Mucorales comprises fungi commonly isolated as saprobes from soil, dung, stored grains and plants. Although these fungi have been studied in several countries, there are relatively a few reports of them in semi-arid areas. Therefore, the aims of the present study were to assess and compare the Mucorales communities in dung from different species and breeds of herbivores in the semi-arid of Pernambuco, based on the frequency of occurrence and species richness of these fungi. Samples of dung collected in the cities of Arcoverde, Serra Talhada and Sertânia were incubated in moist chambers in triplicate. Altogether, 24 taxa of Mucorales distributed in the genera Absidia, Circinella, Cunninghamella, Lichtheimia, Mucor, Pilobolus, Rhizopus and Syncephalastrum were identified. The highest species richness was found in sheep excrement. Mucor circinelloides f. griseo-cyanus was the most common taxon, followed by M. ramosissimus. The similarity of the composition of Mucorales species was greatest between the excrements of Guzerá and Sindi breeds (bovine). All mucoralean species isolated are being cited for the first time from animal dung found in Caatinga and a new species of Mucor was recorded. An identification key for species of Mucorales from dung in the semi-arid region of Brazil is provided.
Mucoromycotina Benny, one of the four subphyla proposed to accommodate certain species of traditional Zygomycota C. Moreau (phylum no longer accepted in the new classification due to its polyphyletic nature),1–3 covers most saprobes fungi characterized by the production of thick-walled spores of sexual origin, known as zygospores. The order Mucorales Fr., the largest in number of species within the Mucoromycotina, includes fungi forming coenocytic hyphae with septa either at the base of reproductive structures or irregularly distributed in older cultures. Species of this order produce asexual structures such as sporangiophores, sporangia, sporangiospores, sporangiola, merosporangia and merospores.4 These fungi are commonly isolated from soil, stored grains, plants, and animal excrement, especially that of herbivores and rodents.5
Coprophilous fungi are those that live in or are associated with fecal material, including soil contaminated with feces. These microorganisms are essential for the maintenance of ecosystems and are directly involved in the decomposition of fecal waste, participating in carbon, nitrogen and energy cycles.6,7 According to Dix and Webster,8 fungi may occur in excrement as obligate and optional coprophilous. The obligate coprophilous have spores that require the action of gastric enzymes to break their dormancy, while secondary or facultative coprophilous spores do not need to go through the digestive tract of animals to germinate.9 Among the Mucorales, only Pilobolus species are considered obligate coprophilous, although other taxa from this order are common in dung.6
The Brazilian semiarid region occupies an area of approximately 969,589.4km2, comprising eight northeastern states: Alagoas, Bahia, Ceará, Paraíba, Pernambuco, Piauí, Rio Grande do Norte, Sergipe and part of the state of Minas Gerais.10 Semiarid regions are formed, mostly by typical vegetation of Caatinga, an exclusively Brazilian domain that consists of heterogeneous phytophysiognomic systems characteristically xerophilic, with vegetation ranging from tree to shrub.11 The diversity of fungi in Semi-arid ecosystems is admittedly higher than previously thought.4,11 However, it is estimated that 41.1% of Caatinga regions have not yet been inventoried.11 Consequently, data related to the fungal community and the ecological relationship between microorganisms and substrates in these ecosystems are still scarce.
Concerning Mucorales in Brazil, 74 taxa, belonging to 20 genera, have been reported. Of these, 42 were isolated from herbivore dung, which is a highly favorable substrate for the growth of these fungi.12 Although a number of authors have documented the occurrence of coprophilous Mucorales in Brazil,13–15 there are no reports addressing the diversity and ecology of these fungi on dung in semi-arid areas. Therefore, the aims of the present study were to assess and compare the Mucorales communities in dung from different species and breeds of herbivores in the semi-arid region of Pernambuco, based on the frequency of occurrence and species richness of these fungi.
Materials and methodsStudy areasSamples of herbivore dung were collected at the Instituto Agronômico de Pernambuco (IPA) in Sertânia-PE (8°03′38″S, 37°13′32″W) [animals: caprine (Capra hircus L., breeds Anglo-Nubiano and Moxotó), and ovine (Ovis aries L., breeds Santa Inês and Morada Nova], and Arcoverde (8°25′00″S, 37°04′00″W) [animal: bovine (Bos taurus L., breeds Holandês, Girolando and Sindi]. Bos taurus L. dung samples (Breed Guzerá Leiteiro) were collected at the IPA in the city of Serra Talhada (7°95′67″S, 38°29′71″W).
