Intracranial aneurysms are abnormal dilations of the cerebral arteries of unknown origin. However, some genes have been linked to their formation, as in the case of NOS3 gene which encodes the endothelial nitric oxide synthase responsible for producing nitric oxide. Several polymorphisms in this gene, in association with a variable number tandem repeat located in intron 4 from eNOS4 gene, can influence the formation of aneurysms. Therefore, the purpose of this study is to determine the genotype frequencies of eNOS3 and eNOS4 genes, and their relationship with intracranial aneurysms.
Material and methodsA prospective case–control study was performed on 79 cases with ruptured intracranial aneurysm and 93 healthy controls. DNA was obtained from all subjects for the study of the eNOS3 and eNOS4 genes by molecular techniques.
ResultsThe GG genotype of eNOS3 gene showed the largest number of patients (n=29) with a large aneurysm. While the intracranial aneurysms of medium size were found in a higher percentage (50%) in patients with genotype GT. In terms of patient outcomes, it was observed that those with genotype GG had the highest percentage (43.13%) recovery, compared to genotype GT (27.27%).
ConclusionsThe present study shows that there is a tendency of an association between genotypes of eNOS3 gene with the mean size of the aneurysm, as well as clinical sequelae of the disease in patients with intracranial aneurysms.
Los aneurismas intracraneales son dilataciones anormales de las arterias cerebrales. La etiología es desconocida, sin embargo algunos genes se han relacionado con su formación; como el caso del gen NOS3 que codifica a la sintetasa de óxido nítrico endotelial encargada de producir óxido nítrico. Varios polimorfismos en este gen en asociación con un número variable de repeticiones en tándem localizado en el intrón 4 del gen eNOS4 pueden incidir en la formación de aneurismas. Por eso, la finalidad de nuestro estudio es conocer las frecuencias genotípicas de los genes eNOS3 y eNOS4 y su relación con aneurismas intracraneales.
Material y métodosSe realizó un estudio prospectivo de casos y controles. Se estudiaron 79 casos con aneurisma intracraneal roto y 93 controles sanos; de todos se obtuvo DNA para el estudio de los genes eNOS3 y eNOS4.
ResultadosEl genotipo GG del gen eNOS3 mostró el mayor número de pacientes (n=29) con aneurisma intracraneal grande. Mientras que los de tamaño mediano se encontraron en mayor porcentaje (50%) en pacientes con genotipo GT. En cuanto a la evolución de los pacientes, se observó que aquellos con genotipo GG presentaron el mayor porcentaje (43.13%) de recuperación, en comparación con los de genotipo GT (27.27%).
ConclusionesNuestro estudio demuestra que en los casos con aneurismas intracraneales existe una tendencia de asociación entre los genotipos del gen eNOS3 con el promedio del tamaño del aneurisma, así como con las secuelas clínicas de la enfermedad.
Intracranial aneurysms form part of the group of cerebrovascular disorders, and are defined as abnormal dilations of the cerebral arteries, generally located in the bifurcation of the arteries which form the Circle of Willis. The incidence in the general population is 2–8%.1–3
Depending on how they present, intracranial aneurysms can be classified as unruptured, which in most cases are incidental findings on cerebral angiography for a different diagnostic purpose, or at autopsy1–3; and ruptured, which usually cause a subarachnoid haemorrhage with manifestations which include: intense headache, nausea, vomiting, focal neurological deficit, and loss of consciousness, amongst others.4 The mortality rate for ruptured intracranial aneurysms is high (50%), while 25% will suffer permanent disability, paralysis, loss of speech, vision and motor coordination.1
