The gastrointestinal stromal tumours (GIST) are the most common soft tissue sarcomas of the digestive tract. They are usually found in the stomach (60–70%) and small intestine (25–30%) and, less commonly, in the oesophagus, mesentery, colon, or rectum.
The symptoms present at diagnosis are, gastrointestinal bleeding, abdominal pain, abdominal mass, or intestinal obstruction. The type of symptomatology will depend on the location and size of the tumour. The definitive diagnosis is histopathological, with 95% of the tumours being positive for CD117.
Clinical casesThis is an observational and descriptive study of 5cases of small intestinal GIST that presented with gastrointestinal bleeding as the main symptom. The period from the initial symptom to the diagnosis varied from 1 to 84 months. The endoscopy was inconclusive in all of the patients, and the diagnosis was made using computed tomography and angiography. Treatment included resection in all patients. The histopathological results are also described.
ConclusionGIST can have multiple clinical pictures and unusual symptoms, such as obscure gastrointestinal bleeding. The use of computed tomography and angiography has shown to be an important tool in the diagnosis with patients with small intestine GISTs.
Los tumores del estroma gastrointestinal (GIST) son los sarcomas más comunes del tracto digestivo. Las localizaciones más frecuentes son estómago (60–70%) e intestino delgado (25-30%).
Los síntomas más comunes son hemorragia de tubo digestivo, dolor abdominal, tumor abdominal y obstrucción intestinal. Estos dependen de la localización y del tamaño del tumor. El diagnóstico es histológico. El 95% de los GIST son positivos para CD117.
Casos clínicosEstudio observacional y descriptivo en el que se reportan 5 casos de GIST de yeyuno e íleon, que tuvieron como manifestación clínica principal hemorragia de tubo digestivo de origen oscuro. El periodo de tiempo al diagnóstico varió de 1 a 84 meses. La endoscopia, en todos los pacientes, no fue concluyente y el diagnóstico se hizo por angiotomografía abdominal. El tratamiento incluyó, en todos los casos, resección.
ConclusionesLos GIST pueden tener formas de presentación y síntomas inusuales como la hemorragia de origen oscuro. La tomografía con medio de contraste y la angiografía son herramientas que han demostrado ser útiles para el diagnóstico certero de este tipo de lesiones.
Gastrointestinal stromal tumours (GIST) are the most common mesenchymal tumours of the digestive tract at 82%. Its annual incidence is approximately 6.8 patients per million inhabitants, slightly more prevalent in males. These tumours can be located anywhere in the digestive tract, from the oesophagus to the rectum. Most are located in the stomach (60–70%) and small bowel (25–30%).1,2
The symptoms they cause are non-specific and relate to their location and the size of the tumour. The most common sign is gastrointestinal bleeding in 50% of cases. Other symptoms described are abdominal pain, abdominal mass and intestinal obstruction.3
Three patterns are described histologically: spindle cell (70%), epithelioid cells (20%) and mixed pattern.4 Characteristically GIST have a specific immunohistochemical profile. Approximately 95% of these are c-KIT positive, although other markers that are present in fewer numbers have been described.5
The most important prognostic factors are the mitotic index, tumour size and the location. Those located in the stomach have a better prognosis.3,5
The objective of this study is to present 5 cases of patients with a diagnosis of GIST of the small bowel, who presented clinically with digestive tract bleeding of obscure origin.
