Colorectal surgery has advanced notably since the introduction of the mechanical suture and the minimally invasive approach. Robotic surgery began in order to satisfy the needs of the patient–doctor relationship, and migrated to the area of colorectal surgery. An initial report is presented on the experience of managing colorectal disease using robot-assisted surgery, as well as an analysis of the current role of this platform.
Material and methodsA retrospective study was conducted in order to review five patients with colorectal disease operated using a robot-assisted technique over one year in the initial phase of the learning curve. Gender, age, diagnosis and surgical indication, surgery performed, surgical time, conversion, bleeding, post-operative complications, and hospital stay, were analysed and described. A literature review was performed on the role of robotic assisted surgery in colorectal disease and cancer.
ResultsThe study included 5 patients, 3 men and 2 women, with a mean age of 62.2 years. Two of them were low anterior resections with colorectal primary anastomoses, one of them extended with a loop protection ileostomy, a Frykman–Goldberg procedure, and two left hemicolectomies with primary anastomoses. The mean operating time was 6h and robot-assisted 4h 20min. There were no conversions and the mean hospital stay was 5 days.
ConclusionThis technology is currently being used worldwide in different surgical centres because of its advantages that have been clinically demonstrated by various studies. We report the first colorectal surgical cases in Mexico, with promising results. There is enough evidence to support and recommend the use of this technology as a viable and safe option.
La cirugía colorrectal ha evolucionado desde el advenimiento de la sutura mecánica y el abordaje de mínima invasión. El inicio de la cirugía robótica obedece a la satisfacción de necesidades del binomio médico-paciente, migrando al área de la cirugía colorrectal. Reportamos la experiencia inicial en el abordaje de la patología colorrectal asistida por robot y analizamos el papel actual de esta plataforma.
Material y métodosEstudio retrospectivo de 5 pacientes con patología colorrectal intervenidos con la plataforma Da Vinci durante un año, en la fase inicial de la curva de aprendizaje. Se describe y analiza el género, la edad, el diagnóstico y la indicación quirúrgica, la cirugía realizada, el tiempo quirúrgico y el de consola, la conversión, el sangrado, las complicaciones postoperatorias, y los días de estancia hospitalaria. Se realiza un análisis de la bibliografía sobre el papel que desempeña la cirugía asistida por robot. Se usan porcentajes como medida de resumen para las variables cualitativas.
ResultadosCinco pacientes, 3 masculinos y 2 femeninos, con una edad promedio de 62.2 años; se realizan 2 resecciones anteriores bajas con anastomosis colorrectal, una de ellas extendida con ileostomía en asa de protección, un procedimiento Frykman-Goldberg, y 2 hemicolectomías izquierdas con anastomosis primaria. El tiempo quirúrgico promedio fue de 6h y el de consola de 4h y 20min. Ningún paciente requirió conversión, y la estancia hospitalaria promedio fue de 5 días.
ConclusiónA nivel mundial diversos centros quirúrgicos emplean la cirugía asistida por robot sobre la base de ventajas teóricas, las cuales se han confirmado en la práctica mediante diferentes estudios. Reportamos los primeros casos de cirugía colorrectal en México, con resultados prometedores. Hay suficiente evidencia para respaldar y recomendar su uso en nuestras instituciones como una opción factible y segura.
Colorectal surgery has evolved notably since the advent of the mechanical suture and subsequently with the minimally invasive approach. Robot-assisted surgery was developed to satisfy the doctor–patient relationship and migrated to the area of colorectal surgery. Greater evidence is required for its use in this area, which appears promising. Based on a revision of the literature we describe below the role of robot-assisted surgery in the field of minimally invasive colorectal surgery and our initial experience in a private third level care centre.
The beginnings of robot-assisted surgery marked a new era in the history of surgery, in minimally invasive procedures in particular. The National Aeronautics and Space Administration (NASA) developed the first remotely controlled robot in 1985 at the request of the United States Department of Defense with the objective of reducing the number of deaths in the Vietnam war.1,2
The initial model of the da Vinci system was launched in 1999. Since then it has undergone a series of improvements until the development of the better performing da Vinci Xi version. The da Vinci system consists of a console and a robot with 4 interactive robotic arms connected to the console and controlled by the surgeon (Fig. 1). One of the arms carries an endoscopic camera, which has 2 lenses that provide a 3 D image with high-definition stereoscopic vision. The other 3 arms are used to adjust the instruments (Fig. 2).
