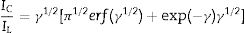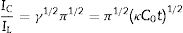In this work, a new catalyst based on carbon paste (CP) modified by spinel NiCo2O4 nanoparticles and zeolite-4A was proposed and used for the electrochemical oxidation of methanol. NiCo2O4@zeolite-4A was obtained through the calcination of the physical mixture of NiCo2O4 and zeolite-4A. Their physicochemical properties were characterized by X-ray diffraction (XRD), scanning electron microscopy-energy dispersive X-ray spectra (SEM-EDS), and FT-IR techniques. The electrocatalytic performance of modified electrode based on NiCo2O4@zeolite-4A was tested using cyclic voltammetry (CV) and chronoamperomerty (CA). The result of cyclic voltammetry indicated a significant rise in the oxidation current on the surface of NiCo2O4@zeolite-4A/CPE modified electrode. The rate constant for the catalytic reaction (k) of methanol was calculated by means of a chronoamperometric technique. To sum up, the incorporation of NiCo2O4 nanoparticles into zeolite-4A provided the binary electroactive sites for catalysis of methanol oxidation as it exhibited a long-term stability for catalytic reaction, and thus a promising application.
En este trabajo se propuso un nuevo catalizador basado en pasta de carbono (CP) modificado por espinela de nanopartículas de NiCo2O4 y zeolita-4ª, y se utilizó para la oxidación electroquímica del metanol. Se obtuvo NiCo2O4@zeolita-4A a través de la calcinación de la mezcla física de NiCo2O4 y zeolita-4A. Sus propiedades fisicoquímicas se caracterizaron por difracción de rayos X (XRD), espectros de rayos X de barrido de energía con microscopía electrónica de barrido (SEM-EDS) y técnicas de FT-IR. El rendimiento electrocatalítico del electrodo modificado basado en NiCo2O4@zeolita-4A se probó utilizando la voltametría cíclica (CV) y cronoamperometría (CA). El resultado de la voltametría cíclica indicó un aumento significativo de la corriente de oxidación en la superficie del electrodo modificado con zeolita-4A/CPE de NiCo2O4. La constante de velocidad para la reacción catalítica (k) del metanol se calculó mediante una técnica cronoamperométrica. En resumen, la incorporación de nanopartículas de NiCo2O4 en la zeolita-4A proporcionó los sitios electroactivos binarios para la catálisis de la oxidación de metanol, ya que exhibió una estabilidad a largo plazo para la reacción catalítica y, por lo tanto, una aplicación prometedora.
Fuel cells (FCs) are among the most common technologies in energy storage. They prove to be environmentally friendly and highly efficient in solving the energy problems of the modern world [1]. Although hydrogen is currently the predominant fuel as, by way of example, fuel cell vehicles under commercial development all use hydrogen fuel, methanol could be a promising alternative owing to its simplicity of operation, transportation and storage [2]. The most commonly used electrodes in direct methanol fuel cell (DMFC), however, exhibit low kinetic and high overpotential. Likewise, Pt-based metal, bimetal and their alloys such as Pt, Pt-Ru, Pt-Fe-Ru are also known to be the best catalysts in investigating the mechanism and kinetic of electrocatalytic oxidation of methanol [3–9] as they are very active in the oxidation of methanol. But these catalysts are expensive and have low natural abundance. Therefore, there is a need to develop alternative low-cost transition metal oxides based catalysts that could overcome these drawbacks.
Transition metal oxides are utilized in heterogeneous catalysis and electrocatalysis by virtue of their intrinsic redox and tunable chemical, morphological, and textural properties, which can be rigidly bound on the substrate materials, i.e., nanoporous architectures with high surface areas and pore volumes [10–12]. Several substrate materials such as carbon nanotube, graphene oxide, and mesoporous silica have been hitherto exploited to improve electro-catalytic activity of metal species [13–17]. The ordered mesoporous materials with high surface area and porous nature known as zeolites are not only among the most commonly used nanoporous supports and substrate but also considered among the best solutions to overcome the pitfalls of catalytic oxidation of alcohol for fuel cells [18]. It should be noted that zeolite comes in different types, of which zeolite-4A is the most widely studied and employed framework, functioning as a substrate for different catalytic reactions [10–24].
Similarly, spinel crystal structure of the type AB2O4 has been used in a broad range of disciplines, from electrochemistry, through catalysis to electronics. In a normal spinel, the A cations occupy the tetrahedral sites while the B cations fill the octahedral sites in the cubic closed packing formed by the anions O2−. In an inverse spinel structure, in contrast, part of the B cations occupy the tetrahedral sites, possibly distorting the lattice by their size, while the A cations fill the octahedral sites [24–26]. One of the binary metal oxides known as NiCo2O4 adopts an inverse spinel structure in which nickel ions fill the octahedral sites whereas cobalt ions are scattered across the tetrahedral and octahedral sites of the structure. NiCo2O4 demonstrates higher electronic conductivity and electrochemical activity than those of nickel and cobalt oxides and hence higher redox chemistry than each single component [27–30]. That being so, NiCo2O4 has been utilized in such diverse fields as magnetic materials, electro-catalysts, optical limiters and switches, chemical sensors, and lithium ion batteries [31].
In the present research, nickel cobaltite nanoparticles (NiCo2O4 NPs) was synthesized via a facile hydrothermal method in the presence of urea and NiCo2O4 nanoparticles were incorporated into the zeolite-4A. In the end, electrocatalytic behavior of NiCo2O4@zeolite-4A modified electrode toward methanol oxidation was observed using the cyclic voltammetry and chronoamperomerty in a conventional three-electrode electrochemical setup. The finding of this study indicated that the electrode modified by NiCo2O4@zeolite-4A might be more efficient in the oxidation of methanol than other catalysts.
ExperimentalMaterialsAll chemicals used in this study were of analytical grade and utilized as received without undergoing any further purification. Sodium aluminate, Cetyl Trimethyl Ammonium Bromide (CTAB), NaOH pellets and Tetraethylorthosilicate (TEOS) were purchased from Sigma–Aldrich; and urea, cobalt (II) chloride hexahydrate and nickel (II) chloride hexahydrate were obtained from Merck.