To date, the aetiology of intracranial aneurysms is unknown. However, various environmental factors are associated, such as smoking, systemic arterial hypertension and alcoholism.3,4 Similarly, genetic factors are associated with a predisposition; although there is no defined Mendelian inheritance pattern for this disease, epidemiological studies demonstrate higher autosomal transmission; the first degree relatives of a person with an intracranial aneurysm have a 3–6 times greater risk than that of the general population.3,4 Genetic studies also show potential sites of susceptible genes, or loci, for the formation of intracranial aneurysms; however, in many populations these results have been contradictory, as in the case of the gene which codes the nitric oxide synthase enzyme (NOS), which is responsible for producing nitric oxide.3,5–9
Endothelial nitric oxide synthase (eNOS) is one of the 3 isoforms of NOS, found in the body constitutively10; the significance of the production of nitric oxide synthase by eNOS is that it is the most powerful endogenous vasodilator known.10,11 eNOS is coded by the NOS3 gene and is expressed principally in the endothelial cells. Various polymorphisms in the gene have been described in association with a variable number tandem repeat (VNTR), located in intron 4 in the eNOS4 gene, which might affect the expression of the enzyme, and alter the circulating levels of nitric oxide. As an example, it is known that eNOS participates in both haemodynamic and structural functions, and its absence or reduction might cause the vascular wall to weaken or rupture, conditioning susceptibility to the formation of coronary and cerebral aneurysms.7,10,12–16
In 2008, Özüm et al.14 reported that the TT genotype of the eNOS3 gene G894T polymorphism is significantly frequent in patients with ruptured intracranial aneurysm compared with the healthy population, and is associated with a risk of rupture (p<0.05). However, other authors did not find this same association, probably due to the ethnic differences in the genotype distribution.14
Studies oriented towards gaining a deeper knowledge of human variability help us to understand the genetic structure of our population, compare it with other populations, and to propose new techniques for diagnosis, and even specific treatments aimed at stabilising the patient.
Over the past decade both Mexico, and clinical genetics have undergone major development, which coincides in part with the changes in the national health profile. Thus, the purpose of our work is to gain an understanding of the genotype frequencies of the polymorphisms of the eNOS3 and eNOS4 genes, and their relationship with ruptured intracranial aneurysms in the Mexican population.
We know that a delay in the diagnosis of an intracranial aneurysm increases the risk of rebleed, especially if more than 6h have passed since the initial haemorrhagic event. Early intervention, known as “haemorrhage prevention”, is the best strategy to reduce the incidence and prevalence of this disorder.
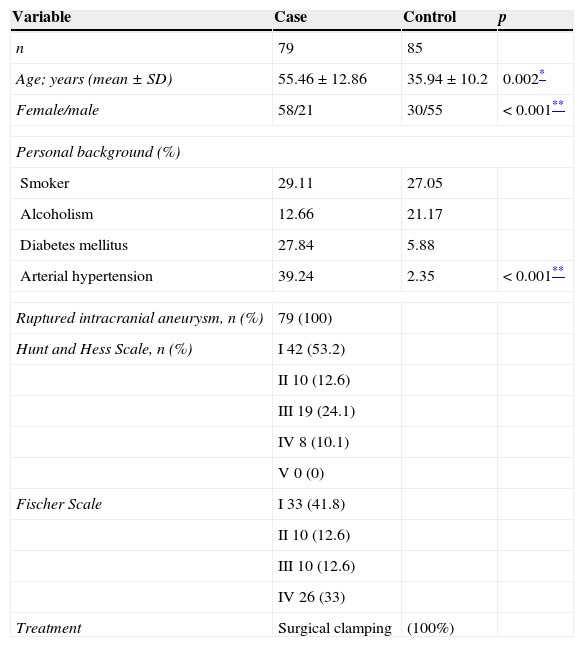
Material and methodsA prospective case–control study was undertaken, evaluated and approved by the Research and Ethics Committees of the Centro Médico Nacional “20 de Noviembre” (CMN), Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE); all of the participants signed their informed consent. A total of 172 patients, of whom 79 had been diagnosed with ruptured intracranial aneurysm, confirmed by panangiography with digital subtraction; their general and clinical features are described in Table 1. Ninety-three individuals were included as control from the same geographical area, selected at random from the Blood Bank; their clinical history, blood count and blood chemistry were taken, and a physical examination performed. They were classed as healthy if they met the requirements. A 5ml venous blood sample was taken from all of them in Vacutainer blood collection tubes with EDTA anticoagulant, for DNA extraction by salting out17 and stored at −20°C, for subsequent use.