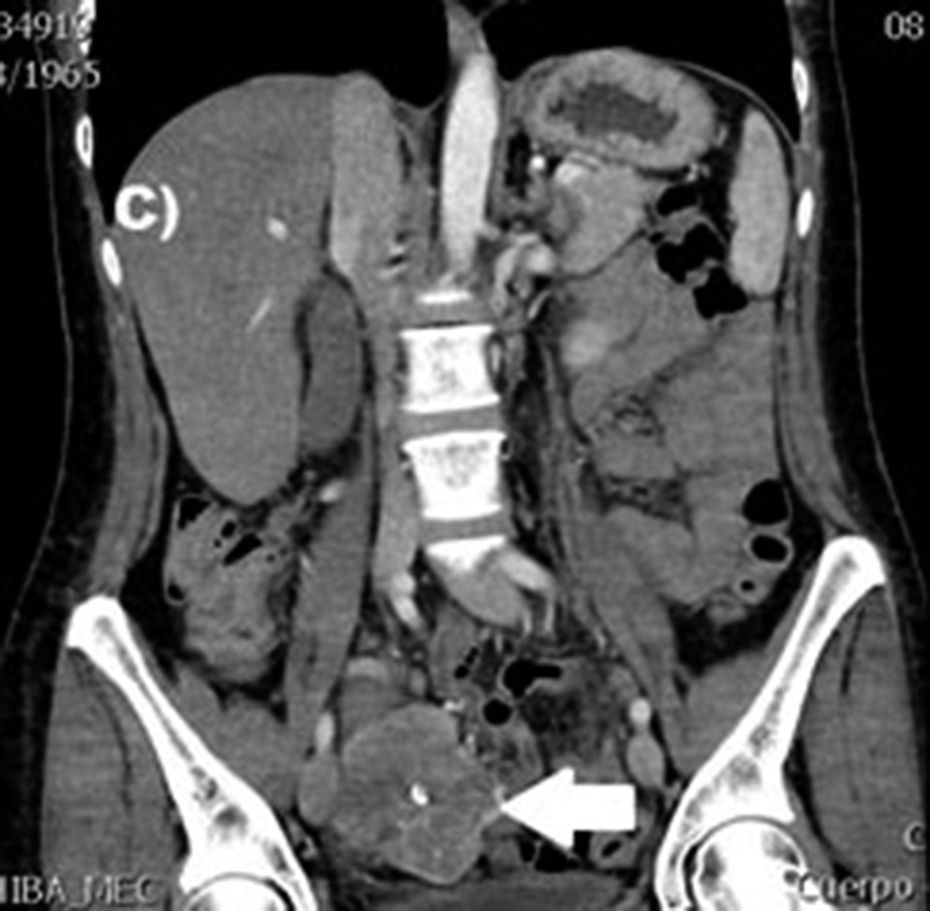
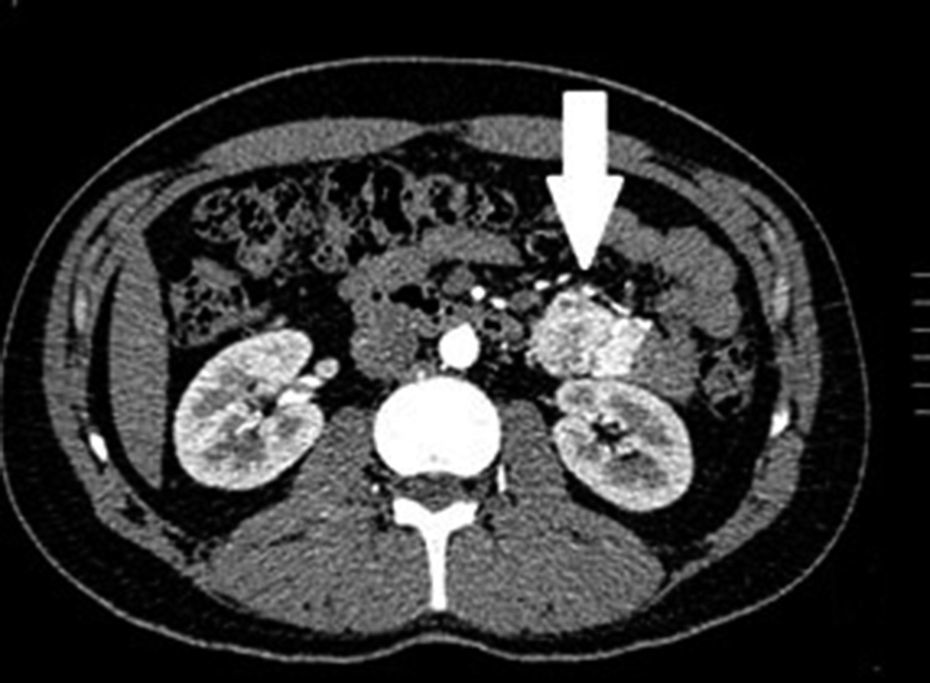
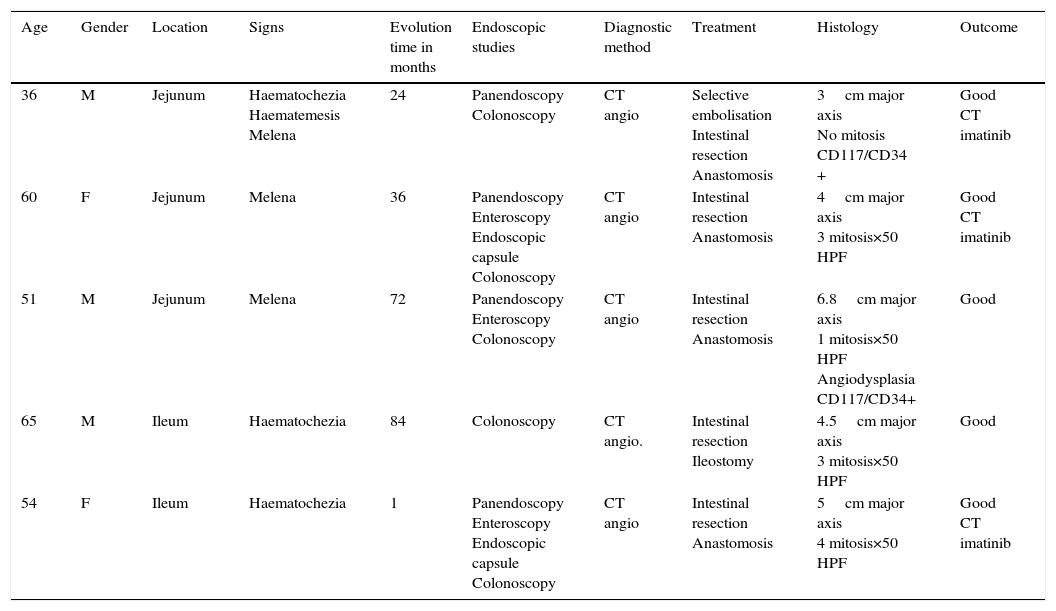
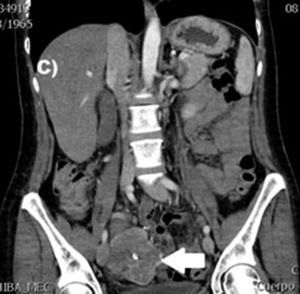
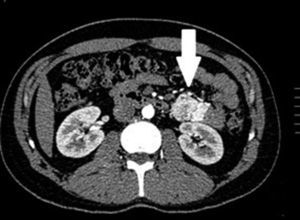
Clinical casesThis is an observational, descriptive case series study, of 5 patients diagnosed with GIST over a 4-year period. The mean age was 53.2 years (range 36 to 65 years). Three patients were male and 2 female. Three of the tumours were located in the jejunum and the rest in the ileum (Fig. 1). All the cases presented with gastrointestinal bleeding of obscure origin. The time to diagnosis was variable, with a mean of 43.3 months (1–84 months). All of the patients initially underwent endoscopic studies that included panendoscopy, colonoscopy or capsule endoscopy. However, the site of bleeding could not be conclusively determined by endoscopy and was subsequently determined by abdominal angiotomography in all cases.
