Filariasis is a parasitic disease caused by nematodes. It is generally benign and endemic to tropical regions such as Nigeria, India and Indonesia. The life cycle of the filaria begins with the mosquito biting the human host and introducing larvae into the bloodstream, which then migrate to the local lymphatic vessels. The larvae develop into adult worms in about 9 months, reaching a maximum of 80–100mm in length that varies according to the species and sex of the nematode. The adult parasites reside in the lymph nodes. Their half-life is 5 years, and they generate waves of microfilariae with nocturnal periodicity reported in most species. The microfilariae actively migrate through the lymphatic vessels and blood capillaries, and the cycle is completed when a mosquito ingests them when biting an infected host. The microfilariae grow until the larva stage in the muscles of the mosquito, which is infective when the larvae reach their third stage.
It has been suggested that the mechanism of vascular extravasation due to lymphatic obstruction could explain the presence of microfilariae in tissues such as the mammary gland.1 When the parasites die, visible calcifications may be observed on mammograms, with specific characteristics that are not contemplated in the BI-RADS2 classification system. In stages of active disease, there may be varying symptoms, including characteristic cutaneous erythema, fibrosis and lymphedema.
The definitive diagnosis can be made with a peripheral blood smear to detect the microfilariae, and, unlike the detection of circulating antigen, this is valid for all species. Other diagnostic criteria include the presence of filaria DNA in the blood, the detection of the adult worm in the lymphatic system or serological indicators of infection. The most widespread filariasis is caused by Wuchereria bancrofti. This, together with Brugia malawi and Brugia timori, are the most common causes of lymphatic filariasis.3
The eradication of filariasis in endemic areas involves annual campaigns of massive chemoprophylaxis with diethylcarbamazine and albendazole.4 It is estimated that over the last 15 years these programs have managed to avoid 36 million chronic cases of lymphatic filariasis worldwide.5 The therapeutic management of lymphatic filariasis involves drugs such as diethylcarbamazine, ivermectin and albendazole.6
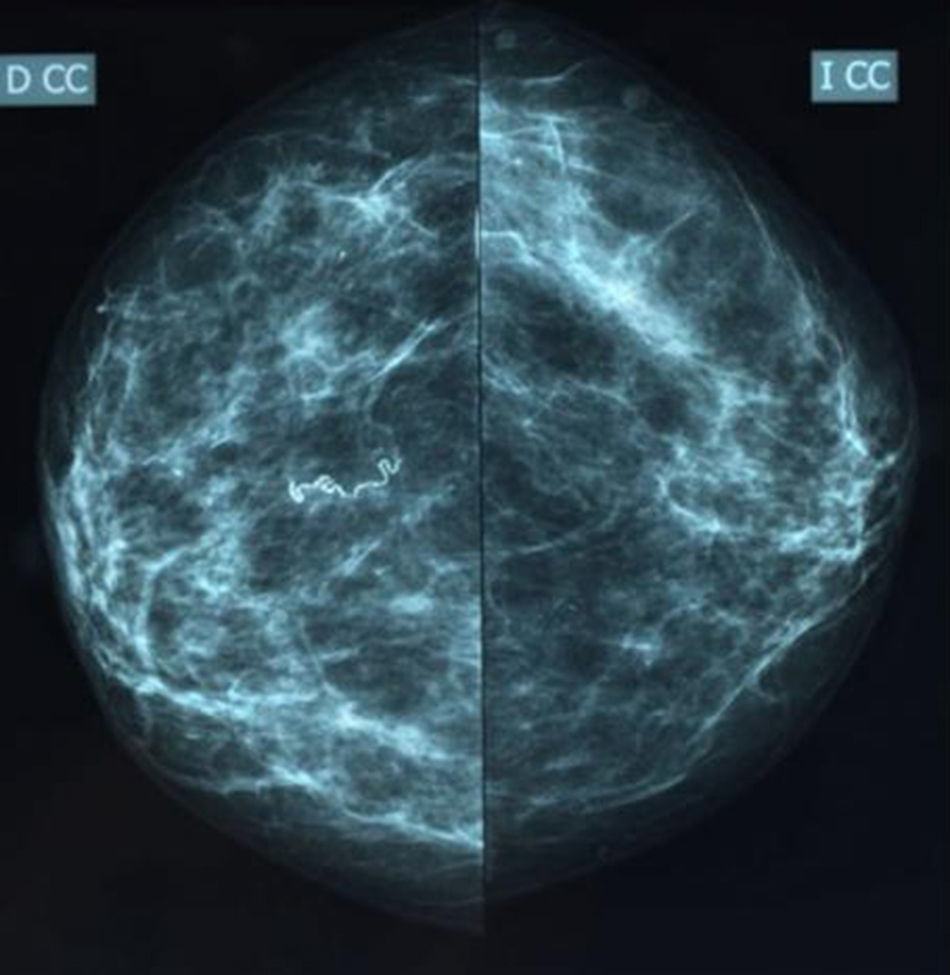
We present the case of a 56-year-old female immigrant from Equatorial Guinea with no family history of cancer. A screening mammogram with craniocaudal and oblique projections (Figs. 1 and 2) identified linear and serpentine calcifications in the right breast, of parasitic infectious origin (category BI-RADS2). The physical examination showed ptotic breasts in a multiparous woman that were symmetrical and showed no notable macroscopic alterations.
The main differential diagnosis should include calcified mammary sutures, benign dystrophic calcifications and carcinoma. Calcifications due to filariasis are distinguishable from malignant microcalcifications because they are not related to the ducts and do not show signs of pleomorphism or irregularity. Dystrophic calcifications present a rough and irregular appearance. In loiasis, we would find fine calcifications in the form of a bubble or corkscrew.7 In trichinosis, the calcifications are not serpiginous in form; they are more numerous, smaller in size and located exclusively in the pectoral muscle. Onchocerciasis is a tangled mass located below the epithelium of the skin.8
Mammary calcifications of filariasis are considered a sequela of chronic infection due to the parasite and appear in late and inactive stages of the process. There are necrosis and fibrosis of the lymphatic ducts of the breast caused by direct contact with the dead nematode. The tissue reaction involves a mixed inflammatory infiltrate including neutrophils, eosinophils and histiocytes, generally without premalignant cellular atypia. They are more frequently found in the upper outer quadrant, although the central or periareolar location has also been described in the literature.9
Diagnosis can be reached by means of an adequate patient medical history and a mammogram compatible with the described calcifications. However, in cases of doubt, fine-needle aspiration (FNA) or biopsy of the lesion are recommended. The diagnosis can be confirmed by measuring microfilariae in peripheral blood, although cases may occur without microfilaremia.10
In our case, peripheral blood microfilariae levels were determined, which were negative. The FNA was inconclusive and the patient refused an open biopsy because of the lack of symptoms.
We determined that it was a case of mammary calcifications from chronic infection of filariasis, and the patient is in periodic follow-up at present.
It is necessary to understand this and the other parasitoses described, especially due to the predictable increase in their incidence in our setting as a consequence of immigration. It is estimated that 120 million people are infected throughout the world. However, there are few published cases that illustrate mammary calcifications. The inclusion of specific mammographic descriptors like “serpiginous” or “filamentous” calcifications in the BI-RADS® system could assist the diagnosis and avoid confusion with other diseases.
We would like to thank Dr. Miguel A. Lorenzo Campos for his tireless dedication to mastology.
Please cite this article as: Pareja López Á, López Saro SM, López Molero VJ, Rico Morales MM, Lorenzo Campos MA. Filariasis mamaria. Cir Esp. 2017;95:349–350.