Synthesis of zeolite-4A, NiCo2O4 and NiCo2O4@zeolite-4AZeolite-4A was prepared using a formerly reported method [32]. That is to say, 4g of NaOH pellets and approximately half of the required amount of CTAB were dissolved in deionized water, in which 8g of sodium aluminate was added later. The resulting mixture was stirred until a clear solution was formed (solution A). 15.6g of TEOS was added to the rest of the required amount of CTAB solution. After 2h of continuous stirring, a homogeneous and clear solution was created (solution B). A blend of solutions A and B formed the initial clear solution for synthesis which was then crystallized hydrothermally at 100°C for 24h in a stainless-steel autoclave. Next, the precipitation product was filtered and washed using deionized water. The final product was eventually dried in a vacuum oven at 60°C, and later calcined at 550°C.
NiCo2O4 was synthesized in a similar way [33]. Namely, 10mmol CoCl2·6H2O, 5mmol NiCl2·6H2O and 50mmol urea were dissolved in 70mL deionized water and stirred until a bright pink colored solution was formed. The mixture was then transferred into a Teflon-lined stainless-steel autoclave, and hydrothermally treated at 120°C for 6h. Autoclave was already cooled at room temperature while the reaction mixture was filtered and washed with distilled water and ethanol. The material was later calcined at 300°C for 4h. In the end, NiCo2O4 and zeolite-4A with weight ratio 40 to 60, denoted as NiCo2O4 (40%)/zeolite-4A were grounded uniformly with ethanol using mortar and pastel. Ethanol was slowly removed during the mixing process. The mixture was then heated at 473K in air for 45min to remove the residual ethanol followed by calcination at 613K for 12h.
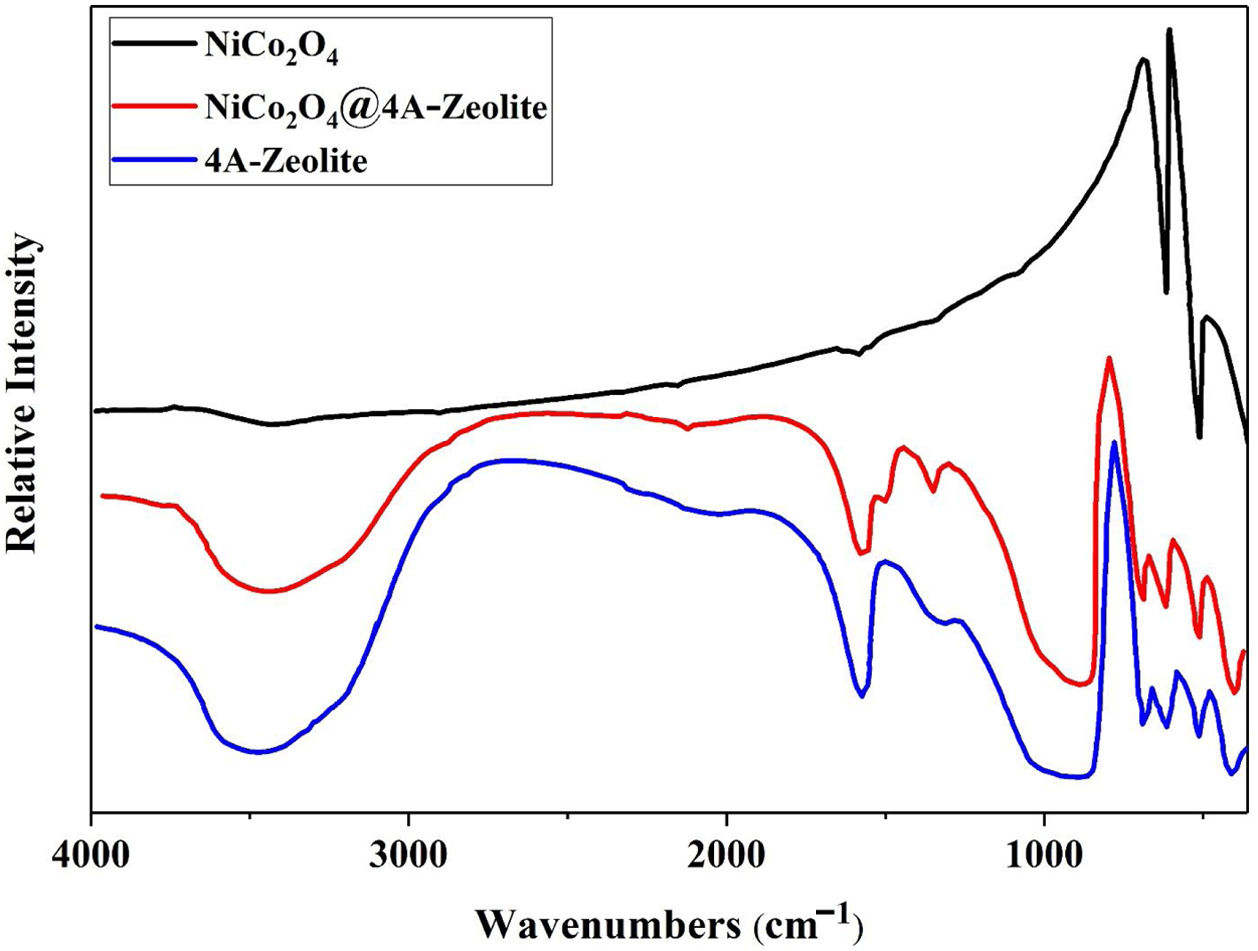
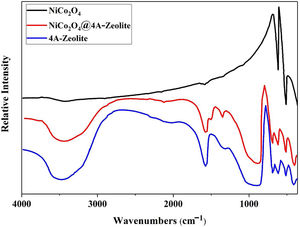
ResultsThe FT-IR spectra of samples are given in Fig. 1. The FT-IR spectra of pristine NiCo2O4 spectrum showed two strong peaks at 557 and 652cm−1, referring to the stretching vibrations of the Ni–O and Co–O bonds in nickel cobalt oxide, respectively [30]. Given the high degree of hydration, the FT-IR spectra of pure zeolite-4A and NiCo2O4@zeolite-4A exhibited a broad peak around 3400cm−1, indicating the presence of hydroxyl groups. All absorption peaks around 450–1000cm−1 alluded to metal–oxygen bonds, owing to the existence of metal complexes or metal oxide nanoparticles brought about by low concentrations of metal loaded on the zeolite-4A. The comparison between FT-IR spectrum of NiCo2O4@zeolite-4A and the pure zeolite-4A spectrum revealed that the original zeolite spectrum about 973cm−1 ((Si, Al)−O) appeared in lower region. This peak shifted 5cm−1 and appeared in 968cm−1 which could be caused by the interaction between zeolite network and nickel cobalt oxide nanoparticles [34–37].