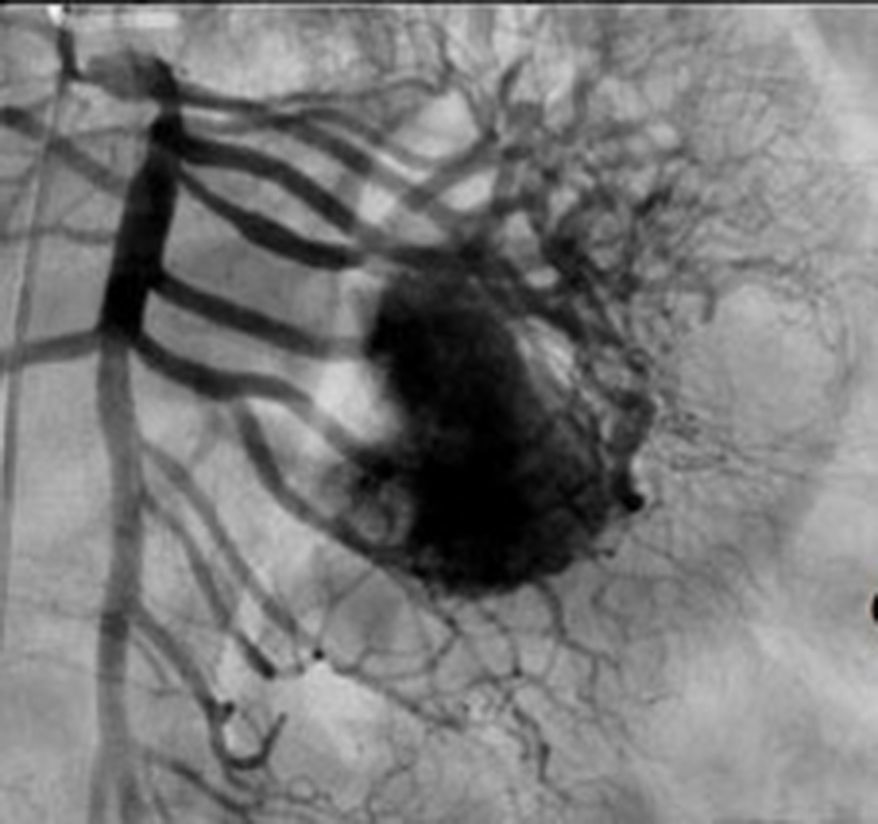
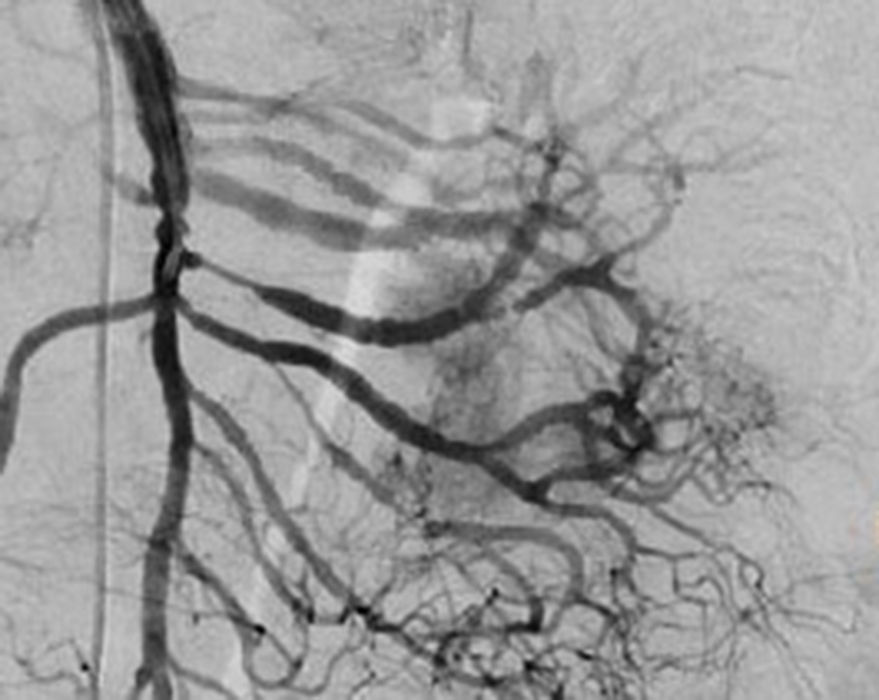
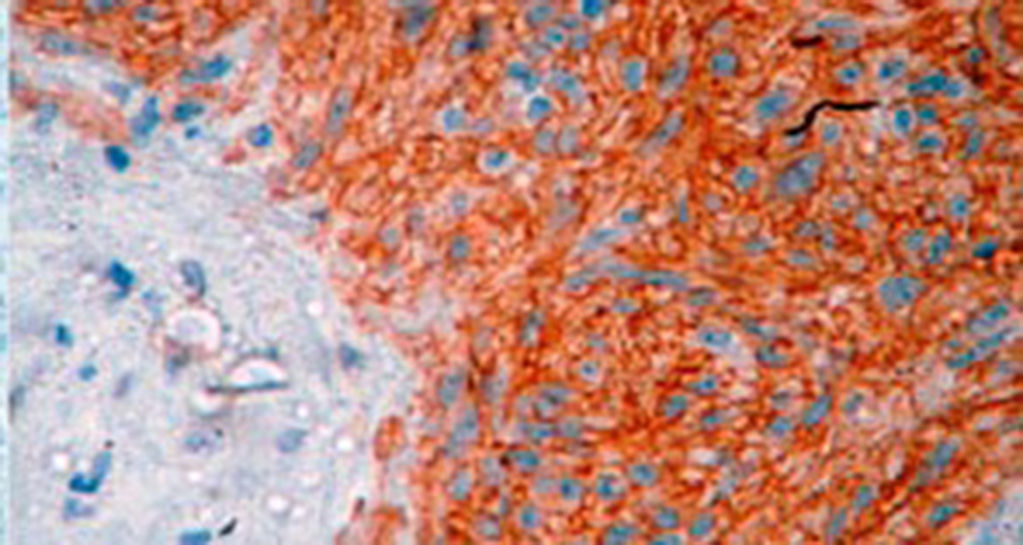
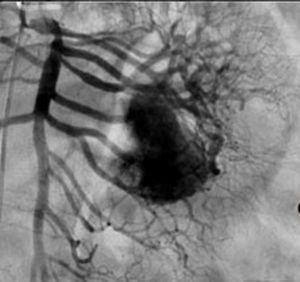
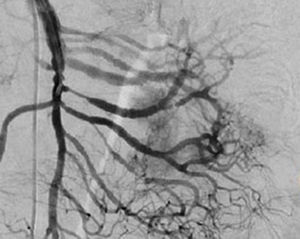
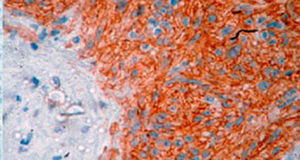
Treatment varied according to the individual characteristics of the tumour and its location. All the patients underwent intestinal resection. One patient (Fig. 2) underwent selective embolisation preoperatively, in order to control the bleeding and was operated the following day (Figs. 3 and 4). A bypass procedure was performed (ileostomy) on one patient alone. All of the patients had good outcomes and are currently being managed by chemotherapy. The patients’ individual data are summarised in Table 1. Definitive diagnosis was corroborated by histopathological studies (Fig. 5).
