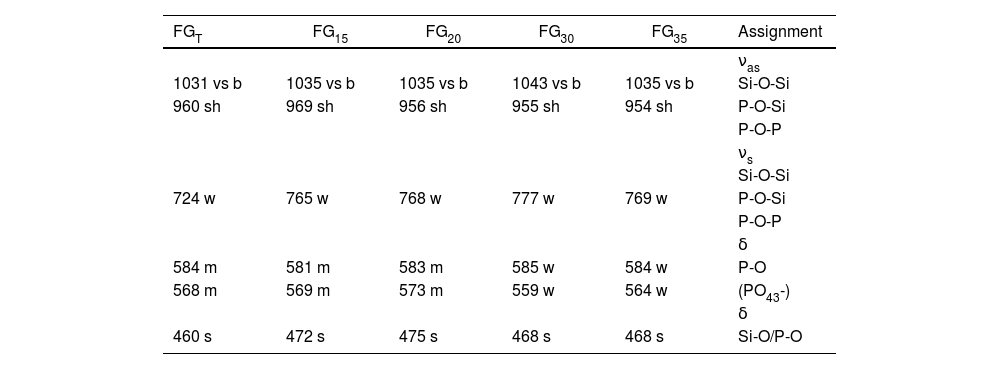
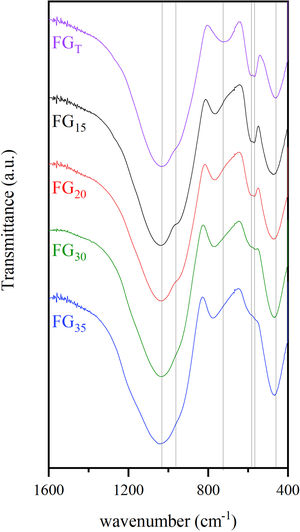
FTIR spectroscopyFig. 9 shows FTIR transmission spectra of glass samples produced by CSP (FG15, FG20, FG30, and FG35) and by an electric furnace (FGT). In general, the spectra of the different samples are quite similar, and they show some specific bands within the range 400–1600cm−1 characteristic of phosphate-silicate glasses. The most intense band appears at 1035cm−1, which is very broad because of the superposition of several bands situated close to each other [33–35]. These bands are attributed to the asymmetric stretching vibrations of bridging oxygen atoms in SiOSi, SiOP, and POP bonds. A shoulder at approximately 960cm−1 can also be detected in all glasses. Bands at approximately 724–777cm−1 can be attributed to symmetric stretching vibrations of the SiOSi, SiOP, and POP bonds. Several differences are observed in the position of these bands according to the use of solar or conventional processes for the melting of glasses. Thus, the band at 724cm−1 in sample FGT is shifted toward higher wavenumbers for samples obtained by CSP. This can be attributed to the higher content of glass framework former, i.e., SiO2 and P2O5, especially SiO2, which is higher in glasses obtained by CSP than in samples obtained by electric furnace (Table 3). The FTIR spectrum of sample FGT shows a set of double bands at 584 and 568cm−1, which are related to the deformation vibrational mode of the tetrahedral phosphate group [35,36]. The higher P2O5 content, the more intensity of this band. Thus, the intensity of this band is higher for samples in which Na3Ca6(PO4) was observed as a crystalline phase (FGT, FG15, and FG20) than in samples FG30 and FG35 with an XRD profile without any crystalline phase. Finally, the bands observed at approximately 460–475cm−1 are assigned to combinations of bending modes of OSiO and OPO bonds. These bands are shifted to higher wavenumbers for samples obtained by CSP, especially for glass with a higher content of glass structure modifiers such as CaO (FG15 and FG20).
Table 6 collects the tentative assignment of the different FTIR bands for all samples according to the literature.
FTIR bands assignment.
| FGT | FG15 | FG20 | FG30 | FG35 | Assignment |
|---|---|---|---|---|---|
| νas | |||||
| 1031 vs b | 1035 vs b | 1035 vs b | 1043 vs b | 1035 vs b | Si-O-Si |
| 960 sh | 969 sh | 956 sh | 955 sh | 954 sh | P-O-Si |
| P-O-P | |||||
| νs | |||||
| Si-O-Si | |||||
| 724 w | 765 w | 768 w | 777 w | 769 w | P-O-Si |
| P-O-P | |||||
| δ | |||||
| 584 m | 581 m | 583 m | 585 w | 584 w | P-O |
| 568 m | 569 m | 573 m | 559 w | 564 w | (PO43-) |
| δ | |||||
| 460 s | 472 s | 475 s | 468 s | 468 s | Si-O/P-O |
A mixture of animal bone flour ash (40wt.%), glassy sand (42wt.%) and K2CO3 (18wt.%) was melted by both a conventional process in an electric furnace and a process involving concentrated solar energy. Based on the results of the characterization tests performed on the materials resulting from melting, the following conclusions can be drawn:
- -
The use of CSP reduces the processing time considerably since the material prepared with a processing time of 15min has a chemical composition and mineralogy similar to that prepared by a conventional process (almost 5h).
- -
An increased CSP processing time leads to increased volatilization of phosphorus and potassium, likely due to reaching working temperatures above 1500°C.
- -
The glass melted in a conventional furnace (FGT), and the FG15 glass prepared by CSP with 15min exposure show a glass-ceramic nature due to the development of Na3Ca6(PO4)5 during the cooling of the melt.
- -
The increased CSP processing time results in a more amorphous nature of the final materials because of the reduced availability of (PO4)3− units for crystal phase formation.
- -
Na3Ca6(PO4)5 develops in the form of spherulites with fibroradiated habits, and the glassy phase presents a phase in phase spinodal decomposition.
- -
The increased CSP processing time also leads to more thermally stable melts and glasses.
In brief, the results of this study highlight the viability of producing ecofriendly glasses in the SiO2–P2O5–CaO–K2O system by using different wastes (viz. animal bone flour ash as the CaO and P2O5 source and glassy sand as the SiO2 source) and concentrated solar energy as the renewable energy source. According to their chemical composition and characteristics, these glasses could be used as matrix-based fertilizers.
The authors thank The European Solar Research Infrastructure for Concentrated Solar Power SFERA-III program (EU. DG RTDs) for providing the infrastructure used to develop project FIGARO-SURPF1904010021. The authors would like to acknowledge Dr. J.I. Robla for his highly valuable and significant assistance in the melting of glasses by concentrated solar power. The collaboration of Ms. P. Díaz and Ms. E. Martín in the preparation of the samples for characterization is also appreciated.