Dung samplesThe samples were collected monthly, from September 2013 to April 2014, using sterilized spatulas. They were placed in cellophane autoclaved paper bags and kept in polystyrene boxes with ice until they arrived in the laboratory. All samples were collected in the morning, usually after the animals’ first meal of the day.
Food supplied to herbivoresThe composition of food supplied to herbivores from Arcoverde, Sertânia and Serra Talhada is provided in Table 1.
Isolation, purification and identificationDung samples from each animal were incubated in moist chambers at 28±2°C for 15 days under alternating light and dark periods, during which time mycelial growth was observed. Fragments of the grown colonies were transferred to malt extract agar (MEA) medium (Merck – EMB),16 supplemented with chloramphenicol (100mgL−1). After growth, the fungi were transferred to test tubes containing the same culture medium without antibiotic.
The specimens were identified by observing their macroscopic (color, appearance and diameter of colony) and microscopic (microstructures) characteristics as described by Hesseltine and Ellis,17 Schipper,18 Hesseltine and Fennel,19 Zheng and Chen,20 Hoffmann et al.,21 Zheng et al.22 and Santiago et al.23
Molecular analysisCulture grown in test tubes containing malt extract were incubated at 28°C for 6 days to obtain fungal biomass. The material was transferred to 2mL microtubes with screw caps. Subsequently, 0.5g acid-washed glass beads of two different diameters (150–212μm and 425–600μm, 1:1; Sigma, USA) were added to each tube. The material was crushed by stirring at high speed in a FastPrep homogenizer. The genomic DNA extraction procedure was conducted as described by Góes-Neto et al.24 The mycelium was washed with chloroform:isoamyl alcohol (24:1) and then homogenized in 2% cetyltrimethylammonium bromide buffer. The DNA was precipitated in isopropanol, washed with 70% ethanol, and resuspended in 50μL ultrapure water.
The primer pairs LR1/LSU2 were used for the amplification of the large subunit (LSU) of nuclear ribosomal DNA (rDNA).23,25 The polymerase chain reactions were carried out as described by Oliveira et al.26 The newly obtained sequence was deposited in the National Center for Biotechnology Information GenBank database (accession number KX133009).
Frequency of occurrence (FO)FO was calculated using the following equation: FO=Ji/k, where FO is the frequency of occurrence of the species i, Ji is the number of samples in which the species i has occurred and k is the total number of soil samples.27
Statistical analysisDifferences in the associations of Mucorales occurring among different herbivores dung were determined using Similarity Analysis (ANOSIM Primer v.6), in which the matrix of Bray–Curtis similarity was plotted as described by Clarke and Gorley.28 Differences between the number of species of Mucorales associated with different breeds of animals and the different months of the year were assessed using the Chi-Square (χ2) test (adjustment of compliance with expected equality proportions), according to the following formula: χ2=Σ[(o−e)2/i], where o is the observed frequency for each class and e is the expected frequency for that class.29 The significance level was set at 0.05 in the analysis.