Another advantage of the da Vinci system is that it allows the surgeon to control the movements of the camera. The system also filters and deciphers the surgeon's hand movements into stable and precise movements, thus eliminating physiologic tremor.
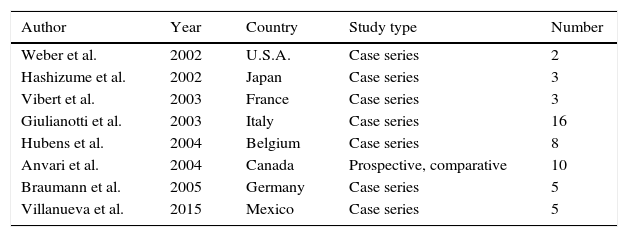
In 2000 the Federal Drug and Administration Agency (FDA) approved the use of the da Vinci robotic system for surgical treatment. This revolutionary approach reached the area of colorectal surgery in 2002, when the first right hemicolectomy was performed.3 Although more than a decade has passed since the first report of robot-assisted laparoscopic colorectal surgery, the role of this technique continues to develop.3 Like us, several authors worldwide have reported their experience and initial results over several years3–9 (Tables 1 and 2).
First robotic surgery reports in the area of colorectal surgery worldwide.
| Author | Year | Country | Study type | Number |
|---|---|---|---|---|
| Weber et al. | 2002 | U.S.A. | Case series | 2 |
| Hashizume et al. | 2002 | Japan | Case series | 3 |
| Vibert et al. | 2003 | France | Case series | 3 |
| Giulianotti et al. | 2003 | Italy | Case series | 16 |
| Hubens et al. | 2004 | Belgium | Case series | 8 |
| Anvari et al. | 2004 | Canada | Prospective, comparative | 10 |
| Braumann et al. | 2005 | Germany | Case series | 5 |
| Villanueva et al. | 2015 | Mexico | Case series | 5 |
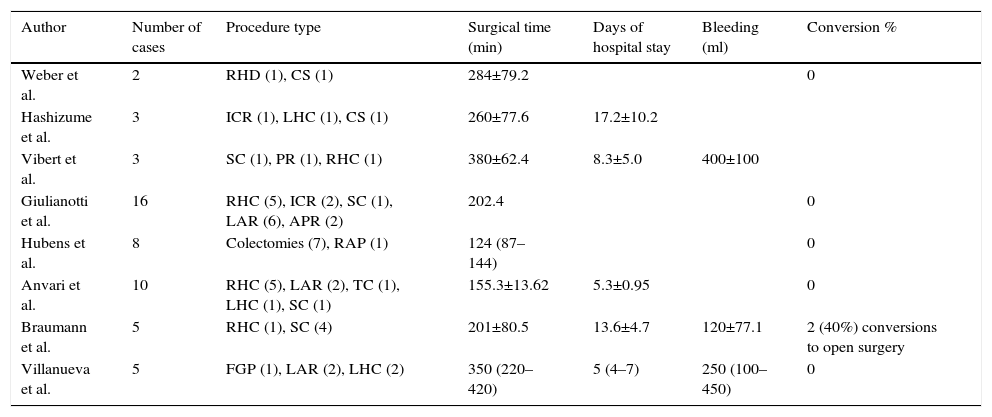
Results of the first reports on robotic surgery in the area of colorectal surgery worldwide.
| Author | Number of cases | Procedure type | Surgical time (min) | Days of hospital stay | Bleeding (ml) | Conversion % |
|---|---|---|---|---|---|---|
| Weber et al. | 2 | RHD (1), CS (1) | 284±79.2 | 0 | ||
| Hashizume et al. | 3 | ICR (1), LHC (1), CS (1) | 260±77.6 | 17.2±10.2 | ||
| Vibert et al. | 3 | SC (1), PR (1), RHC (1) | 380±62.4 | 8.3±5.0 | 400±100 | |
| Giulianotti et al. | 16 | RHC (5), ICR (2), SC (1), LAR (6), APR (2) | 202.4 | 0 | ||
| Hubens et al. | 8 | Colectomies (7), RAP (1) | 124 (87–144) | 0 | ||
| Anvari et al. | 10 | RHC (5), LAR (2), TC (1), LHC (1), SC (1) | 155.3±13.62 | 5.3±0.95 | 0 | |
| Braumann et al. | 5 | RHC (1), SC (4) | 201±80.5 | 13.6±4.7 | 120±77.1 | 2 (40%) conversions to open surgery |
| Villanueva et al. | 5 | FGP (1), LAR (2), LHC (2) | 350 (220–420) | 5 (4–7) | 250 (100–450) | 0 |
SC: sigmoid colostomy; TC: total colectomy; RHC: right hemicolectomy; LHC: left hemicolectomy; FGP: Frykman–Goldberg procedure; PR: proctectomy; LAR: low anterior resection; APR: abdominoperineal resection; HR: Hartmann's reversal; ICR: ileocaecal resection; S: sigmoidectomy.