General features in case and control groups.
| Variable | Case | Control | p |
|---|---|---|---|
| n | 79 | 85 | |
| Age; years (mean±SD) | 55.46±12.86 | 35.94±10.2 | 0.002* |
| Female/male | 58/21 | 30/55 | <0.001** |
| Personal background (%) | |||
| Smoker | 29.11 | 27.05 | |
| Alcoholism | 12.66 | 21.17 | |
| Diabetes mellitus | 27.84 | 5.88 | |
| Arterial hypertension | 39.24 | 2.35 | <0.001** |
| Ruptured intracranial aneurysm, n (%) | 79 (100) | ||
| Hunt and Hess Scale, n (%) | I 42 (53.2) | ||
| II 10 (12.6) | |||
| III 19 (24.1) | |||
| IV 8 (10.1) | |||
| V 0 (0) | |||
| Fischer Scale | I 33 (41.8) | ||
| II 10 (12.6) | |||
| III 10 (12.6) | |||
| IV 26 (33) | |||
| Treatment | Surgical clamping | (100%) | |
SD, standard deviation.
G894T polymorphism of the eNOS3 gene was analysed by polymerase chain reaction–restriction fragment length polymorphism. The DNA was used as a template at a concentration of 5μg/ml per sample. Subsequently it was amplified using the following oligonucleotides: 5′ cat gag gct cag ccc cagaac 3′ and 5′ agt caa tcc ctt tgg tgc tca c 3′. The polymerase chain reaction (PCR) test used the following: 1.25U of TaqDNA polymerase (Invitrogen®), 1× buffer reaction, MgCl2 3mM, dNTP's 0.2mM and oligonucleotides 10μM, for a reaction total of 25μl. The PCR conditions were as follows: an initial cycle of denaturation at 95°C for 7min, followed by 35 amplification cycles (denaturation at 94°C for 30s, annealing at 57°C for 30s, extension at 72°C for 30s) and a final extension step at 72°C for 7min. A 7μl aliquot was used to detect the final PCR product from 206 base pairs, and subjected to horizontal electrophoresis, using 2% agarose stained with ethidium bromide as a medium. The rest of the amplicon was directed with the restriction enzyme Mbol at 37°C for 4h; the fragments were analysed by electrophoresis in 2% agarose gels stained with ethidium bromide.
Polymerase chain reactionVNTR polymorphism of the eNOS4 a/b gene was detected by PCR using genomic DNA (5μg/μL) as a template amplified with the following oligonucleotides: 5′ agg ccc tat ggt agt gcc ttt 3′ and 5′ tct ctt agt gct gtg gtc at 3′. The general conditions for the PCR were: 1× buffer reaction, 2.5mM of MgCl2, 0.4μM of dNTP's, 0.5μM of oligonucleotides and 1.25U of TaqDNA polymerase for a final volume of 25μL. The PCR was started with denaturation for 4min at 95°C, followed by 35 amplification cycles (denaturation at 94°C for 30s, annealing at 58°C for 30s, extension at 72°C for 30s) and a final extension step at 72°C for 7min. VNTR polymorphism of the eNOS4 a/b gene was analysed by electrophoresis in 2% agarose gels stained with ethidium bromide.
Statistical analysisThe information was gathered using Excel software (Microsoft Corporation, USA). Graph Pad Prism V-4.00 (GraphPad Software, San Diego, CA, USA) was used for the statistical calculations. The distribution of genotypes was determined in each polymorphism; the χ2 test was used to compare the genotype frequencies between cases and controls; the Kruskal–Wallis and ANOVA tests were used to study the association of genotypes with categorical data. In all cases the p value <0.05 was used as statistically significant.
FindingsThe general feature of the cases with intracranial aneurysms and the controls included in our study (Table 1) show that the female:male ratio is higher in the case group compared to the control group (p<0.001). Furthermore, with regard to personal pathological background, the case group presented a higher percentage of patients with diabetes mellitus and systemic arterial hypertension, the latter being known to significantly increase the risk of a ruptured aneurysm.