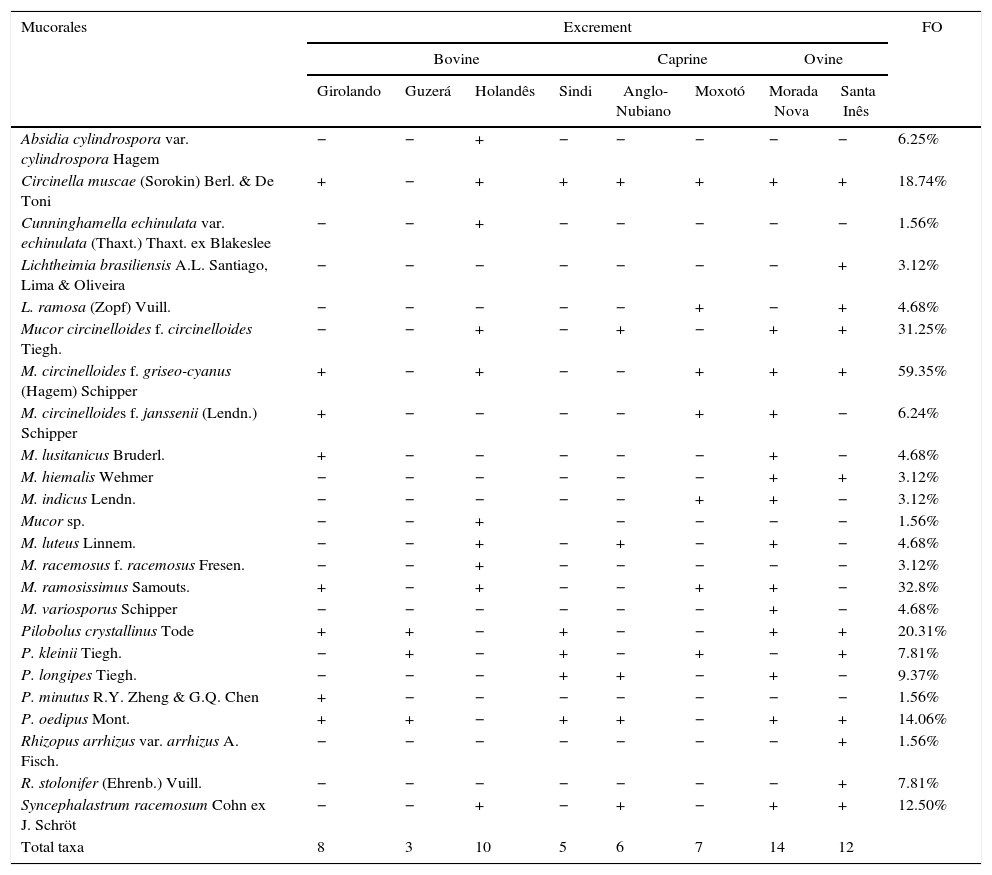
ResultsTwenty-four taxa within the genera Absidia, Circinella, Cunninghamella, Lichtheimia, Mucor, Pilobolus, Rhizopus and Syncephalastrum were identified in bovine, caprine and ovine dung found in the semi-arid region of Pernambuco. The highest number of taxa was observed in samples from Morada Nova (14 taxa) and Santa Inês (12) (ovine), followed by Holandês (10) and Girolando (8) (bovine). The lowest species richness was observed in samples from Guzerá (bovine) (3) (Table 2). Mucor exhibited the highest number of species (9), followed by Pilobolus (5) (Table 2).
Richness and frequency of occurrence (FO) of Mucorales in herbivore dung from Arcoverde, Sertânia and Serra Talhada, PE.
| Mucorales | Excrement | FO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bovine | Caprine | Ovine | |||||||
| Girolando | Guzerá | Holandês | Sindi | Anglo-Nubiano | Moxotó | Morada Nova | Santa Inês | ||
| Absidia cylindrospora var. cylindrospora Hagem | − | − | + | − | − | − | − | − | 6.25% |
| Circinella muscae (Sorokin) Berl. & De Toni | + | − | + | + | + | + | + | + | 18.74% |
| Cunninghamella echinulata var. echinulata (Thaxt.) Thaxt. ex Blakeslee | − | − | + | − | − | − | − | − | 1.56% |
| Lichtheimia brasiliensis A.L. Santiago, Lima & Oliveira | − | − | − | − | − | − | − | + | 3.12% |
| L. ramosa (Zopf) Vuill. | − | − | − | − | − | + | − | + | 4.68% |
| Mucor circinelloides f. circinelloides Tiegh. | − | − | + | − | + | − | + | + | 31.25% |
| M. circinelloides f. griseo-cyanus (Hagem) Schipper | + | − | + | − | − | + | + | + | 59.35% |
| M. circinelloides f. janssenii (Lendn.) Schipper | + | − | − | − | − | + | + | − | 6.24% |
| M. lusitanicus Bruderl. | + | − | − | − | − | − | + | − | 4.68% |
| M. hiemalis Wehmer | − | − | − | − | − | − | + | + | 3.12% |
| M. indicus Lendn. | − | − | − | − | − | + | + | − | 3.12% |
| Mucor sp. | − | − | + | − | − | − | − | 1.56% | |
| M. luteus Linnem. | − | − | + | − | + | − | + | − | 4.68% |
| M. racemosus f. racemosus Fresen. | − | − | + | − | − | − | − | − | 3.12% |
| M. ramosissimus Samouts. | + | − | + | − | − | + | + | − | 32.8% |
| M. variosporus Schipper | − | − | − | − | − | − | + | − | 4.68% |
| Pilobolus crystallinus Tode | + | + | − | + | − | − | + | + | 20.31% |
| P. kleinii Tiegh. | − | + | − | + | − | + | − | + | 7.81% |
| P. longipes Tiegh. | − | − | − | + | + | − | + | − | 9.37% |