At present it has shown advantages over laparoscopic surgery, the principal of these being: a stable platform, better vision (3D HD) and better access to reduced spaces. These advantages have made the robot an attractive tool for many specialties, specifically in procedures in the extraperitoneal rectum and pelvis.1,10–12
Material and methodsA retrospective study reviewing the clinical records of 5 patients with colorectal disease operated using the da Vinci platform over one year, in the initial phase of the learning curve. We describe gender, age, diagnosis and indication for surgery, surgery time, console time, conversion, bleeding, postoperative complications and days of hospital stay. We also review the literature on the role of robot-assisted surgery.
ResultsFive patients were operated, 3 males and 2 females, with a mean age of 62.2 years. The following were performed: (a) a left hemicolectomy with primary anastomosis due to complicated diverticular disease Hinchey stage IIB, with a surgery time of 5h, of which 3h and 30min corresponded to the console, and haemorrhage of 150cc with no complications; (b) an extended low anterior resection with colorectal anastomosis and protective loop ileostomy for mid rectal cancer after chemo and radiotherapy, with a duration of 6h, of which 4h 20min were on the console, and bleeding of 400cc; (c) Frykman–Goldberg procedure with hysterectomy due to complete rectal and uterine prolapse, with a duration of 7h, of which 3h 30min were for the rectopexy and sigmoidectomy – with 3h on the console – and 3h for the gynaecological procedure, with bleeding of 450cc; (d) left hemicolectomy plus colorectal anastomosis due to adenocarcinoma at the recto-sigmoid junction with a duration of 3h 40min – with 3h on the console – and bleeding of 100cc, and (e) low anterior resection with resection of terminal ileum and protective ileostomy due to an adenocarcinoma in the recto-sigmoid junction fistulised to the ileum, with a duration of 6h 20min, with 4h 20min on the console, bleeding of 150cc, and terminal ileostomy due to resection of the terminal ileum. The mean surgery time for the 5 patients was 5h 50min, with a time on the console of 3h and 50min, and mean bleeding of 250cc. None of the patients required conversion and the mean hospital stay was 5 days13 (Table 3).
General features of the patients.
| Patient number | Gender/age | Diagnosis | Surgery time (h) | Surgery performed | Console time (h) | Conversion | Complication | DHS | Bleeding (ml) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/58 | Complicated diverticular disease Hinchey IIB | 5:00 | LHC+MEECA | 3:30 | No | No | 7 | 150 |
| 2 | F/67 | Rectal and uterine prolapse | 7:00 | FGP+AHT | 3:00 | No | No | 5 | 450 |
| 3 | F/55 | Adenocarcinoma middle third of rectum | 6:00 | Extended RAB +MEECA+protective loop ileostomy | 4:20 | No | No | 5 | 400 |
| 4 | M/58 | Adenocarcinoma recto-sigmoid junction | 3:40 | LHC+MEECA | 3:00 | No | No | 4 | 100 |
| 5 | M/73 | Adenocarcinoma recto-sigmoid junction with colon-ileal fistula | 6:20 | LAR+MEECA+terminal ileum resection+protective ileostomy | 4:20 | No | No | 4 | 150 |
| Mean | 62.2 | 5:50 | 3:50 | 0 | 0 | 5 | 250 |
MEECA: mechanical end-to-end colorectal anastomosis; DHS: days of hospital stay; F: female; LHC: left hemicolectomy; AHT: abdominal hysterectomy; M: male; FGP: Frykman–Goldberg procedure; LAR: low anterior resection.