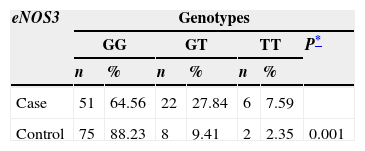
Similarly, the distribution of the genotypes of the eNOS3 and eNOS4 genes was studied in both the case group and the control group (Table 2). In the eNOS3 gene the GG genotype was observed to predominate in both the case group, and the control group (64.56% and 88.23%; respectively); while the GT genotype presented more frequently in the case group (p=0.0012). Furthermore, the a/b and b/b genotypes of gene eNOS4 showed similar proportions in each study group, the b/b genotype presenting in over 90% in both the case and the control group.
Distribution of genotypes for eNOS3 and eNOS4 in case and control groups.
| eNOS3 | Genotypes | ||||||
|---|---|---|---|---|---|---|---|
| GG | GT | TT | P* | ||||
| n | % | n | % | n | % | ||
| Case | 51 | 64.56 | 22 | 27.84 | 6 | 7.59 | |
| Control | 75 | 88.23 | 8 | 9.41 | 2 | 2.35 | 0.001 |
| eNOS4 | a/b | b/b | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Case | 5 | 6.33 | 74 | 93.67 | |||
| Control | 1 | 1.18 | 84 | 98.82 | 0.079 | ||
GG: guanine, guanine; GT: guanine, thymine; TT: thymine, thymine.
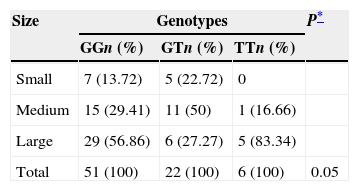
Due to the distribution of the genotypes of gene eNOS3, it showed greater heterogeneity in the case group, a correlation was made between genotypes and frequency of presentation of intracranial aneurysm according to its size; classified as small (<5mm), medium (6–9mm) and large (>10mm) (Table 3). As can be observed, the GG genotype showed the greater amount of patients (n=29) with a large aneurysm; however the TT genotype had the greater percentage (83.34%) of patients with this type of aneurysm. Whereas medium-sized intracranial aneurysms were the highest percentage (50%) in patients with GT genotype. This heterogeneity in the size of aneurysms between the genotypes produced a significant tendency (p=0.05) between them.
Distribution of the size of the aneurysms according to the genotypes of the eNOS3 gene in cases with intracranial aneurysms.
| Size | Genotypes | P* | ||
|---|---|---|---|---|
| GGn (%) | GTn (%) | TTn (%) | ||
| Small | 7 (13.72) | 5 (22.72) | 0 | |
| Medium | 15 (29.41) | 11 (50) | 1 (16.66) | |
| Large | 29 (56.86) | 6 (27.27) | 5 (83.34) | |
| Total | 51 (100) | 22 (100) | 6 (100) | 0.05 |
GG: guanine, guanine; GT: guanine, thymine; TT: thymine, thymine.
