This paper describes the laboratory process of synthesis of wollastonite based on calcining at 1050°C a diatom-rich marl from waste tips of diatomite deposits of Hellín (Albacete. Spain) and its potential use in porous white-ware. The results show that it is viable to obtain wollastonite using as a source rock diatom-rich marls from a natural deposit and that the use of this synthetic material in the compositions for white-ware porous tiles substituting calcium carbonate or natural wollastonite, has not only produced viable products, but has greatly reduced the firing temperature of the raw mixes.
Este artículo describe el proceso de síntesis de wollastonita en laboratorio basada en la calcinación a 1050°C de una marga rica en diatomitas procedente de las escombreras de los yacimientos de diatomita de Hellín (Albacete, España) y su uso potencial en la fabricación de pastas blancas porosas. Los resultados indican que es viable obtener wollastonita utilizando como materias primas las margas ricas en diatomitas de un depósito natural y que el uso de este material sintético en las composiciones de pastas blancas porosas en sustitución del carbonato cálcico o de wollastonita natural, no solo produce productos viables, sino que ha reducido en gran medida las temperaturas de cocción de los crudos.
The ceramic industry is very important for the European economy (€28 billion production value, 200,000 direct jobs; €4.6 billion positive trade balance: 80% SMEs) and is a leading technological sector. Tile production in Spain in 2019 reached 510Msqm [1] of which 100Msqm where white-ware tiles. White-ware tiles average composition [2] includes 40–60% siliceous clays, 0–15% plastic clays, 0–20% kaolin, 10–15% calcium carbonate and 15–25% of silica sand. Calcium is included in the compositions usually as CO3Ca to increase tile porosity, thus water absorption, which contributes to increase dimensional stability, and also produces a delay in the sintering process by liquid phase formation, although when sintering happens this occurs more rapidly. Spain imports annually around 2000tpa of wollastonite for the ceramic tiles industry mostly used in glazes.
Wollastonite is a white natural calcium silicate that has a theoretical composition of CaSiO3 (which may also be written CaO·SiO2). The chemical composition of wollastonite is about 48.3% calcium (CaO) and about 51.7% silica (SiO2). β-Wollastonite (β-CaSi03) is the high temperature form of the calcium metasilicate. It's cristallographically triclinic and is commonly referred to as pseudowollastonite [3–5].
Ceramic applications probably account for 30–40% of wollastonite sales worldwide, followed by polymers (plastics and rubber) with 30–35% of sales, and paint with 10–15% of sales [6].
The production of synthetic wollastonite has been studied by many authors, and there are several patents [7] and multiple papers [8–10], as well as studies on the use of wollastonite in the production of ceramic bodies [10–12].
The objective of this paper is to demonstrate the advantages of using synthetic wollastonite produced from natural diatom-rich marls sourced from waste tips exiting in a well-known and currently active diatomite deposit in south-east Spain, in the industrial production of white-ware replacing the use of natural wollastonite imports, thus reducing the ecological imprint of the diatomite exploitations and the trade balance of the country and taking advantage of the principles of the circular economy.
Materials and methodsIn this paper we have studied two different processes: first we produce synthetic wollastonite out of waste natural raw materials, then we use the synthetic wollastonite in the production of white-ware. Thus the materials and the methods must cover both processes.
Raw materials usedThe raw materials used in the process where:
Natural products:
- •
Diatomaceous marl RM-A (from waste tips of quarries in Hellín. Albacete. Spain)
Commercial products (analytical results included in the cited web sites):
- •
Hymod clay (Source ECC, now Imerys) (https://www.imerys-ceramics.com/sites/default/files/2018-03/Hymod/HSM/E.pdf)
- •
HP-71 Clay (Source: ECC, now Imerys)
- •
Quartz SE-6 (Source: Sibelco S.A.)
- •
Talc (Source: Luzenac) (https://digitalfire.com/material/luzenac+talc+00s)
- •
Ground calcium carbonate (Source: Moltuval)
- •
Natural wollastonite (Source: Comercial Química Massó) (http://www.arciresa.es/wollastonita.pdf)
The chemical analysis of the natural diatomaceous marl employed from a diatomite exploitation waste tip near Hellín (Albacete) (yet to estimate huge reserves (mt) of waste material available around the quarries) is included in Table 1. This determination was carried out by wavelength dispersive X-ray fluorescence spectrometry, using reference materials to guarantee the measurements traceability. The commercial products analysis is included in the web sites cited above.
Characterization techniquesThe crystalline structures were identified by X-ray diffraction of the powdered sample, using a BRUKER Theta-Theta model D8 Advance diffractometer (testing conditions: 40mV and 30mA). The mineralogical species present were identified using the ICDD files for pure crystalline phases. The crystallization evolution of the powdered samples fired at several temperatures was monitored by scanning electron microscopy using a FEI Quanta 200F Field Emission Gun-SEM. The samples were observed and photographed with the backscattered electron signal using a voltage of 20kV. This signal provides information on topography and composition.
The synthetic wollastonite obtained at 1050°C was subjected to the following additional tests:
- •
Soluble salts test
- •
Grain size distribution by laser diffraction
- •
Specific surface area by the BET method
A reference white-ware porous tile composition was selected, in which the calcium carbonate was substituted by natural wollastonite in one experiment and by synthetic wollastonite milled to a standard commercial grain size in a ball mill to between 45 and 35μm (mesh 400), with different percentages in another two compositions. The compositions used are included in Table 2.
White-ware compositions used.
| Reference composition | Composition 1 | Composition 2 | Composition 3 | |
|---|---|---|---|---|
| Hymod clay (%) | 40 | 40 | 40 | 40 |
| HP-71 Clay (%) | 20 | 20 | 20 | 20 |
| Quartz SE-6 (%) | 20 | 15 | 10 | 20 |
| Talc (%) | 5 | – | – | – |
| Ground calcium carbonate (%) | 15 | – | – | – |
| Synthetic wollastonite (%) | – | 25 | 30 | – |
| Natural wollastonite (%) | – | – | – | 20 |
In order to carry out the test, clays were dry milled using a hammer mill to a grain size distribution under 1mm. The non-plastic raw materials were introduced in the compositions without previous treatment as they were supplied with a suitable particle size distribution. The synthetic wollastonite produced was milled to a standard commercial grain size in a ball mill to between 45 and 35μm (mesh 400). Compositions were mixed and wet milled in a laboratory ball mill until 3–4% in weight reject over the 60μm screen were reached.