Clinical and diagnostic characteristics of the patients.
| Age | Gender | Location | Signs | Evolution time in months | Endoscopic studies | Diagnostic method | Treatment | Histology | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 36 | M | Jejunum | Haematochezia Haematemesis Melena | 24 | Panendoscopy Colonoscopy | CT angio | Selective embolisation Intestinal resection Anastomosis | 3cm major axis No mitosis CD117/CD34 + | Good CT imatinib |
| 60 | F | Jejunum | Melena | 36 | Panendoscopy Enteroscopy Endoscopic capsule Colonoscopy | CT angio | Intestinal resection Anastomosis | 4cm major axis 3 mitosis×50 HPF | Good CT imatinib |
| 51 | M | Jejunum | Melena | 72 | Panendoscopy Enteroscopy Colonoscopy | CT angio | Intestinal resection Anastomosis | 6.8cm major axis 1 mitosis×50 HPF Angiodysplasia CD117/CD34+ | Good |
| 65 | M | Ileum | Haematochezia | 84 | Colonoscopy | CT angio. | Intestinal resection Ileostomy | 4.5cm major axis 3 mitosis×50 HPF | Good |
| 54 | F | Ileum | Haematochezia | 1 | Panendoscopy Enteroscopy Endoscopic capsule Colonoscopy | CT angio | Intestinal resection Anastomosis | 5cm major axis 4 mitosis×50 HPF | Good CT imatinib |
CT angio: computed tomography angiography; HPF: high power fields; CT: chemotherapy.
Gastrointestinal bleeding of obscure origin is defined as bleeding that persists or recurs after negative panendoscopy and colonoscopy, that can present as occult blood in faeces, iron deficiency anaemia or recurrent bleeding manifesting with melena stools or haematochezia.6 The aetiology varies according to the age of the patient. This type of bleeding presents a diagnostic challenge to the gastroenterologist and a therapeutic challenge to the surgeon.6,7
Small bowel tumours comprise 5% to 10% as causes of gastrointestinal bleeding of obscure origin. Leiomyomas and GIST are most commonly associated with this symptom.6
The most common presentation of GIST is acute or chronic gastrointestinal bleeding and manifests according to the location of the tumour.7 A particular characteristic that was observed in this study was that 80% of the patients had a recorded history of upper or lower gastrointestinal over at least one year (with a mean of 3.6 years and range of one month to 7 years) before the source of the bleeding was identified, which meets the criteria for gastrointestinal bleeding of obscure origin.
Manrique and Degrate comment that, in cases of gastrointestinal bleeding secondary to GIST identified after resection and pathological and immunohistochemical study, these GIST tumours should be considered the cause of the bleeding.7,8
Endoscopic and radiological studies are used to complement the study of patients with bleeding of obscure origin to establish the location and size of the tumour, and to rule out associated complications such as metastatic disease.
Endoscopic procedures are very important in diagnosis. There are many options with different sensitivity and specificity and the choice of procedure will depend on the clinical manifestations, diagnostic suspicion or failure to reach a diagnosis with other studies. The American Society for Gastrointestinal Endoscopy recommends a specific diagnostic algorithm for gastrointestinal bleeding of obscure origin suggesting, in the first instance, upper endoscopy or colonoscopy and if the results are negative, capsule endoscopy, angiotomography or enterography by tomography.9 Of all the endoscopic studies, the primary indication for capsule endoscopy is bleeding of obscure origin, whether it is occult in faeces or manifest (melena, haematochezia, haematemesis).10 The total range of diagnostic effectiveness for capsule endoscopy varies from 40% to 75%. In the case of gastrointestinal bleeding of obscure origin, this effectiveness ranges from 55% to 75%.11
When endoscopic studies show no lesion or conclusive site of bleeding, other diagnostic studies are used, including computed axial tomography.12 Lupescu et al. reported that a diagnosis of GIST can be suspected using tomography, although there is the disadvantage that it is difficult to differentiate from other tumours of similar characteristics with this radiographic tool.13
The most frequent findings reported on computed axial tomography in patients with GIST of the small bowel is the presence of lesions with well-defined borders that show homogeneous enhancement, originate in the intestinal wall, grow away from the intestinal lumen and project towards the abdominal cavity.14 An intramural hypervascular mass larger than 3cm should lead to suspicion of a GIST as the first option in differential diagnosis, followed by carcinoid tumour, glomus tumour or ectopic pancreas.15 It is important to underline that the hypervascular mass observed in the small bowel using angiotomography in the cases we present is not the classic presentation of GIST; however there are reports in the literature of arteriovenous and angiodysplasia malformations inside the tumour.16 Shiozawa et al. recently reported the case of a patient with abdominal pain whose tomography showed a hypervascular tumour with vascular drainage through a jejunal vein, where arterial phase enhancement and early venous return indicated an arteriovenous malformation. However, after histopathological study, the definitive diagnosis was GIST.17
Despite the fact that endoscopy is the study of choice and offers the most information in order to diagnose gastrointestinal bleeding, in our patients, suspected diagnosis was reached through abdominal angiotomography. This indicates that in patients with gastrointestinal bleeding of obscure origin with normal endoscopy, abdominal angiotomography is a useful resource for diagnosis. Magnetic resonance imaging, with enteroclysis specifically, has also proved very useful in diagnosing small bowel tumours, with sensitivity and specificity above 88% and 93%, respectively.18 However, as in our experience, it use is not yet widespread.