| P. minutus R.Y. Zheng & G.Q. Chen | + | − | − | − | − | − | − | − | 1.56% |
| P. oedipus Mont. | + | + | − | + | + | − | + | + | 14.06% |
| Rhizopus arrhizus var. arrhizus A. Fisch. | − | − | − | − | − | − | − | + | 1.56% |
| R. stolonifer (Ehrenb.) Vuill. | − | − | − | − | − | − | − | + | 7.81% |
| Syncephalastrum racemosum Cohn ex J. Schröt | − | − | + | − | + | − | + | + | 12.50% |
| Total taxa | 8 | 3 | 10 | 5 | 6 | 7 | 14 | 12 | |
Mucor circinelloides f. griseo-cyanus occurred in high prevalence (FO=59.35%) in dung from the bovines, caprine and ovine examined, followed by M. ramosissimus (FO=32.8%) and M. circinelloides f. circinelloides (FO=31.25%). Cunninghamella echinulata var. echinulata, Mucor sp., P. minutus and R. arrhizus var. arrhizus were the least common (FO=1.56%) (Table 2).
The highest number of taxa was observed in dung samples from Morada Nova (14) and Santa Inês sheep breeds (12) (Table 2). However, according to the χ2 test, there were no significant differences in the species richness of Mucorales in dung from the different breeds of animals (p=0.0926), but differences in the number of species isolated across sampling per months of sampling were significant (p=0.0458).
Most Mucorales occurred in the excrement of bovine, caprine and ovine, with the exception of Absidia cylindrospora var. cylindrospora, C. echinulata var. echinulata, Mucor sp. and P. minutus, which were only found in bovine dung, while Lichtheimia brasiliensis, M. variosporus, R. arrhizus var. arrhizus and R. stolonifer were only observed in sheep feces.
Considering that L. ramosa is difficult to be separated from L. corymbifera (Cohn) Vuill. and L. ornata (A.K. Sarbhoy) Alastr.-Izq. & Walther based exclusively on morphological characters, the LSU rDNA region of our L. ramosa was sequenced. The Blastn analysis showed that our sequence (KX133009) was 99% similar to L. ramosa (CBS 582.65 – NG042518.1), confirming the identity of our specimen.
The species composition was most similar between Guzerá and Sindi (bovine) (75%), followed by Girolando (bovine) and Morada Nova (ovine) (63.63%) and Holandês (bovine) and Anglo-Nubiano (caprine) (60%) (Fig. 1).
Dendrogram of Bray–Curtis similarity for species composition of Mucorales from the herbivore dung of different animals. Species composition was more similar between Guzerá and Sindi dung, followed by Girolando and Morada Nova dung. Excrements of Anglo-Nubiano and Holandês were less similar.