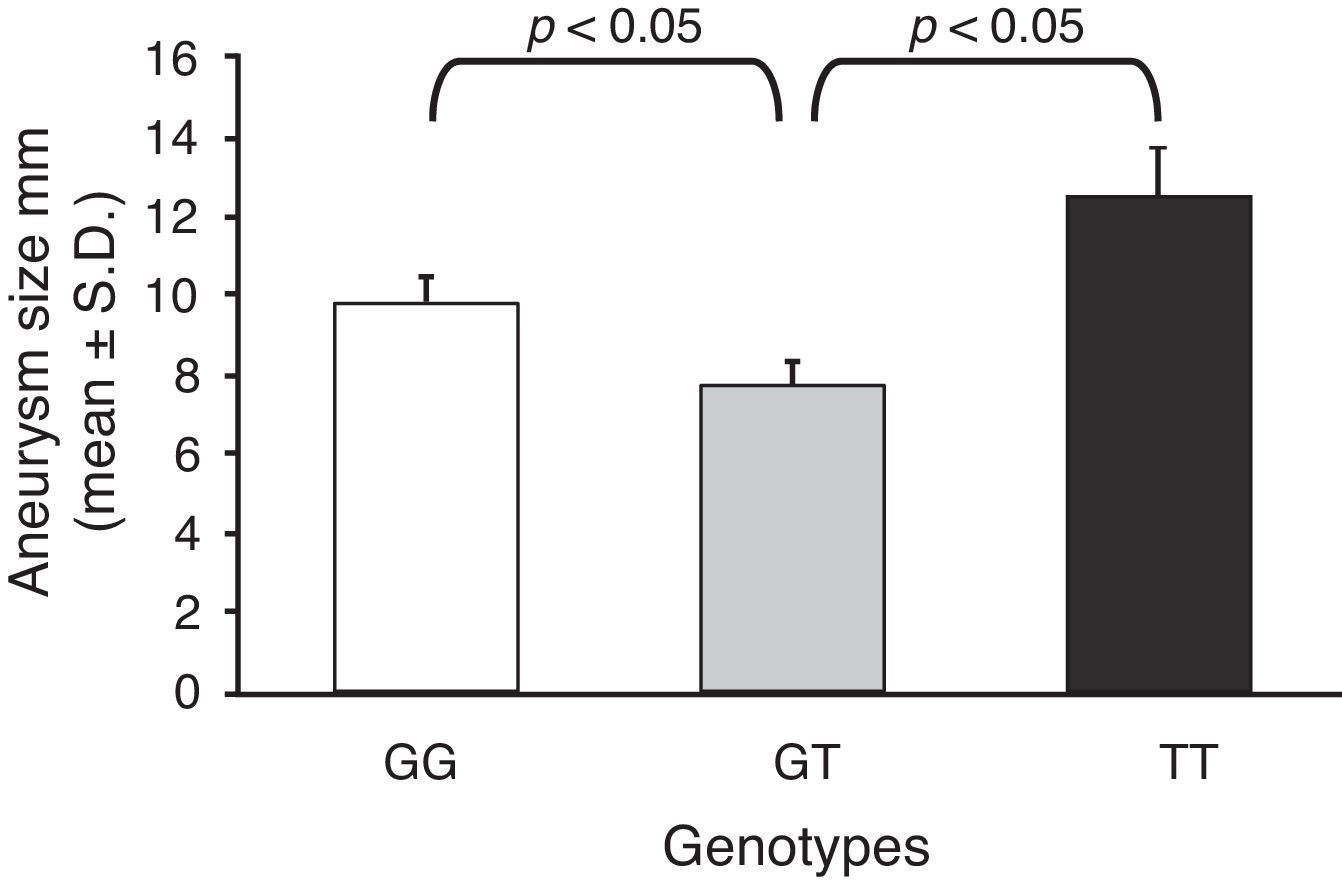
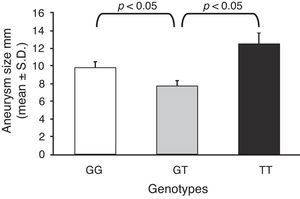
Furthermore, taking into account the size in millimetres of each aneurysm for each genotype, the mean size of the aneurysm was calculated, and they were compared with one another (Fig. 1). As can be seen, the GT genotype presents the lowest mean of the 3 genotypes (7.73±0.76 vs 9.81±0.54; and 7.73±0.76 vs 12.5±1.12; for GT vs GG and GT vs TT; respectively).
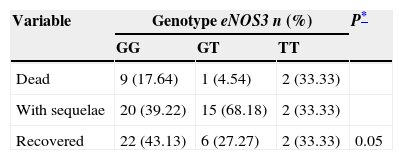
Taking the patients’ outcomes during their hospitalisation into account (death, sequelae or recovery), it was observed that there was greater heterogeneity of results in the GG and GT genotypes (Table 4). Thus it was noted that the patients with GG genotype presented the highest percentage (43.13%) of recovery, compared to the patients with GT genotype (27.27%). By contrast, the patients with GT genotype presented the highest percentage (68.18%) of sequelae and the lowest percentage of deaths (4.54%).