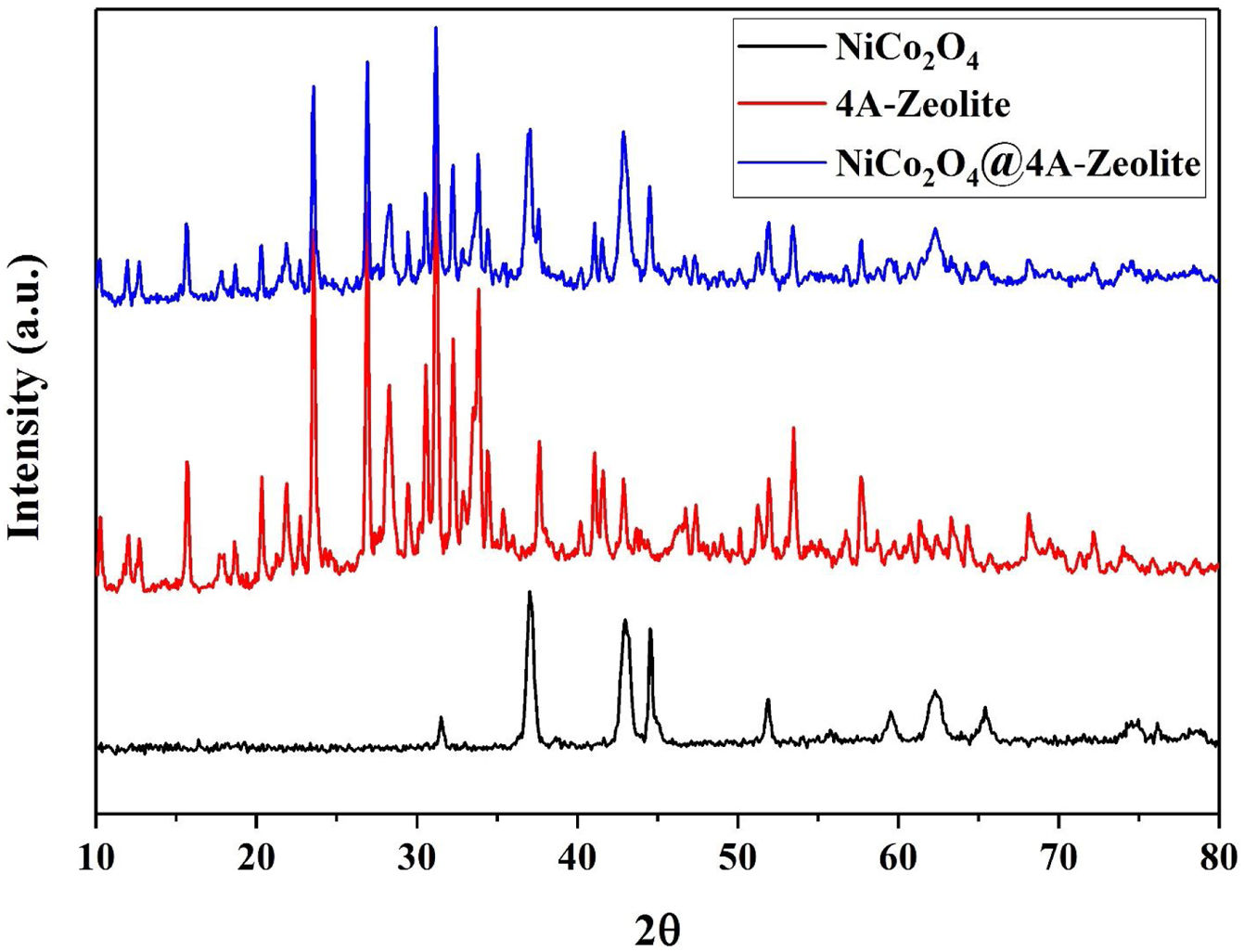
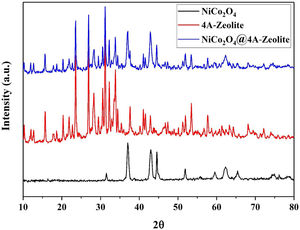
The X-ray diffraction (XRD) pattern of all samples are shown in Fig. 2. As depicted in XRD, all the diffraction peaks for NiCo2O4 and the space groups Fd-3m corresponded to the standard patterns of the spinel NiCo2O4 phase (JCPDS card No. 73-1702) and all the diffraction peaks of zeolite-4A with the space groups Fm-3c were, likewise, confirmed by (JCPDS card No. 39-222). Diffraction peaks of NiCo2O4 NPs were observed at XRD pattern of zeolite-4A after loading NiCo2O4 NPs on zeolite-4A, which indicated that NiCo2O4 NPs were well loaded into zeolite-4A.