| 1. | Obligatory coprophilous species; sporangiophores bearing subsporangial vesicles and trophocysts | 2 |
| 1. | Facultative coprophilous species; sporangiophores without subsporangial vesicles and trophocysts | 6 |
| 2. | Sporangiospores globose, subglobose or ovoid | 3 |
| 2. | Sporangiospores ellipsoid | 5 |
| 3. | Subsporangial vesicles ovoid; sporangiospores globose or subglobose, thin-walled | 4 |
| 3. | Subsporangial vesicles obovoid; sporangiospores thick-walled, spherical to broad ovoid | Pilobolus oedipus |
| 4. | Trophocysts short, 180–610×125–270μm; columellae cylindrical; sporangiospores subglobose, 7–11μm in diam. | P. minutus |
| 4. | Trophocysts long, 410–1800×240–420μm; columellae conical; sporangiospores subglobose, 10–18.5μm in diam. | P. longipes |
| 5. | Columellae nipple-like; sporangiospores ellipsoid, pale yellow or hyaline 7.5–12×4.5–6.5μm | P. crystallinus |
| 5. | Columellae conical-cylindrical; sporangiospores ellipsoid, intensely yellow 10.5–17.5×5.5–8μm | P. kleinii |
| 6. | Sporangiophores bearing sporangia | 8 |
| 6. | Sporangiophores bearing merosporangia or sporangiola | 7 |
| 7. | Merospores produced in merosporangia | Syncephalastrum racemosum |
| 7. | Pedicellate unispored sporangiola produced on a fertile vesicle | Cunninghamella echinulata var. echinulata |
| 8. | Sporangiophores simple or with erect or curved branches; sporangia without sterile spines | 9 |
| 8. | Sporangiophores with circinate branches; sporangia with a sterile spine | Circinella muscae |
| 9. | Sporangia unapophysate; giant cells not produced | 10 |
| 9. | Sporangia apophysate; giant cells present or absent | 20 |
| 10. | Sporangiospores regular in shape and size | 11 |
| 10. | Sporangiospores with varied shapes and sizes, globose, ovoid, cylindrical or fusiform | Mucor variosporus |
| 11. | Sporangiophores unbranched or slightly branched | 12 |
| 11. | Sporangiophores repeatedly branched | 13 |
| 12. | Columellae obovoid; sporangiospores ellipsoidal, plano-convex 2.5–10×2–7.5μm | M. hiemalis |
| 12. | Columellae globose; sporangiospores long-elliptical and fusiform 2.5–8.1 (–12.5)×1–5μm | M. luteus |
| 13. | Mesophilic species, not growing at 40°C | 14 |
| 13. | Thermotolerant, growing at 40°C | M. indicus |
| 14. | Columellae regular in shape; sporangiospores globose, subglobose or ellipsoid | 15 |
| 14. | Columellae of several shapes; sporangiospores subspherical to ellipsoid | Mucor sp. |
| 15. | Sporangiophores with swellings or not; sporangia with short lateral branches; columellae flattened, globose or obovoid; chlamydospores present or not, when present never abundant and never formed in reproductive structures | 16 |
| 15. | Sporangiophores without swellings and with long lateral branches; columellae subglobose, ovoid or ellipsoid; abundant chlamydospores produced in sporangiophores and in a few columellae | M. racemosus f. racemosus |
| 16. | Colonies high (up to 10mm); sporangiophores curved or not; swellings under sporangia absent; columellae obovoid, globose or subglobose | 17 |
| 16. | Colonies low (up to 2mm); sporangiophores curved or not; swellings often viewed under sporangia; columellae flattened | M. ramosissimus |
| 17. | Colonies initially white, turning gray in older cultures; sporangiophores up to 7 (–10)μm in diam.; sporangia black | 18 |
| 17. | Colonies initially yellow, turning brownish in older cultures; sporangiophores up to 14 (–17)μm in diam.; sporangia gray-brownish | 19 |
| 18. | Sporangiophores sympodially branched; dark sporangia, globose (15–) 20–72.5 (–75)μm; sporangiospores ellipsoid | M. circinelloides f. griseo-cyanus |
| 18. | Sporangiophores sympodially or monopodially branched (rarely); sporangia dark brown, globose to slightly subglobose, 20–90μm; sporangiospores globose to slightly subglobose | M. circinelloides f. janssenii |
| 19. | Sporangia dark brown and globose, 35–85μm; columellae obovoid; sporangiospores ellipsoid | M. circinelloides f. circinelloides |
| 19. | Sporangia brown and globose, 21.5–92.5μm; columellae globose; sporangiospores ellipsoid and sometimes irregularly shaped | M. lusitanicus |