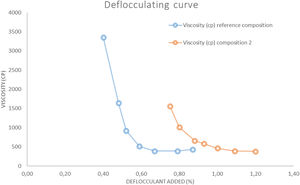
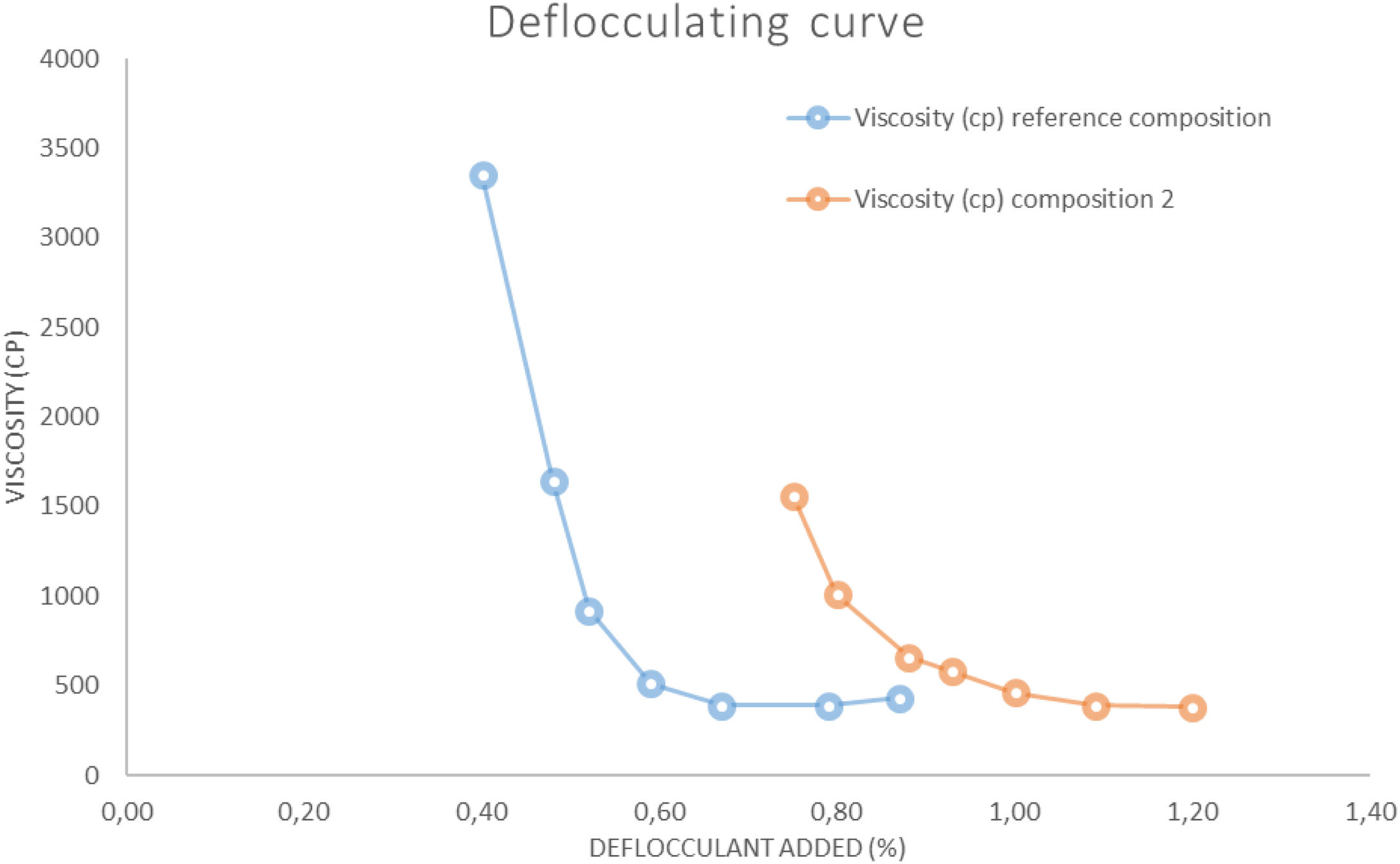
The deflocculating curve (Fig. 3) was carried for each composition using a slip solid content that provides the minimum viscosity between 300 and 700cP. The deflocculant used was a mixture of sodium metasilicate and sodium tripoliphosphate in 1:1 proportion. The test was carried out with a torsion wire GALLENKAMP viscometer.
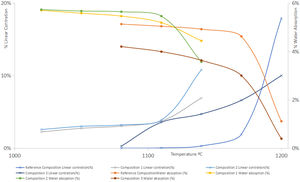
To carry out the linear shrinkage/water absorption diagram (Fig. 4), test specimens were prepared using a cylindrical mould and pressing conditions were 250kg/cm2 and 5.5% humidity. The specimens were then dried in an electric oven at 110°C with air recirculation until constant weight. Then they were calcined at different temperatures in an electric furnace, remaining 6min at the maximum temperature performing firing cycles of 50min.
To determine the dry and calcined bending strength, prismatic test specimens with similar pressing conditions (250kg/cm2 and 5.5% humidity) were prepared. The load was applied at constant deformation speed using the universal tests machine INSTRON.
The study of the whiteness of the calcined pieces was carried out with a spectrophotometer COLOR WXE 7000 MACBETH using a D65 type illuminant and standard observer at 10°.
The adsorbed water in autoclave by the calcined specimens at different temperatures was carried out at a vapour pressure of 10kg/cm2 during 5h.
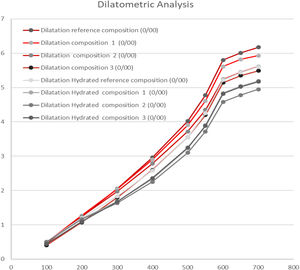
The dilatometric analysis was performed on previously calcined specimens at a specific temperature for each composition. The tested temperatures were 1110°C for the reference composition and for the composition 3, and 1080°C for composition 1 and 2. The test was carried out with an ADAMEL dilatometer at a heating speed of 5°C/min.