The field of colorectal surgery has evolved notably with the advent of the mechanical suture and minimally invasive procedures. Some minimally invasive colorectal surgery series show improvements compared to the open technique. The benefits demonstrated to date include a shorter hospital stay, rapid return to daily activities, better aesthetic result, lower hernia rate, less postoperative pain, and less risk or bleeding and ileus.14–16 However, the minimally invasive approach requires wide surgical experience, and a long learning curve with skilled assistants. There is also loss of in-depth vision, a reduced sense of touch and limited range of movement. This is why it has not been generally adopted.17
The study, Colon Cancer Laparoscopic or Open Resection (The COLOR Trial) concludes that laparoscopic colectomy is associated with less blood loss, prompt recovery of bowel function, less use of analgesics and short hospital stay. However, the operating time is longer, and there is almost 20% conversion to open surgery.18
A meta-analysis of 4 randomised controlled studies on patients with colon cancer allocated at random for open or laparoscopic colectomy concluded that laparoscopic surgery of the colon is oncologically safe and viable.19,20
Laparoscopic total mesorectal excision has a longer learning curve for rectal cancer,21 from approximately 50–70 cases.20–22 Bladder function appears not to be altered, however there is a high incidence of sexual dysfunction due to inadvertent injury to the autonomous nerves as a consequence of the mesorectal excision, lack of 3D vision, and lower mobility of the instruments, especially in the pelvis. This may explained by the increasing use of the laparoscopic approach.23
The viability and safety of laparoscopic rectal surgery is not clear, especially with the circumferential margin, according to the data from the study on Conventional versus Laparoscopic-Assisted Surgery in Colorectal Cancer (CLASICC).19 This study reports a greater number of conversions when using the laparoscopic approach, higher morbidity and mortality when there is conversion to open surgery, with no difference in local recurrence at 3 years.19,24,25 The reasons for conversion are voluminous tumours and technical complexity.15,25 This is where the robot is of great value since it removes some of the technical difficulties that present during dissection of rectal tumours treated laparoscopically, principally in accessing narrow areas such as the pelvis. After 10 years, the right colon showed a higher propensity for local recurrence compared with cancer of the left colon (p=0.019).19
Finally, the Korean study compared open surgery to laparoscopic surgery in mid or low rectal cancer after neoadjuvant chemotherapy. In this study there is a conversion rate of 1.2% compared to the 34% of the CLASICC study. The low conversion rate is attributed to the greater experience of the surgeons.26
In our cases we had no conversion to open or laparoscopic surgery. All the procedures were robot-assisted with the 3D, high definition vision and the mobility offered by the robotic instruments using Endowrist technology. All our cases had tumour-free surgical margins and appropriate lymph node harvest.
Evidence in robot-assisted colorectal surgeryInterest in robot-assisted colorectal surgery has been increasing throughout the world. The first publication on the subject was by Weber et al.3 in 2002. Since then there have been increasing numbers of publications.
There is growing interest in robot-assisted surgery, often without the back-up of appropriate data on its cost-effectiveness. This approach is considered to provide better results in terms of continence and sexual function due to the reduction in damage to the autonomic pelvic plexus.27,28 The study RObotic versus LAparoscopic Resection for Rectal Cancer (ROLARR) is currently underway. This is a multicentre, controlled prospective, randomised, non-blinded study of parallel groups of robot-assisted surgery versus laparoscopic surgery for curative treatment of rectal cancer. The study analyses the conversion rate to open surgery, pathological involvement of the circumferential margin, local recurrence at 3 years, disease-free period, overall survival, morbidity, mortality, quality of life and cost-effectiveness of both approaches. We are still awaiting the results.29
The learning curve in robot-assisted colorectal surgery is shorter and is achieved after 15–20 cases. It has been observed that the optimal skill level is achieved is after 25 cases.30–32
Robot-assisted surgery is superior, safe and feasible in narrow areas such as the pelvis, with three dimensional vision and zoom. It eliminates the tremor in open surgery and laparoscopy. It provides 7 degrees more motion than the wrist, which means less fatigue on the part of the surgeon after the procedure. It also has an ergonomic console enabling surgical procedures to be undertaken more easily. It has better pathological and functional results, with fewer complications and conversions, less postoperative pain and shorter hospital stay, shorter time until recovery of bowel function, first flatus and resumption of oral feeding. The areas where it is used have a lower rate of postoperative bleeding and ileus. The double console enables students to participate in the surgical procedure with simulators that can be connected to the console to practice the various skills prior to undertaking procedures. The disadvantages of robot-assisted surgery are the high cost of purchasing and maintaining the equipment and the learning curve required to perform procedures, which entails increased surgery times in the first cases.1,12,25,33–39
Patient selection is vitally important, particularly in the first stages of the learning curve. It is suggested that patients are selected that have tumours under 7cm, surgical risk ASA 1–3, BMI <30, aged under 75 years, with no previous pelvic or abdominal surgery, with T1/T2 tumours located in or just above the rectal peritoneal reflection, with no neoadjuvant chemo-or radiotherapy and able to tolerate the Trendelenburg position.30–32,40 Caution should be taken with complicated diverticular disease since this can be technically more demanding.