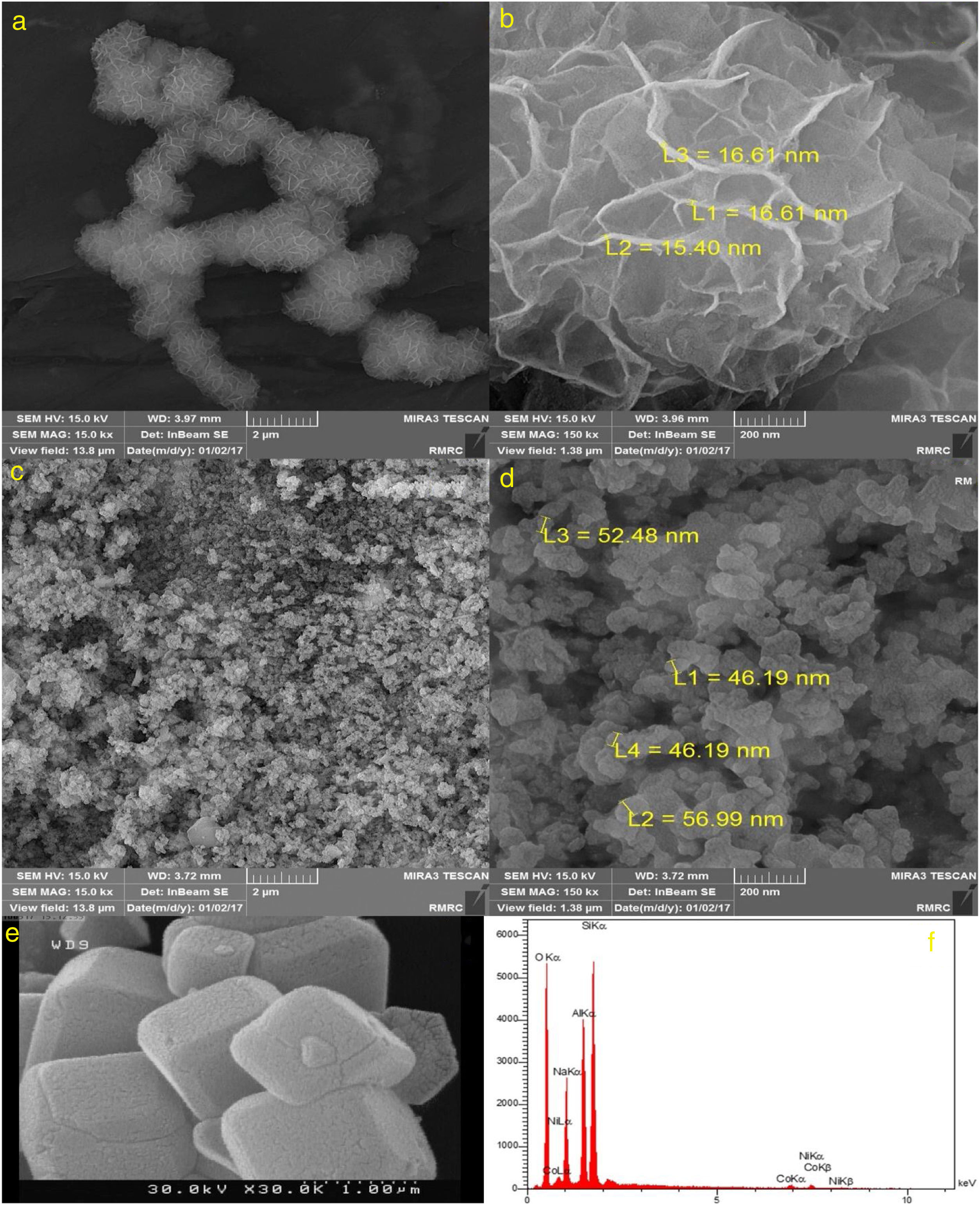
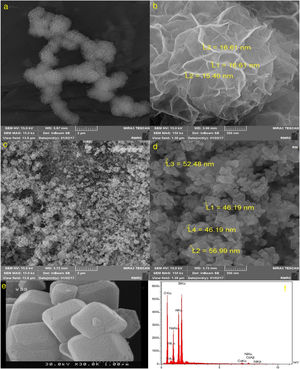
Fig. 3 illustrates SEM images of NiCo2O4, zeolite-4A and NiCo2O4@zeolite-4A. As can be seen, the NiCo2O4 nanoparticles were composed of uniform flower-like structures whose average size is 1.6μm in diameter. These 3D flower-like structures were assembled by a large number of nanosheets with an average thickness of 16nm (Fig. 3a and b). Fig. 3c and d indicates that NiCo2O4@zeolite-4A had the spherical structure with particle diameter ranging from 46.2nm to 57nm. The comparison between Fig. 3c, d and e revealed that the cubic structure morphology of zeolite transformed into a spherical structure subsequent to the loading of NiCo2O4 nanoparticles.
It can be inferred from the EDX analysis that the obtained NiCo2O4@zeolite-4A (Fig. 3f) had the purity of the samples containing only Si, Al, O, Na, Ni and Co elements. The loading of NiCo2O4 nanoparticles to the zeolite-4A had already been shown in Fig. 3c.
Preparation of the working electrodesElectrochemical measurements were carried out by means of a Sama-500 electrochemical workstation while the electrocatalytic performance was tested using cyclic voltammetry (CV) and chronoamperometry (CA). All the measurements were performed in a conventional three-electrode system containing Ag/AgCl as the reference electrode (3M KCl) a platinum wire (both from Azar Electrode Co., Iran) as the counter electrode and NiCo2O4 modified carbon paste electrode (CPE) as the working electrode. The NiCo2O4@zeolite-4A and zeolite-4A modified carbon paste electrode were prepared by mixing NiCo2O4@zeolite-4A and zeolite-4A in a ratio of 30:70% (w/w) with the graphite powder and then with diethyl ether. After solvent evaporation, paraffin was blended by hand in a mortar, the resulting paste of which was inserted in the bottom of a glass tube (with internal radius 1.5mm). The electrical connection was implemented by a Cu wire. The unmodified CPE were produced in a similar manner, except for the addition of an appropriate amount of NiCo2O4 to the graphite powder. The electrodes were ultimately polished on a very fine smooth paper.