The evaluation of the moisture expansion of the compositions has been carried out by dilatometric analysis. In order to do that the thermal expansion at 700°C of the calcined specimens, at the working temperatures above mentioned, as well as their thermal expansion after autoclave treatment (10kg/cm2 of vapour pressure during 5h) were determined. Assuming the reversibility of the process, the difference in thermal expansion between both pieces will be directly related with the moisture expansion, which the material might suffer during its usage time. On the other hand, the measure of adsorption water in the autoclave (at the conditions above described) as a function of the firing temperature can be used to estimate the variation of the phenomenon of moisture expansion with temperature.
Production of synthetic wollastoniteSynthetic wollastonite was obtained exclusively from calcining the diatomaceous marl RM-A at different temperatures (1000, 1050 and 1100°C) in order to select the optimum thermal cycle. No other addition was made to the raw product as the idea is to use the product as it comes from the waste tips. The obtained samples were subsequently subjected to XRD (components identification) and determination of soluble salts (Ca and Mg).
In order to observe the evolution of the formation of wollastonite at the different firing temperatures (1000, 1050 and 1100°C) of the diatomaceous marl a Scanning Electron Microscope (SEM) was employed.
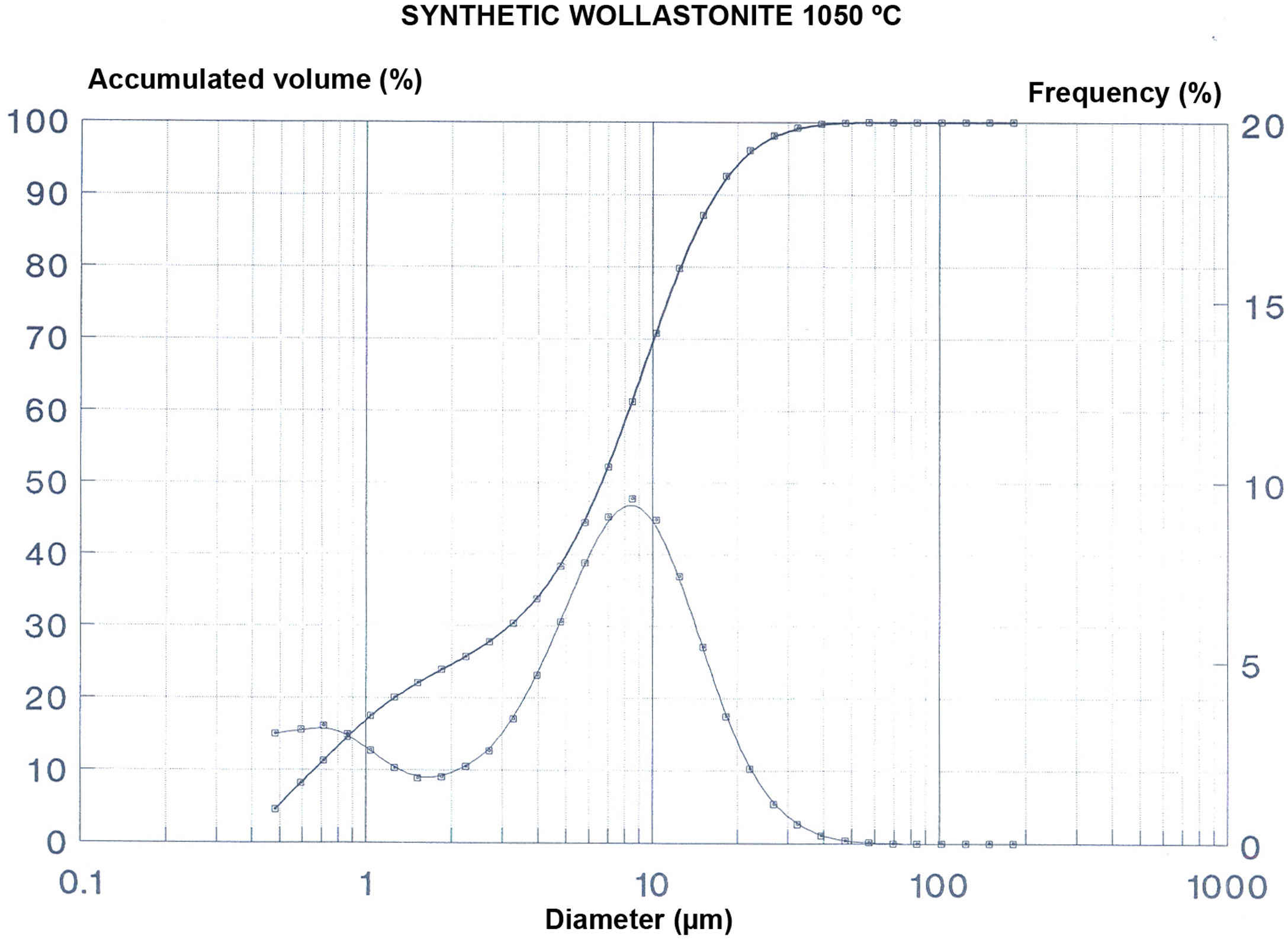
The wollastonite produced at the selected temperature of 1050°C was later studied to obtain soluble salts content, grain size analysis by laser ray diffraction and specific surface using the BET method.