Angiography is another tool used as part of the study of patients with gastrointestinal bleeding of obscure origin and GIST, which has been demonstrated as excellent for diagnosis and treatment, since it enables the use of embolisation for preoperative management.19 Because of the vascular nature and bleeding associated with many digestive tract cancers, the use of preparatory embolisation reduces the size of the tumour and therefore the extent of resection and intra-operative bleeding.20,21 This has been commonly demonstrated in GIST. In one of our patients selective embolisation was used to reduce the vascularity and size of the mass, and we achieved successful intra-operative management. After embolisation the mass was surgically resected.
A definitive diagnosis of GIST is made by histopathological and immunohistochemical study. Three of our patients were c-KIT positive, this is a marker that is present in 95% of GIST and enables an almost unequivocal diagnosis (Fig. 5). In some cases the stain is negative, therefore the use of markers and molecular analysis of mutations in some proto-oncogene exons which codes for CD117 enables a definitive diagnosis.22,23 In our cases, the pattern of immunohistochemical markers used were key in the differential diagnosis. While GIST are strongly CD117 and CD34 positive, leiomyoma and leiomyosarcoma are positive for smooth muscle actin and desmin, but negative for CD34 and CD117. Schwannomas are strongly positive for S100 protein although are sometimes focally positive for CD117, to mention a few.24
Prognosis depends on size, mitotic activity and location. In the study by Miettinem et al. an overall mortality of 39% is reported for GIST located in the small bowel.25 Three of the patients in this study were deemed low risk according to Fletcher's classification for malignant potential, amended by anatomical site, and 2 deemed intermediate. According to the guidelines of the European Society of Medical Oncology (ESMO) for the management of GIST, the rate of metastasis or death related to tumours of the stomach, small bowel, grouped according to size and mitosis index is 4.3% in tumours with low risk and 24% in those with intermediate risk.26
Surgical resection is the treatment of choice for GIST. There are complementary therapies such as imatinib or sunitib that improve prognosis and survival, especially in patients with unresectable or metastatic tumours.27,28
ConclusionsGIST can have different ways of presenting and differing symptoms. In the cases we present in this study, the patients had hypervascular masses demonstrated by angiotomography, and gastrointestinal bleeding of obscure origin. Contrast-enhanced tomography and angiography are tools of demonstrated effectiveness for the accurate diagnosis of this type of lesion, especially in patients with negative endoscopic study results. Although there are a great many diseases that should be considered in differential diagnosis, analysis of clinical history, previous endoscopic tests and the clinical picture should guide the appropriate therapeutic approach.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Romero-Espinosa L, Martínez-Ordaz JM, Romero-Hernández T, de la Fuente-Lira M, Arellano-Sotelo J. Hemorragia gastrointestinal de origen oscuro por tumores de estroma gastrointestinal. Cir Cir. 2017;85:214–219.