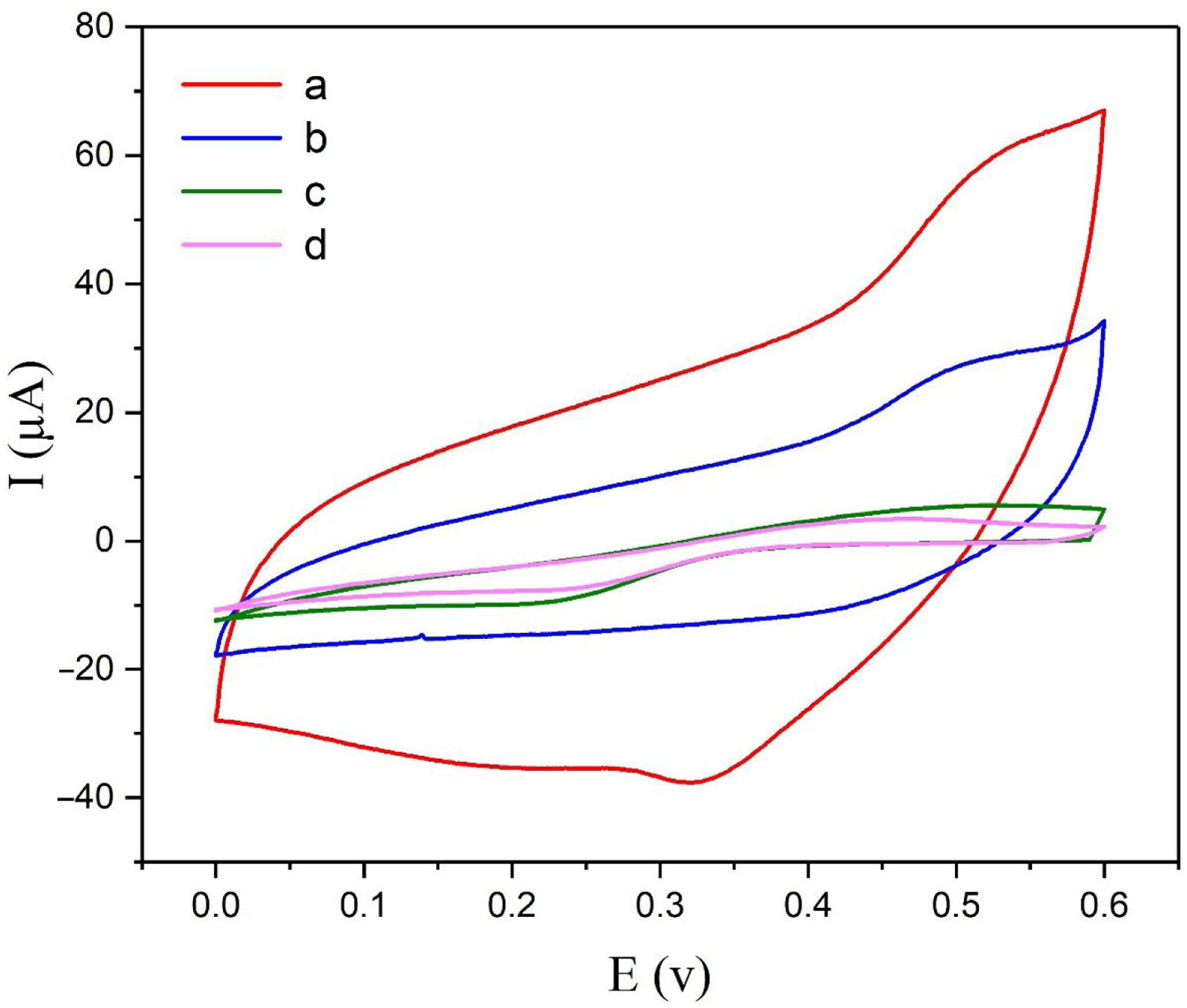
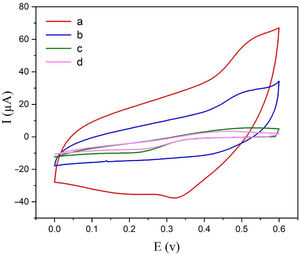
Electrochemical behavior of modified electrodeThe electrochemical behaviors of different modified electrodes were examined by cyclic voltammetry recorded at a potential sweep rate of 25mVs−1 for methanol oxidation (Fig. 4). As shown in Fig. 4, NiCo2O4@zeolite-4A/CPE displayed large oxidation current with the oxidation peak current of 60μA (curve a). On the surface of zeolite-4A/CPE and CPE (curve c, d), however, no redox peaks were observed under the same condition. It can be inferred from Fig. 4c and d that the zeolite-4A played no significant role in the oxidation of methanol. The oxidation peak current on the surface of NiCo2O4/CPE was 28μA (curve b) which proved that the presence of NiCo2O4 in CPE could increase methanol oxidation peak currents. The findings revealed that the presence of synthesized NiCo2O4 nanoparticles and zeolite-4A on NiCo2O4@zeolite-4A/CPE surface were significantly improved by virtue of electrochemical oxidation response of methanol.
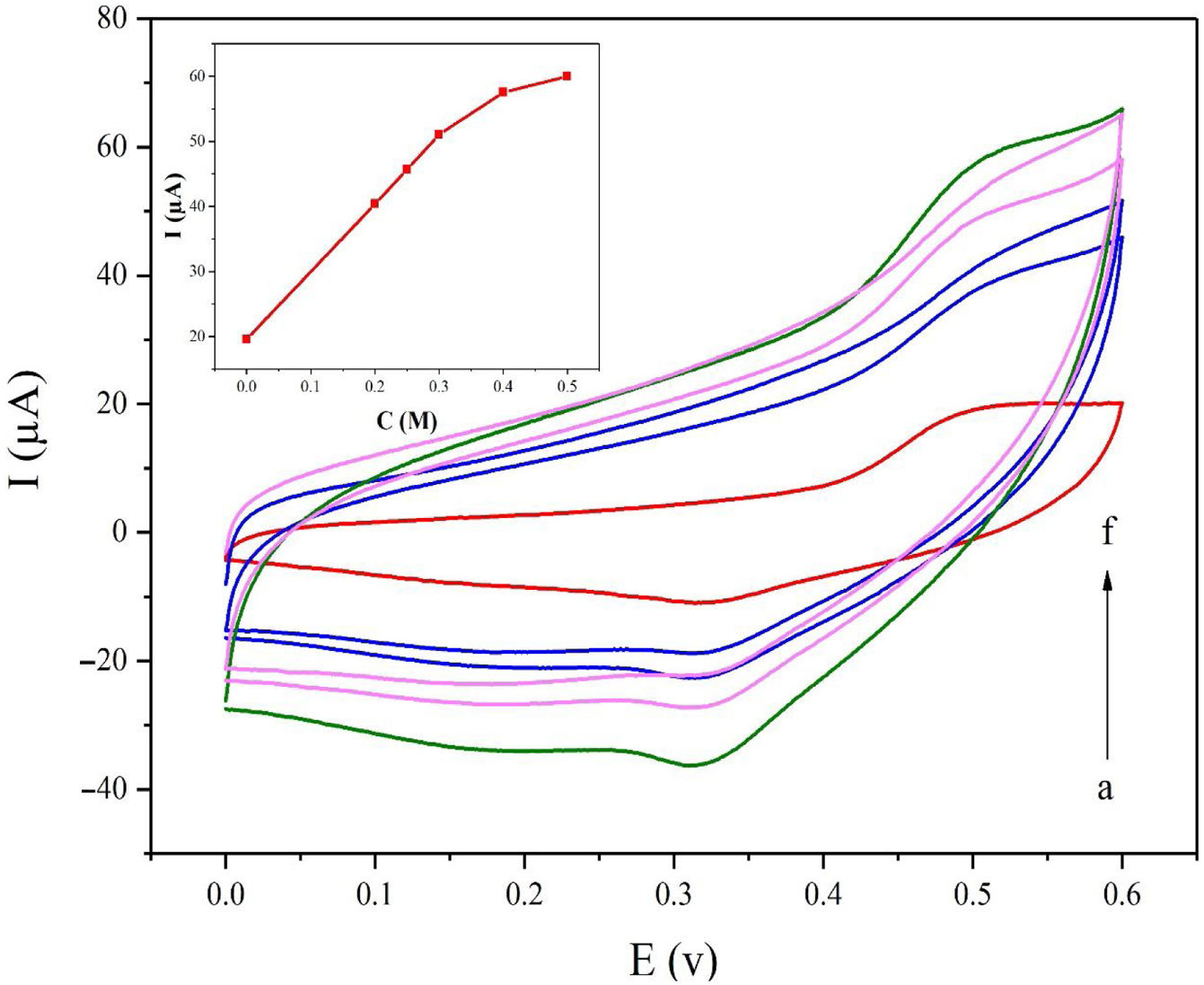
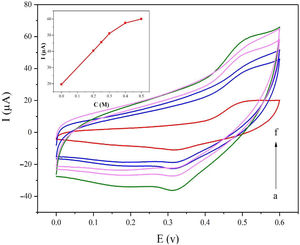
Fig. 5a displays the cyclic voltammetry behavior of the NiCo2O4@zeolite-4A/CPE toward the methanol concentrations in the range of 0–0.6V at the scan rate of 25mVs−1. The concentration of methanol changed from 0 to 0.5M in 0.1M NaOH solution. As evident in Fig. 5, the amount of electrocatalytic current increased after adding the concentration of methanol until 0.5M which confirmed the excellent electrocatalytic behavior of NiCo2O4@zeolite-4A/CPE.