Clinical course of the cases with intracranial aneurysm divided by genotype.
| Variable | Genotype eNOS3 n (%) | P* | ||
|---|---|---|---|---|
| GG | GT | TT | ||
| Dead | 9 (17.64) | 1 (4.54) | 2 (33.33) | |
| With sequelae | 20 (39.22) | 15 (68.18) | 2 (33.33) | |
| Recovered | 22 (43.13) | 6 (27.27) | 2 (33.33) | 0.05 |
GG: guanine, guanine; GT: guanine, thymine; TT: thymine, thymine.
In recent years, the Mexican Health Secretary has reported cardiovascular and cerebrovascular disease as principal causes of death in the general adult population, occupying the second and third place respectively.18 Currently, there are no serum and/or genetic markers in Mexico to enable us to identify these diseases in advance. On a global level, various groups have studied genetic markers with clinical relevance for intracranial aneurysms.6,19–21 This research indicates that the presence of G894T polymorphisms of the eNOS3 gene, and VNTR a/b polymorphisms of the eNOS4 gene are associated with the development of intracranial aneurysms,5,14 and with other diseases of vascular-endothelial tissue.16,22
The presence of these 2 polymorphisms was studied in the Caucasian population of the USA, observing a greater frequency of the GT genotype of the G894T polymorphism, followed by the GG and TT genotypes, whereas, in the black population of Colombia the GG genotype was more frequent, followed by the GT and TT genotypes.12,23 The latter findings were also found in our population.
In the case of the VNTR a/b polymorphism, the Americans and the Colombians had a greater frequency of the genotype b/b, followed by the genotypes b/a and a/a.12,23
In our study, the b/b genotype also presented with greater frequency; however, we had no case with a/a genotype. This discrepancy in the distribution of the genotypes could be attributed to ethnic differences which should be taken into account for our population, because ancestrally, we have a different proportion of American Indian, European and African ethnicities.24,25
Song et al.5 recently described the relationship between ruptured aneurysms and genotypes; they found that in patients with intracranial aneurysms, the heterozygous genotype was associated with the size of the aneurysm compared with wild-type homozygotes and mutant homozygotes. Our data show that the patients with medium-sized aneurysms and GT heterozygous genotype had the highest percentage of sequela due to rupture (68.18%) compared to GG and TT homozygotes, therefore the data put forward by Song et al.5 and our findings indicate that genotypes can be used to predict the risk of the disorder.
There are currently few publications in Mexico which link polymorphisms of the eNOS gene with the size, location, and consequent rupture of aneurysms, therefore further studies are necessary to complement, confirm or discount our findings to provide greater evidence of the usefulness of these polymorphisms in our population.
ConclusionsOur study demonstrated that ruptured intracranial aneurysm occur more frequently in women with a mean age of 55, and a history of system arterial hypertension. It was also found that there is a significant tendency between the G894T polymorphisms of the eNOS3 gene, and the VNTR polymorphisms of the eNOS4 gene with the size and clinical course of intracranial aneurysms, and therefore the eNOS gene appears to play an important role in the pathogenesis of this disease.
This information could be used by neurological doctors and neurosurgeons, to generally anticipate the course of the disorder, and help to optimise the management, monitoring, treatment and recovery of patients with intracranial aneurysms.
Therefore, genetic screening for individuals at high risk, and their potential complications might be a clinical test available in the future.
Conflict of interestsThe authors have no conflict of interests to declare.
Dr Liliana García-Ortiz thanks CONACyT (Sectoral Fund for Health; health project 2012-01-181582) for their partial support in this study. She would also like to thank the Hospital Records Division of the Centro Médico Nacional “20 de Noviembre”, ISSSTE under C. Enrique Díaz Carrillo, for their statistical support, and to M.A. Hendrik Goss for his comments.
Please cite this article as: García-Ortiz L, Gutiérrez-Salinas J, Guerrero-Muñiz S, Chima-Galán MC, Sánchez-Hernández J. Aneurismas intracraneales y su comportamiento clínico-genético. Cirugía y Cirujanos. 2015;83:467–472.