| 20. | Sporangiophores arising from stolons, never opposed to rhizoids, spherical sporangia, piriform or subpiriform and apophysed; giant cells present | 21 |
| 20. | Sporangiophores arising from the aerial mycelium and/or stolons, opposed to rhizoids, sporangia apophysed, globose and subglobose; giant cells absent | 23 |
| 21. | Thermophilic species, growing at 40°C; subsporangial septum absent or rare; sporangiospores globose, subglobose or ellipsoid; giant cells present | 22 |
| 21. | Mesophilic species, not growing at 40°C; subsporangial septum present; sporangiospores cylindrical; giant cells absent | Absidia cylindrospora var. cylindrospora |
| 22. | Columellae globose, subglobose and spatulate, often exhibiting projections; giant cells present | Lichtheimia ramosa |
| 22. | Columellae subglobose and short hemispheric, without projections; giant cells absent | L. brasiliensis |
| 23. | Rhizoids present, well developed, abundant and rhizopodiform; sporangiophores reaching 3mm in length; columellae ovoid | Rhizopus stolonifer |
| 23. | Rhizoids, undeveloped, simple or rarely branched when present; sporangiophores reaching 1.7mm in length; columellae subglobose, hemiglobose, rarely oblong-ovoid | R. arrhizus var. arrhizus |
Altogether, 24 taxa of Mucorales were identified in the dung of herbivores from Arcoverde, Sertânia and Serra Talhada, PE, Brazil. All species identified in the present study are being cited for the first time in the dung of herbivores in Caatinga areas. However, most of the species identified herein have been reported by other authors in excrement from other domains in Brazil, indicating that they are not endemic to the Caatinga.14,15,30
With the exception of M. indicus and Mucor sp., all taxa of this genus isolated in the present study as well as Circinella muscae, P. crystallinus, P. kleinii, P. longipes, R. arrhizus var. arrhizus and S. racemosum were reported by Alves et al.14 and by Santiago et al.15 in the dung of herbivores in the Atlantic Forest in Recife, PE. Lichtheimia ramosa (as A. ramosa), C. muscae, M. hiemalis, P. crystallinus, P. kleinii and P. longipes were described by Trufem30 and Trufem and Viriato13 in the Atlantic Forest in São Paulo state. Fifteen species reported by the above mentioned authors were observed in the present study, although feces from different herbivores were analyzed in different studies, indicating that the majority of Mucorales do not exhibit specificity for a particular type of animal dung.31–33
A recent phylogenetic study on Mucorales that included critical species of this order34 strongly evidenced that M. ramosissimus is a synonym of M. circinelloides f. circinelloides or f. janssenii. In fact, both species are morphologically very similar to each other. However, considering that our isolates exhibited morphological characteristics, such as small colonies up to 2mm high, sporangiophores with a frequent swelling below the sporangia and columellae applanate, that were very similar to those described by Schipper35 for M. ramosissimus, and considering that M. ramosissimus is still a valid species, we prefer to maintain M. ramosissimus in our manuscript.
Some of the species isolated in the present study have also been reported in animal dung from other countries. Masunga36 isolated C. elegans and P. crystallinus from the dung of elephants in Africa, while Abdullah37 reported P. kleinii in donkey, sheep and camel dung collected in Iraq. Studies have shown that this group of fungi has a wide distribution and can adapt to different environmental conditions.31,38,39
Lichtheimia brasiliensis and M. indicus have been commonly isolated from soils, although they have not as yet been found in dung.23,40,41 However, these species are now reported for the first time in the dung of herbivores, thereby expanding our knowledge about the diversity of Mucorales. Mucor sp. exhibit morphological and genetic characteristics that differ from other taxa of the genus and will be described and published as a new species in a subsequent paper.