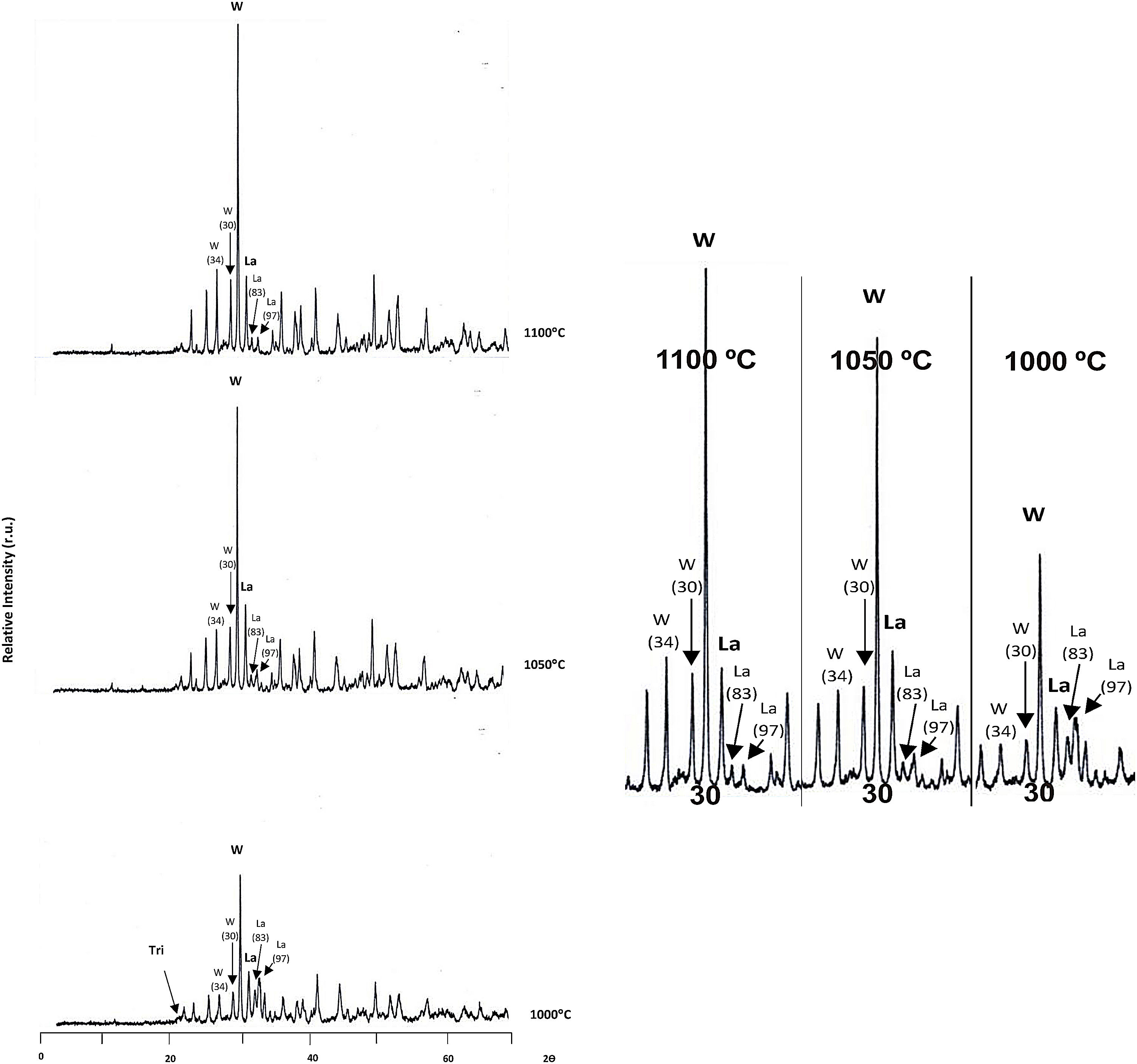
Firing processThe XRD mineralogical analysis obtained following the procedures indicated in “Characterization techniques” are included in Fig. 1.
At 1000°C the main crystalline phases detected were wollastonite and larnite, although a small peak corresponding to trydimite was also observed. When the temperature raised, trydimite disappeared and wollastonite progressively increased, peaked at 1100°C. The larnite peak also increased at 1050°C, but stabilized from this temperature on.
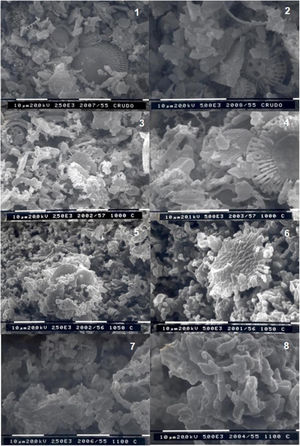
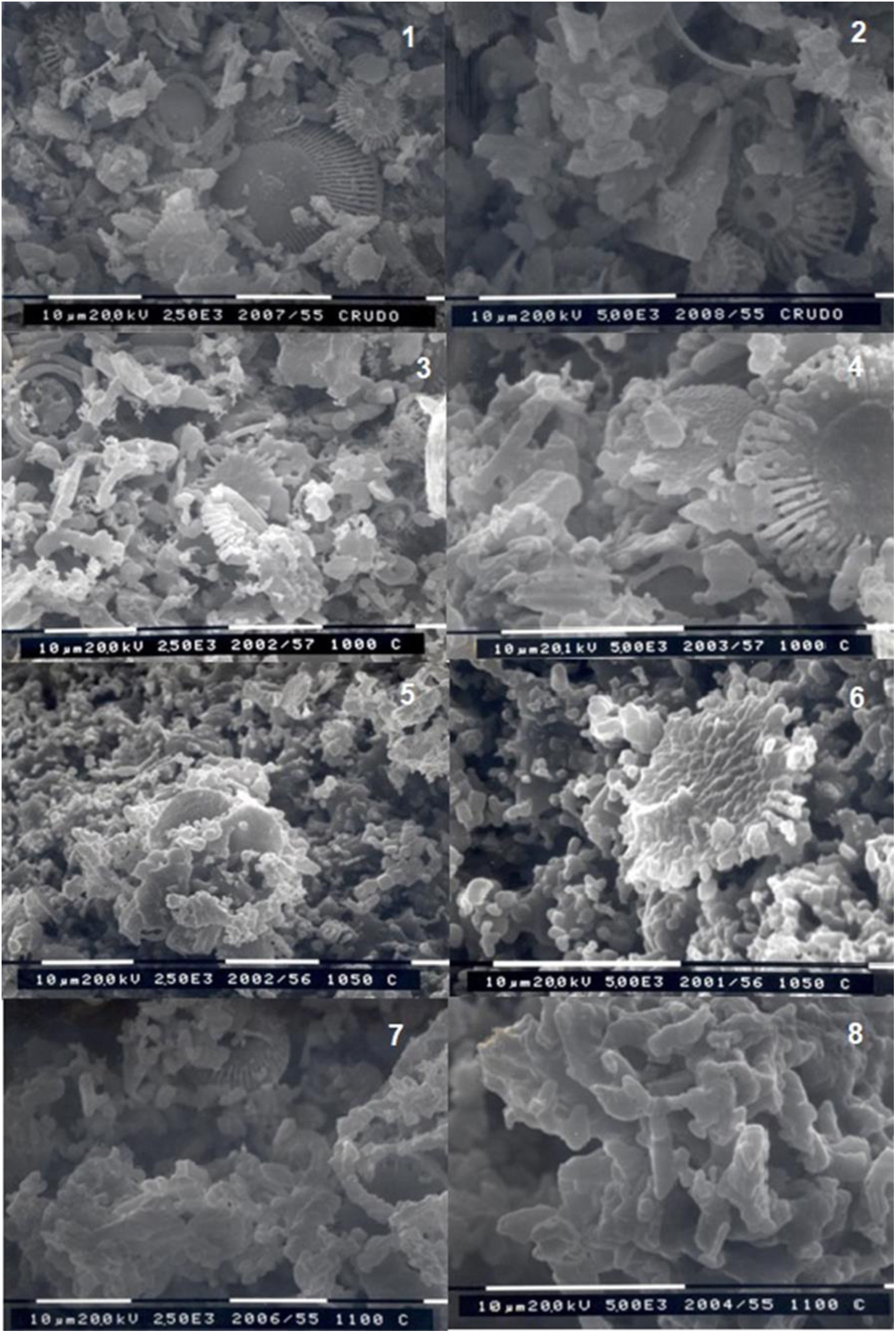
Fig. 5 shows some SEM microphotographs made at 2500 and 500 magnifications of the raw material and of the material after firing at different temperatures (1000, 1050 and 1100°C).