Cyclic voltammograms of the NiCo2O4@zeolite-4A/CPE at the scan rate of 25mVs−1 with different concentrations of methanol: (a) 0.0, (b) 0.2, (c) 0.25, (d) 0.3, (e) 0.4, and (f) 0.5M, respectively in 0.1M NaOH. Inset shows plot of Ipa vs. ν1/2 for the oxidation of methanol on NiCo2O4@zeolite-4A/CPE.
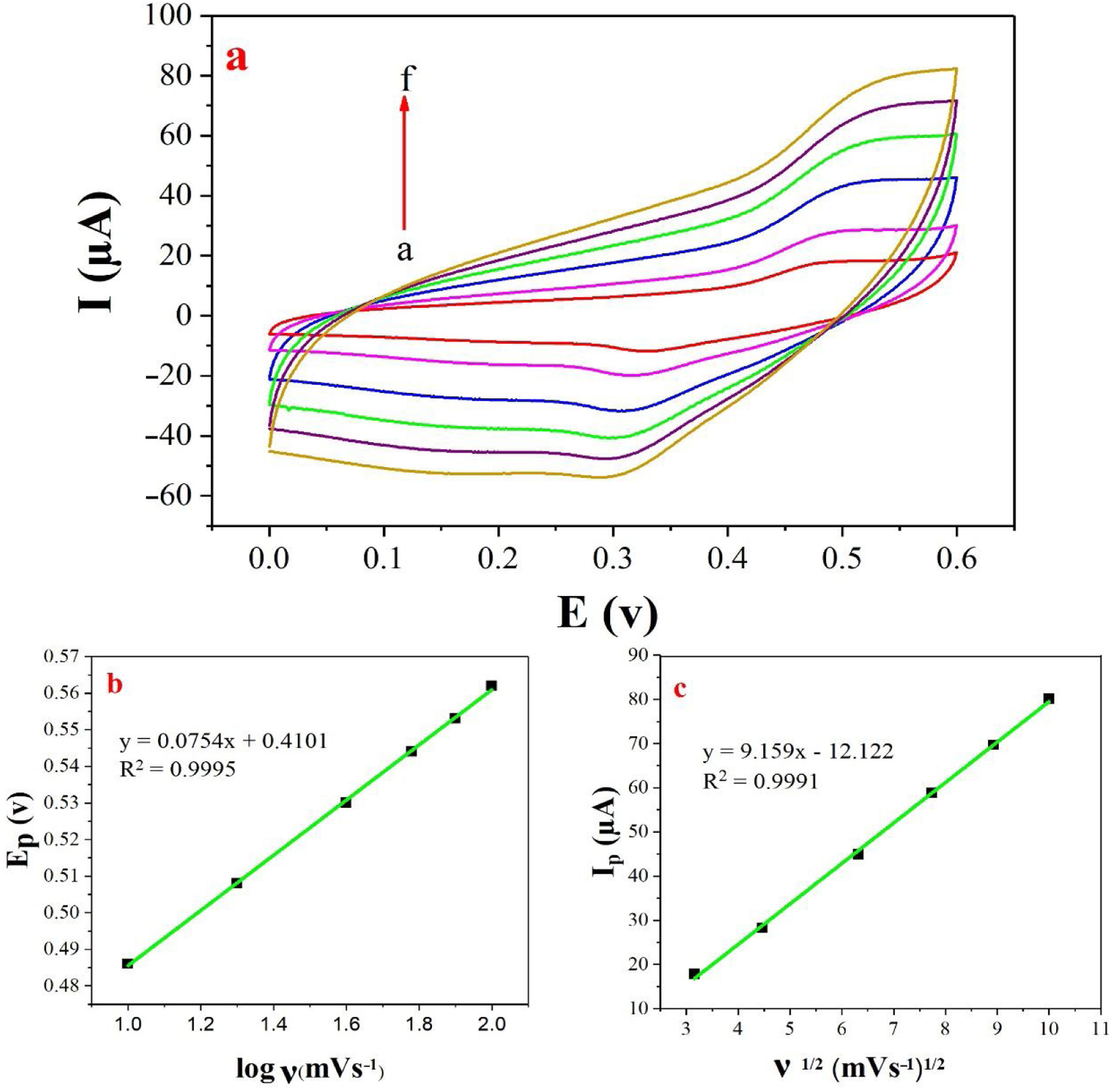
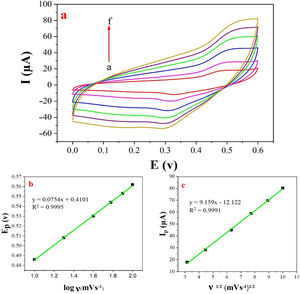
The examination of scan rate can shed more light on electrochemical mechanisms and kinetic characteristics of methanol. Fig. 6a depicts the cyclic voltammograms of methanol on NiCo2O4@zeolite-4A/CPE in 0.1M NaOH between 0 and 0.6V at various scan rates. As can be seen in Fig. 6c, the anodic oxidation current (Ip) increased linearly proportional to the square roots of scan rates from 10 to 100mVs−1 indicating a probable occurrence of a diffusion-controlled process in the oxidation of methanol on NiCo2O4@zeolite-4A/CPE. To obtain further information on the rate determining step, a Tafel plot was developed for the methanol on the surface of NiCo2O4@zeolite-4A/CPE using the data derived from the raising part of the logν–voltage curve (Fig. 6b). The slope of the Tafel plot is equal to n(1−α)F/2.3RT and α is 0.59.