The mean surgery time in our patients was 6h. This was longer compared to open surgery, and not as significant compared to laparoscopic surgery. It is worth mentioning that surgery time is longer in robotic surgery, especially at the start, but better results are achieved in the surgical procedure. These times are part of the first part of the learning curve and therefore are expected to reduce significantly in subsequent cases. The mean hospital stay was 5 days. Mean bleeding was 250cc, which is less than open or laparoscopic surgery and taking into account the type of surgical procedures that were performed. We had no conversion to open or laparoscopic surgery. All the surgical procedures were completely performed with a robot thanks to the 3D high-definition vision and mobility offered by the robotic instruments with Endowrist technology. All the specimens we obtained had tumour-free margins and appropriate lymph node harvest. We had no complications in any of our cases. These results coincide with those of the abovementioned studies and are expected to improve since these are the first cases in the learning curve.
Anaesthetic considerations in robotic surgeryFrom an anaesthetic perspective, we are faced with various challenges in maintaining the patient under safe operative conditions. The main challenge is prolonged pneumoperitoneum, since it increases intra-abdominal pressure which creates mechanical effects compressing the large vessels, as occurs in the inferior vena cava, reducing preload. It also has a direct impact on renal blood flow, causing patients to present oliguria or even anuria during these procedures. Elevated carbonic acid levels produce an increase in arterial resistance with the indirect consequence of a fall in cardiac output. Reduced venous return together with each patient's particular risk factors increases the possibilities of deep venous thrombosis.
Patients who undergo these types of procedures are generally put into a forced Trendelenburg position. This limits pulmonary distensibility and because the abdominal mass also limits diaphragmatic movements, this encourages greater CO2 absorption and the appearance of atelectasis.
To lessen the adverse effects of a prolonged pneumoperitoneum, the main objective is to keep intra-abdominal pressure between 12 and 15mmHg. With regard to ventilation, we should maintain normocapnia or mild hypercapnia in order to prevent a state of acidaemia. As far as is possible, a peak pressure of 30cmH2O should not be exceeded in order to prevent barotrauma. The formation of alectasias, which might compromise appropriate oxygenation in the transoperative period and trigger adverse respiratory events postoperatively, might be reduced using fractions of inspired oxygen between 50% and 70%. The use of PEEP preceded by pulmonary recruitment manoevres is vitally important.
Antibiotic prophylaxis is recommended according to the type of procedure to be undertaken. Temperature monitoring and control with forced air warming is necessary because the exposure time is still very prolonged. Hypothermia alters the pharmacokinetics of our drugs, and has direct repercussions on the enzymatic processes, in addition to increasing postoperative O2 consumption. We also recommend that a deep neuromuscular block is maintained in the patient from the start to finish of the procedure, in order to complete the ventilatory manoeuvres, facilitate vision and prevent any movement in the approach to the surgical site. And last but not least, anti-thrombotic measures should be taken for all patients in line with their features and requirements.
ConclusionSeveral surgical centres worldwide use robot-assisted surgery based on the theoretical advantages, which have been confirmed in practice through various studies. Robot-assisted surgery is a costly health procedure, which merits meticulous evaluation. The ROLARR study is a pragmatic test that will provide a complete assessment of both surgical procedures for curative resection of recto-sigmoid cancer. We report the first cases of colorectal surgery in Mexico, with promising results. There is sufficient evidence to support and recommend the use of the robot in these cases and use this new technology in our institutions as a feasible and safe option.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Villanueva-Sáenz E, Ramírez-Ramírez MM, Zubieta-O’Farrill G, García-Hernández L. Experiencia inicial en cirugía colorrectal asistida por robot en México. Cir Cir. 2017;85:284–291.