From these results it can be observed that the raw diatomaceous marl RM-A sample showed no wollastonite crystals. Small wollastonite crystals were observed in the sample calcined at 1000°C, being this crystalline phase the main component of the sample calcined at 1100° C since only small remnants of the original diatomaceous marl could be observed.
Analysis of the product obtainedThe synthetic wollastonite obtained at 1050°C was subjected to the following additional tests:
- •
Soluble salts test
- •
Grain size distribution by laser diffraction
- •
Specific surface area by the BET method
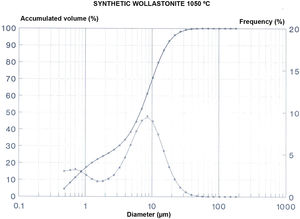
The results of those tests are included in Fig. 2 and Tables 3–5.
Specific surface. Synthetic wollastonite 1050°C. Method: B.E.T. Equipment: Accusorb 2100E Micromeritics. Degasification temperature: 200°C.
| V [cm3 (STP)/g] | X=P/P0 | X/(V·(1−X)) [g/cm3] |
|---|---|---|
| 0.2545 | 0.0396 | 0.1621 |
| 0.2826 | 0.0870 | 0.3371 |
| 0.3014 | 0.1290 | 0.4914 |
| 0.3186 | 01667 | 0.6280 |
| 0.3331 | 0.2013 | 0.7565 |
| Specific surface: 1.18m2/g | ||
Note: V: adsorbed gas amount at standard temperature and pressure. P and P0: equilibrium and the saturation pressure of adsorbed gases at the temperature of adsorption.
This tests show that the Ca2+ is the most abundant cation, together with a great proportion of the sulphate anion. The grain size distribution shows a maximum around 12μm with the higher proportion of particles in a relatively close range of sizes, which results in a low value of the specific surface of the sample.
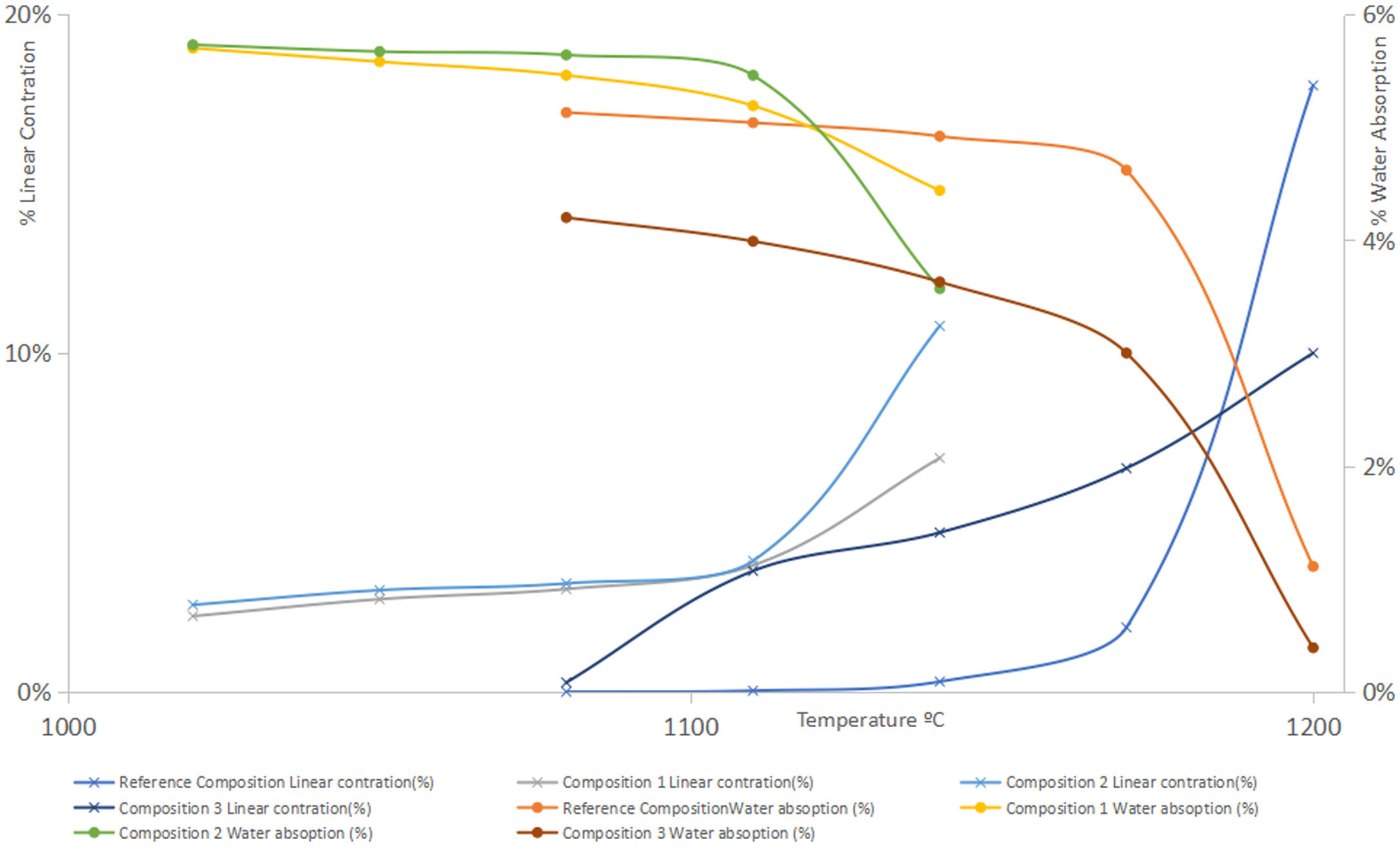
Results on the production of white-ware porous tilesThe figures and tables included below show the results obtained in the above mentioned test carried out on the different compositions prepared: Fig. 3. Deflocculating curve, Fig. 4. Linear shrinkage/Water absorption/Temperature, Fig. 6. Dilatometric analysis, Table 6. Dry mechanical strength, Table 7. Physical properties of Reference Composition calcined, Table 8. Physical properties of composition 1 calcined, Table 9. Physical properties of composition 2 calcined, Table 10. Physical properties of composition 3 calcined, Table 11 Moisture expansion measured by dilatometry.