(a) Cyclic voltammograms of the NiCo2O4@zeolite-4A/CPE in the presence of 0.1M methanol, at different scan rates of (a) 10, (b) 20, (c) 40, (d) 60, (e) 80 and (f) 100mVs−1 in 0.1M NaOH. (b) and (c) Plot of Ep vs. logν and Ipa vs. ν1/2 for the oxidation of methanol on NiCo2O4@zeolite-4A/CPE.
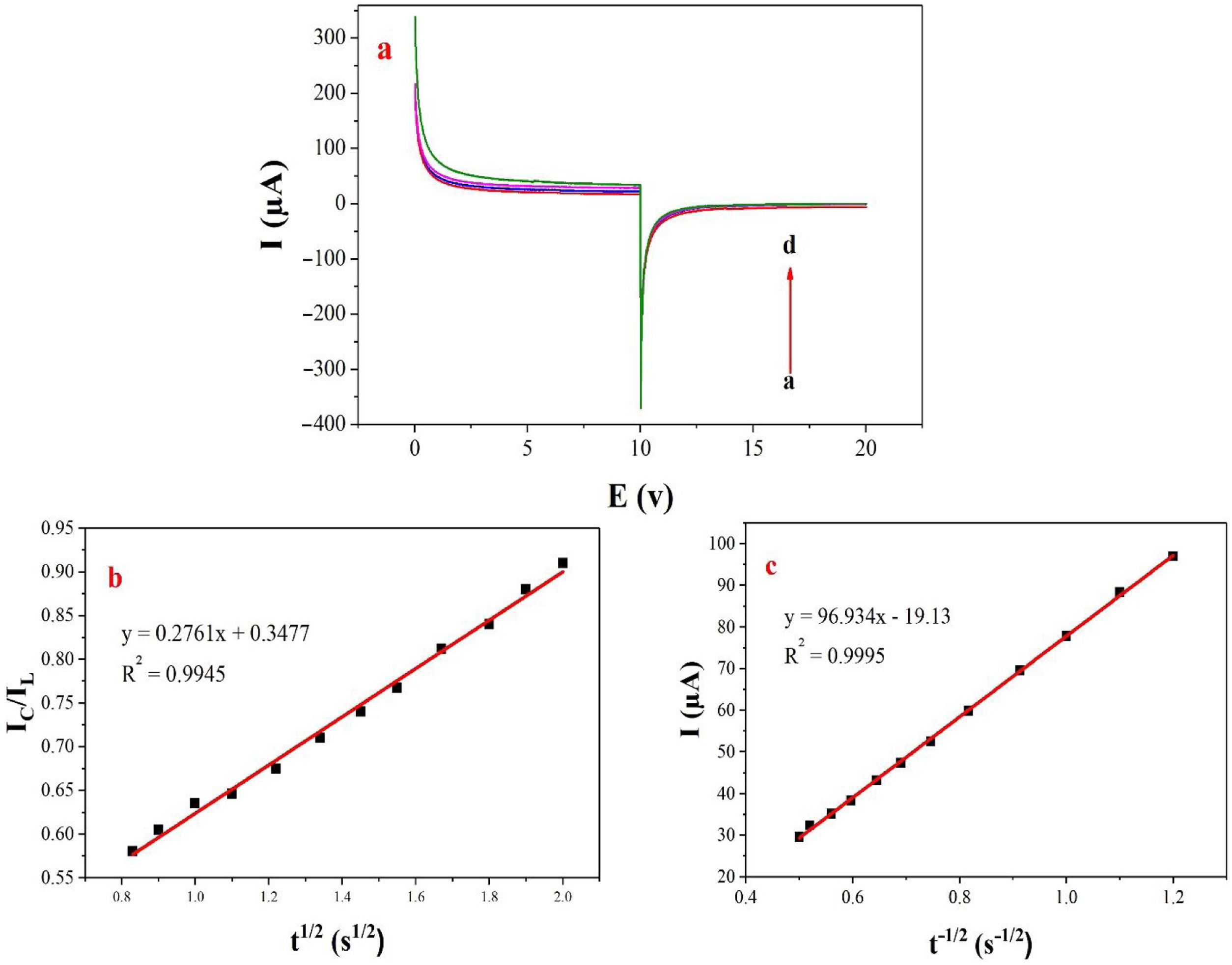
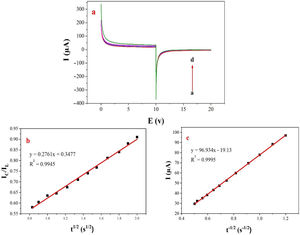
Double potential step chronoamperometry along with some other electrochemical methods were used to examine the catalytic rate constant of the methanol oxidation on the NiCo2O4@zeolite-4A/CPE. As depicted in Fig. 7, the current–time profiles recorded by setting the potential of modified electrode at 520mV (in first step) and 320mV (in second step) vs. Ag/AgCl (KCl 3M) were determined based on peak potential of redox process at various concentrations of methanol. The plot of I vs. t−1/2 showed a linear dependence in the absence of methanol (Fig. 7c). From exponential I–t amperograms (Fig. 7a) a diffusion controlled process occurs which can be modeled by a Cottrell equation [38]. From chronoamperograms, the rate constant for the chemical reaction between the methanol and redox sites of NiCo2O4@zeolite-4A/CPE modified electrode can be evaluated according to following equation [39]:
where IC is the catalytic current of NiCo2O4@zeolite-4A/CPE in the presence of methanol, IL is limiting current in the absence of methanol and γ=kC0t (C is the argument of error function). C0 in the argument refers to the concentration of methanol in bulk solution. When γ is more than 2, the error function is almost equal to 1 and the above equation can be summarized as follows:where k, C0 and t are the catalytic rate constant (cm3mol−1s−1), methanol concentration (molcm−3) and time passed (s), respectively. From the slope linear plot of IC/IL vs. t1/2 obtained from the plot of chronoamperogram of NiCo2O4@zeolite-4A/CPE, the value of k for a given concentration of substrate can be easily calculated. Fig. 7b displays one such plot, constructed from the chronoamperogram of the NiCo2O4@zeolite-4A/CPE in the absence and presence of 0.05M methanol. The mean value of k was found to be approximately 1.1×103cm3mol−1s−1. From Eq. (2), the findings confirmed that NiCo2O4@zeolite-4A/CPE represents a good catalytic activity toward the methanol oxidation.(a) Chronoamperograms obtained at the NiCo2O4@zeolite-4A/CPE in absence (a) and presence of (b) 0.05, (c) 0.1, and (d) 0.2M of methanol, first and second potential steps were 0.52 and 0.32V vs. Ag–AgCl, respectively, in 0.1M NaOH solution. (b) Dependence of current on t−1/2, derived from the data of chronoamperograms. (c) Dependence of IC/IL on t1/2 derived from the data of chronoamperogams of in the main panel.