Among the isolated genera, Mucor was the most representative in number of taxa, with nine species and four forms, followed by Pilobolus, with five species. According to Krug et al.,6Mucor is characterized by facultative coprophilous species, while Pilobolus is characterized by obligatory coprophilous species. Since the species of the former genus are very common in soil samples,42 it is possible that the presence of these taxa in the inventoried excrement was due to the transition of propagules from the soil to the dung, as mentioned by Santiago et al.15 All species of Mucorales reported here are being cited for the first time as coprophilous in the Caatinga. In Brazil, the occurrence of Mucor species and/or Piloloblus in herbivore dung was reported by Batista and Pontual,43 Trufem,30 Trufem and Viriato,13 Richardson,31 Alves et al.14 and Santiago et al.15. The greatest representation of Mucor and Pilobolus in relation to the number of taxa in the dung analyzed was confirmed by Santiago et al.15
Our results indicated that Morada Nova dung (ovine) was the richest in terms of the number of taxa, followed by the dung of Santa Inês (ovine) and Holandês (bovine). However, according to the χ2 test, no significant difference (p=0.0926) was found for the species richness of Mucorales in the dung of the different animals analyzed. According to Ebersohn and Eicker38 and Santiago et al.,15 differences in the composition of a community can be correlated with abiotic and biotic factors that influence the mycobiota of the substrate. The fact that dung samples were kept in similar experimental conditions (temperature, light incidence, moisture) and were free of mycophagous insects could explain the similarities found for the species richness of Mucorales in dung. However, statistical analysis revealed significant differences in the number of taxa isolated from dung between the different sampling months (p=0.0458), indicating that seasonality influences the number of species, but not the composition of the Mucorales. The opposite result was observed by Santiago et al.,15 who reported that the composition of Mucorales species was affected by seasonal changes of the year, differing from the results observed for the number of taxa. Bell44 conducted a three-year study on the mycota in possum dung (Trichosurus Vulpecula Kerr) in New Zealand and attributed the higher incidence of certain fungal species to winter rains.
Concerning the frequency of occurrence of the isolates, M. circinelloides f. griseo-cyanus was the most common taxon (FO=59.35%), while C. echinulata var. echinulata, Mucor sp. and R. arrhizus var. arrhizus were the least common taxa (FO=1.56%). Mucor and Rhizopus include many species, coprophilous or not,6 while Cunninghamella species primarily colonize other substrates.42 Most species identified in the present study exhibited low frequencies of occurrence, not exceeding 32.8%. Similar behavior was observed by Richardson,31 Nyberg and Persson32 and Santiago et al.15 in studies of coprophilous fungi from herbivorous dung. Pilobolus kleinii has been commonly associated with a high frequency in herbivorous animal dung in other domains.31,32 However, this species was uncommon in the samples analyzed in the present study (7.81%). It is possible that this taxon is more sensitive to the water and temperature stress that are characteristic of the semi-arid region.
Considering the different breeds of herbivores, species composition was more similar between Guzerá and Sindi (bovines) (75%), which although not sharing the same environment, were exposed to a similar nutritional status and were the only animals kept on pasture (Table 1). According to Santiago et al.,15 nutritional differences between the animals can influence the mycota of dung. The fact that these animals spent most of their time grazing in the pasture may have contributed to the high similarity found. Curiously, high similarity was also observed between the Girolando (bovine) and Morada Nova (ovine) species, and between Anglo-Nubiano (caprine) and Holandês (bovine) species, despite the fact that they were grazed in different cities (although in the same domain) and have different diets. Studies have shown that although the food given to the animals is a limiting factor for the appearance of certain taxa, other factors, such as the geographic location, humidity and high temperatures may influence the composition of fungal communities in animal dung.31,38,45
The present work reports 24 taxa of Mucorales in herbivore dung from the semi-arid of Brazil and is a pioneer study for coprophilous Mucorales in the Caatinga. Considering the adverse conditions of temperature and humidity, typical of semi-arid regions, and comparing the results described herein with those of other authors, it is clear that the species richness of Mucorales in the inventoried areas is high. Abdullah37 reported only three species of Mucorales in donkey, sheep and camel dung collected in semi-arid regions of southern Iraq. Masunga et al.36 reported six species of this group in semi-arid areas of Botswana, Africa. Upon comparison of the results of the present study with previous studies of the diversity of coprophilous Mucorales in Atlantic Forest areas, Santiago et al.15 reported 39 taxa in this domain in Pernambuco, whereas Trufem and Viriato13 and Viriato and Trufem46 together only obtained 23 taxa in the same domain in São Paulo. Thus, it is relevant to continue surveying the fungal diversity in the Caatinga in order to demystify the erroneous idea that the richness of fungal species in this area is low.
Conflicts of interestThe authors declare no conflicts of interest.
The authors of the present study would like to express their gratitude to the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE – APQ 0842–2.12/14), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – 458391/2014–0), the Programa de Pesquisa em Biodiversidade do semiárido (MCT/CNPQ/PPBio – 457498/2012–9), and the two anonymous reviewers for critical reading the manuscript.