Physical properties of reference composition calcined.
| Temperature (°C) | Linear shrinkage (%) | Water absorption (%) | Apparent density (g/cm3) | Bending strength (kg/cm2) | L* | a* | b* | Ib | Ia | Adsorbed water (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1080 | 0.00 | 17.10 | 1.800 | 87.56 | 0.72 | 10.96 | 67.76 | 22.31 | 2.74 | |
| 1110 | 0.01 | 16.80 | 1.804 | 228.00 | 87.20 | 0.66 | 11.41 | 66.75 | 23.14 | 1.93 |
| 1140 | 0.09 | 6.40 | 1.814 | 86.41 | 0.51 | 12.01 | 65.04 | 27.88 | 1.31 | |
| 1170 | 0.57 | 15.40 | 1.845 | 270.00 | 83.47 | 0.16 | 13.74 | 59.51 | 32.48 | 0.45 |
| 1200 | 5.37 | 3.70 | 2.190 | 76.11 | 0.00 | 15.16 | 50.03 | 2.19 | 0.20 |
Physical properties of composition 1 calcined.
| Temperature (°C) | Linear shrinkage (%) | Water absorption (%) | Apparent density (g/cm3) | Bending strength (kg/cm2) | L* | a* | b* | Ib | Ia | Adsorbed water (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1020 | 0.67 | 19.00 | 1.758 | 90.13 | 0.81 | 9.59 | 72.58 | 19.38 | 4.02 | |
| 1050 | 0.82 | 18.60 | 1.775 | 184.00 | 89.52 | 0.71 | 10.20 | 71.03 | 20.52 | 3.11 |
| 1080 | 0.91 | 18.20 | 1.784 | 89.17 | 0.60 | 10.69 | 69.94 | 21.40 | 2.72 | |
| 1110 | 1.12 | 17.30 | 1.803 | 280.00 | 88.37 | 0.41 | 11.07 | 68.56 | 22.07 | 2.00 |
| 1140 | 2.07 | 14.80 | 1.867 | 83.10 | 0.27 | 13.51 | 59.44 | 27.66 | 0.47 |
Physical properties of composition 2 calcined.
| Temperature (°C) | Linear shrinkage (%) | Water absorption (%) | Apparent density (g/cm3) | Bending strength (kg/cm2) | L* | a* | b* | Ib | Ia | Adsorbed water (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1020 | 0.77 | 19.10 | 1.752 | 90.30 | 0.72 | 9.53 | 72.89 | 19.17 | 3.56 | |
| 1050 | 0.90 | 18.90 | 1.762 | 210.00 | 89.67 | 0.67 | 10.26 | 71.12 | 20.57 | 3.11 |
| 1080 | 0.96 | 18.80 | 1.836 | 89.47 | 0.50 | 10.49 | 70.59 | 20.90 | 2.91 | |
| 1110 | 1.16 | 18.20 | 1.852 | 264.00 | 88.90 | 0.27 | 10.63 | 68.78 | 21.08 | 2.01 |
| 1140 | 3.24 | 11.90 | 2.051 | 82.75 | 0.12 | 13.52 | 59.09 | 27.64 | 0.43 |
Physical properties of composition 3 calcined.
| Temperature (°C) | Linear shrinkage (%) | Water absorption (%) | Apparent density (g/cm3) | Bending strength (kg/cm2) | L* | a* | b* | Ib | Ia | Adsorbed water (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1080 | 0.82 | 14.00 | 1.934 | 87.30 | 1.03 | 10.54 | 68.03 | 21.84 | 2.39 | |
| 1110 | 1.07 | 13.30 | 1.953 | 246.00 | 85.94 | 1.04 | 11.50 | 65.20 | 23.91 | 1.79 |
| 1140 | 1.41 | 12.10 | 1.980 | 83.84 | 0.90 | 12.55 | 61.47 | 26.25 | 1.11 | |
| 1170 | 1.98 | 10.00 | 2.026 | 300.00 | 79.30 | 0.74 | 14.60 | 53.98 | 31.15 | 0.42 |
| 1200 | 3.00 | 1.30 | 2.118 | 74.96 | 0.28 | 15.59 | 48.36 | 33.98 | 0.16 |
| L* | Black-White parameter |
| a* | Green-Red parameter |
| b* | Blue-Yellow parameter |
| Ib | Whiteness index (Hunter 60) |
| Ia | Yellowness Index (ASTM D1925) |
The typical firing temperature of white-firing wall tiles in the industrial roller kiln around 1140°C, that is equivalent to 1110° al laboratory scale. For this reason, 1110°C was chosen to determine other properties such as the bending strength. For the reference composition higher temperatures were tested until an excessive shrinkage was detected (1200°C, 5.34%). Due to the fact that the other compositions showed higher values of the shrinkage, the tested temperatures were limited, as the information obtained is far from the industrial interest due to an excessive shrinkage. The criteria used were not to exceed a shrinkage higher than 2.0%.