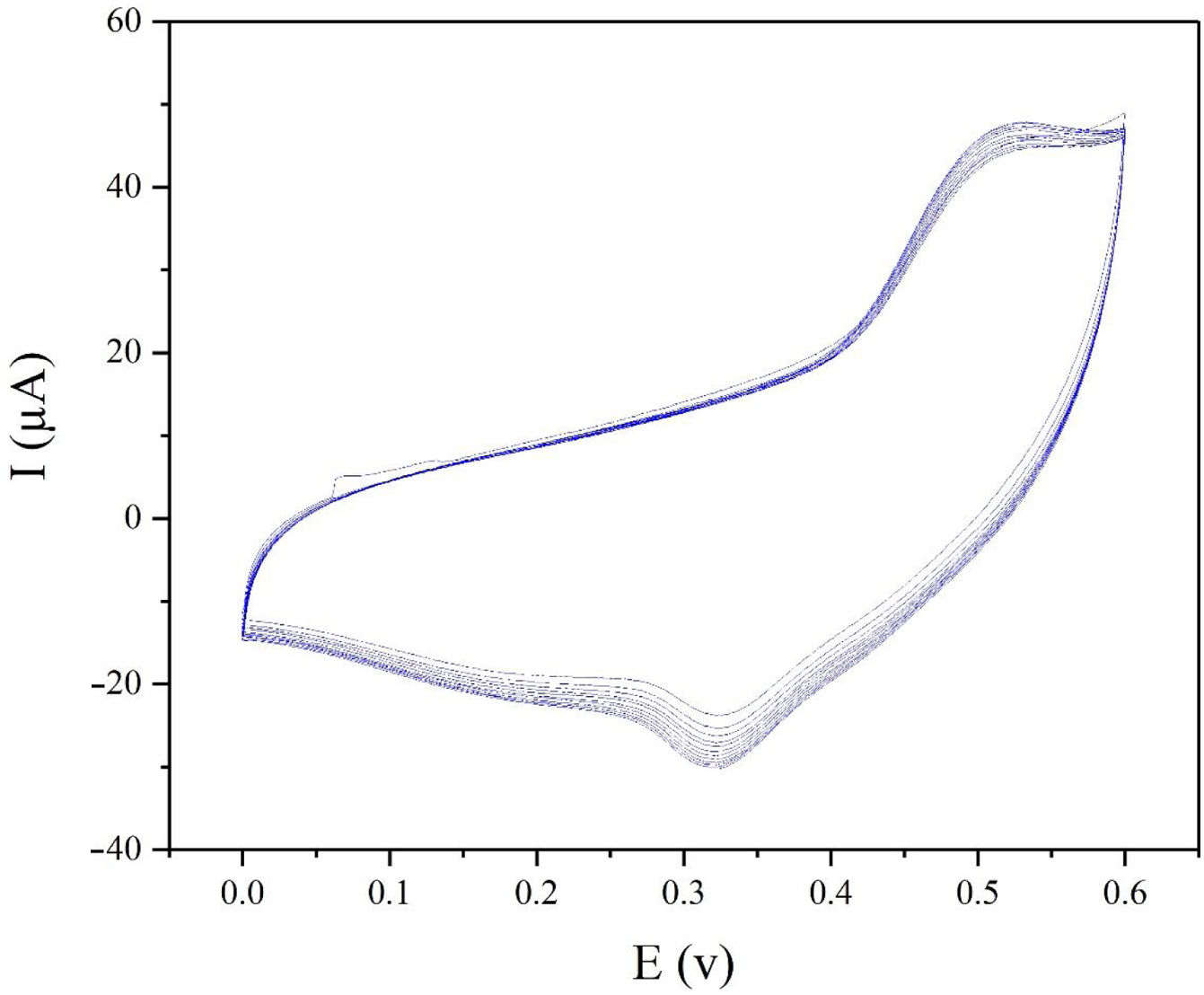
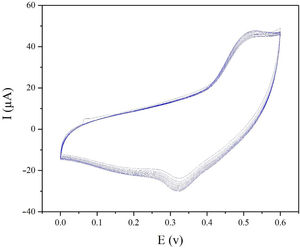
The electrochemical stability of the modified electrode is also a very important factor to be evaluated. In order to investigate the stability of modified electrode, cyclic voltammograms of modified electrode were recorded in presence of 0.1M methanol (Fig. 8). As seen in Fig. 8, the CV plots are quite stable and the current density at 0.55V (vs. Hg/HgO) exhibits 95% retention after 50 cycles at 25mVs−1.
ConclusionIn this study, spinel NiCo2O4 nanoparticles and zeolite-4A were prepared via a facile hydrothermal route. The findings of SEM and XRD revealed that NiCo2O4 nanoparticles of the flower-like 3D structures were assembled by a large number of nanosheets with an average thickness of 16nm while zeolite-4A had the spherical structure with particle diameter ranging from 46.2nm to 57nm. The behavior of modified electrode in electrocatalytic process of methanol oxidation was investigated using cyclic voltammetry and chronoamperometry. From cyclic voltammetry, the modified electrode delivered an anodic oxidation current about 60μA at 0.6V in 1M KOH and 0.5M methanol and displayed a good stability with 95% current retention after 50 cycles at 30mVs−1. The modified electrode could enhance the electro-catalytic oxidation and overcome the low kinetic of reaction of methanol due to its rich binary electro-active sites of Ni and Co species, high intrinsic electron conductivity and superior surface structures.