DiscussionProduction of synthetic wollastoniteAccording to the results obtained in the identification of crystalline components by X-ray diffraction and in the analysis of soluble salts in the various samples of wollastonite obtained, the following features should be highlighted:
- •
Synthetic wollastonite obtained at 1050 and 1100°C, do not show too much differences both in their soluble salts content and in the crystalline phases formed. The sample calcined at 1050°C contains a higher proportion of larnitic phase.
- •
There is a marked difference between the wollastonite produced at 1000°C with regards to the other two. This product contains a great amount of unreacted silica (in the form of trydimite) and the percentage of soluble salts, particularly of calcium type, is higher.
According to the above results, it has been considered the synthetic wollastonite produced at 1050°C as the starting raw material to be used in the white-ware porous tiles, as it has the advantage of having a lower calcining temperature with respect to that formed at 1100°C.
Production of white-ware using the obtained synthetic wollastoniteFrom the results of the tests carried out in the different calcined compositions, we can highlight the following aspects:
Unfired physical propertiesThe deflocculation tests (Fig. 3) were determined at a constant solids content (67%) to compare the effect of wollastonite. This solids content is in the range of the ones used in the industry (65–70%). The results show that in composition 2 the solids content diminishes greatly with respect to the reference composition, also the optimum amount of deflocculant employed rises 0.4–0.5%. The difficulties in deflocculating this composition are probably due to a higher presence of flocculant electrolytes supplied by the synthetic wollastonite (Ca2+ and SO42−), as well as a less adequate distribution of the particle size distribution of the particles resulting from the addition of that raw material.
The apparent dry density (Tables 6–10) of the specimens shows higher values in the reference specimen and in the composition 3, with natural wollastonite. The addition of synthetic wollastonite in composition 1 worsens compaction, as mentioned above, due to the particle size distribution of the synthetic wollastonite, with abundant intermediate particles, less favourable to compaction. As the proportion of this material in composition 2 is increased, the reduction of apparent density is more evident.
The dry bending strength (Tables 6–10) shows adequate values in all compositions, observing the expected reduction in compositions 1 and 2 as the dry apparent density reduces.
Firing range, mechanical strength and firing whitenessFrom the observation of the linear shrinkage-water absorption- firing temperature diagrams (Fig. 4), it can be observed that the introduction of synthetic wollastonite in compositions 1 and 2 increases the firing shrinkage in what it could be considered the “working range” for this type of compositions (temperatures under 1110°C for compositions 1 and 2) as well as a higher variation of this parameter with the temperature in the range previously considered. Both facts could be the result of a lower reactivity of the calcium phases included, when compared with compositions with calcium carbonate. On the other hand, in the synthesis of this type of wollastonite the proportion of vitreous phase obtained were very important (see Fig. 5).
Photo 1. Raw marly diatomite RM-A. Magnification x2500. Photo 2. Raw marly diatomite RM-A. Magnification X5000. Photo 3. Marly diatomite fired at 1000°C. Magnification X2500. Photo 4. Marly diatomite fired at 1000°C. Magnification X5000. Photo 5. Marly diatomite fired at 1050°C. Magnification X2500. Photo 6. Marly diatomite fired at 1050°C. Magnification X5000. Photo 7. Marly diatomite fired at 1100°C Magnification X2500. Photo 8. Marly diatomite fired at 1100°C. Magnification X5000.
Nevertheless, both firing ranges are adequate for this type of products, reducing the firing temperature with regards to the reference composition in approximately 60°C.
The porosities obtained (measured as water absorption) (Tables 6–10) have been even higher with the use of synthetic wollastonite, since although the loss of ignition is much lower, both compositions provide specimens with higher open porosities.
The effect of introducing natural wollastonite has been similar to the use of synthetic wollastonite with respect to the increase of the calcining shrinkage, but the variation of firing parameters with temperature (lineal shrinkage and water absorption) is much higher. In those compositions, the reduction of the porosity of the calcined specimens has been noteworthy, with working temperatures similar or slightly lower than the reference composition.
With regard to the calcined bending strength (Tables 6–10), in all the compositions there is an increase of this parameter as a result of the higher degree of calcic phases formed and the reduction of porosity originated by a higher development of vitreous phases. At the working temperatures, 1050°C for compositions 1 and 2 and 1110°C for the reference composition and composition 3, the higher value of the bending strength corresponded to composition 3 with less calcined porosity followed by the reference composition. The composition with synthetic wollastonite has shown the lower values although adequate for this type of compositions.
The compositions formulated with synthetic and natural wollastonite, have shown a higher degree of whiteness (Tables 7–10) at the different tested temperatures, as well as a lower index of yellowness, although in these particular values apart from influencing the type and amount of formed phases, there must be an influence of the rest of the raw materials employed.
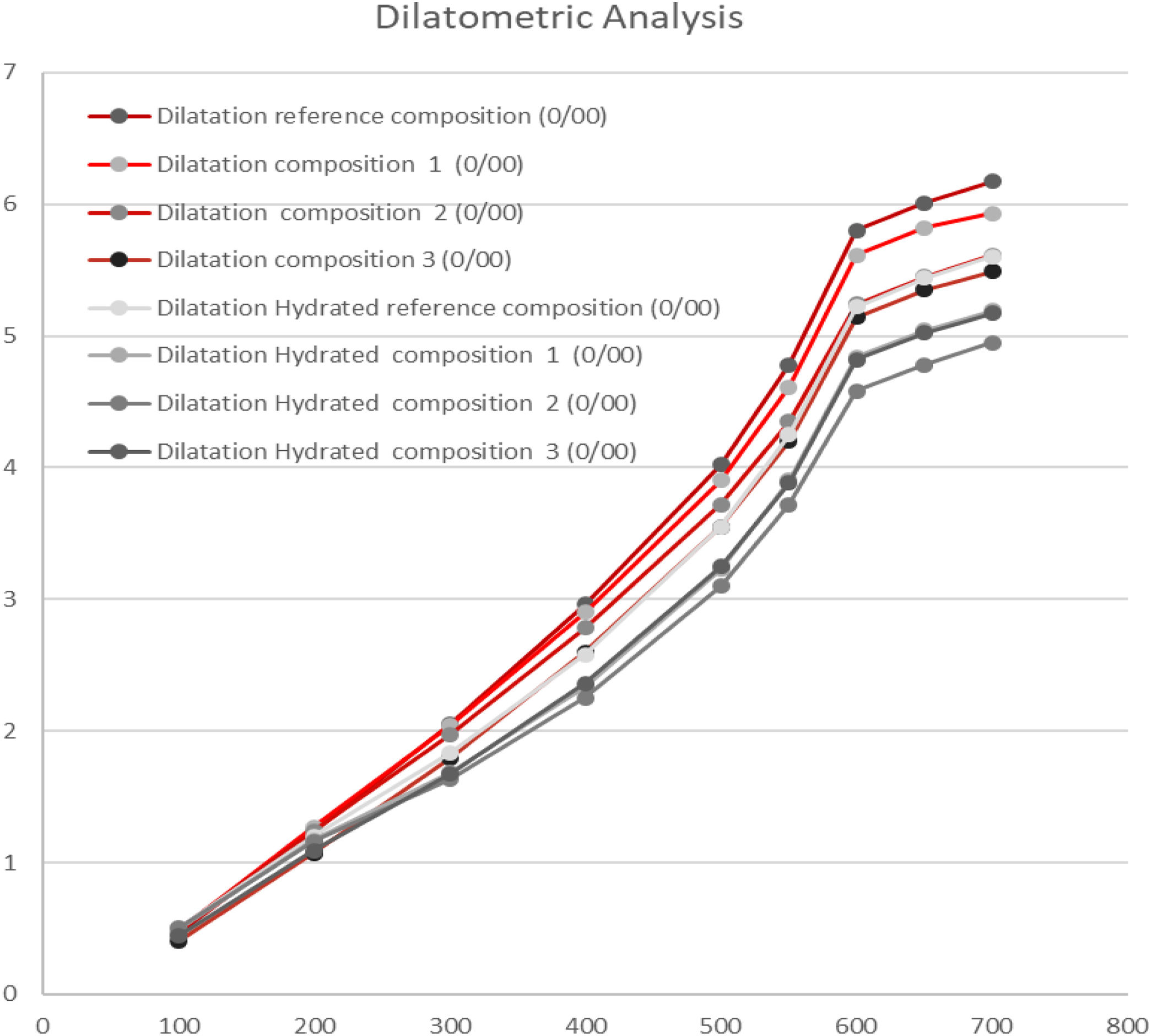
Dilatometry analysisThe coefficient of thermal expansion of the formulated compositions with synthetic wollastonite have resulted adequate to the type of compositions studied, showing even lower values that the reference composition. Nevertheless, these values are greatly influenced by the proportion of non-plastic components such as quartz or talc. Both formers of crystalline phases of high expansion coefficient.
The substitution of calcium carbonate T-50, talc and quartz by synthetic wollastonite in the composition 1 with respect to the reference composition reduces the thermal expansion of the calcined specimens at the working temperature.
When the proportion of synthetic wollastonite is increased in composition 2, with respect to composition 1 in detriment of quartz, the reduction of the thermal expansion is even more evident, due to the high thermal expansion of the quartz.
Composition 3 with natural wollastonite presented an even lower thermal expansion than composition 1 with synthetic wollastonite and with a higher proportion of quartz.
Moisture expansion (Table 11)The results obtained in the water adsorption test in autoclave as a function of the temperature show that in all the compositions the adsorption of water vapour, thus the tendency to expand, diminishes with temperature, resulting, in all cases, from the disappearing of the porosity of the pieces and the reduction of the higher hydratability of the amorphous phases.
When observing the value obtained at the estimated working temperatures estimated for each composition, compositions 1 and 2 (1050°C) show a slighter higher value of the adsorbed water than in the compositions reference and 3 (1110°C). This is probably due to the higher porosity and lower calcium phases obtained from the compositions with synthetic wollastonite.
Fig. 6 details the values of the thermal expansion differences at 700°C of the hydrated and non-hydrated specimens. These values, related with the moisture expansion, show that there is a higher moisture expansion of the reference composition and particularly of the composition 3, due to its lower adsorbed water values as well as its high mechanical properties (measured as bending strength). Thus, compositions with better mechanical properties, seem to produce lower expansion.
Compositions 2 and 3 with synthetic wollastonite, although they have shown higher values that previous compositions, are perfectly adjusted by better fine-tuning the prepared formulations.
ConclusionsFrom the study carried out which included the manufacture of synthetic wollastonite from a natural diatomaceous marl raw material which was later employed in the manufacture of white ware porous tiles, we can conclude that:
Firing at 1050°C during 2h of a sample of diatomaceous marl has produced a material whose main calcic phase is wollastonite, with small contents of larnite. The resulting wollastonitic product was then characterized and showed a narrow particle size range, a low specific surface, and a relatively high proportion of soluble electrolytes particularly of calcium and sulphates. Such set of characteristics have had a great importance in the behaviour or the white ware composition prepared with this material.
The introduction of synthetic wollastonite in white ware porous tiles compositions substituting calcium carbonate or natural wollastonite produced compositions that, albeit presenting some different features during the manufacturing process, have been adequate, both from the point of view of the manufacturing process and of the properties of the finished product. The firing temperature of those compositions has been notably reduced if compared with the reference composition.
The main difference observed in the manufacturing process of compositions with synthetic wollastonite has been the deflocculating stage, as the solids content was quite lower than the usually obtained in this type of compositions. This will result in extraordinary lower atomization process performances respect to the reference compositions. The reason for this underperformance is the higher content in flocculants electrolytes and the narrow particle size distribution of the synthetic wollastonite.
The increase in wollastonite proportions from 25 to 30% does not seem to have produced excessive differences with respect to the characteristics of the compositions obtained, thus might be more adequate, due to the problems introduced by this material during deflocculation, to reduce the synthetic wollastonite proportion in the composition.
In order to solve the above-mentioned problems, we suggest washing the synthetic wollastonite to eliminate soluble salts. This could be done by wet milling the product and later filter pressing it. Other alternative solution could be using other inorganic or organic deflocculating mixtures.





![DRX of the products obtained by firing the diatomaceous marl RM-A at different temperatures, Tri (trydimite), W (wollastonite), La (larnite), [34] expected relative intensity. DRX of the products obtained by firing the diatomaceous marl RM-A at different temperatures, Tri (trydimite), W (wollastonite), La (larnite), [34] expected relative intensity.](https://static.elsevier.es/multimedia/03663175/0000006100000006/v2_202212200715/S0366317521000376/v2_202212200715/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)