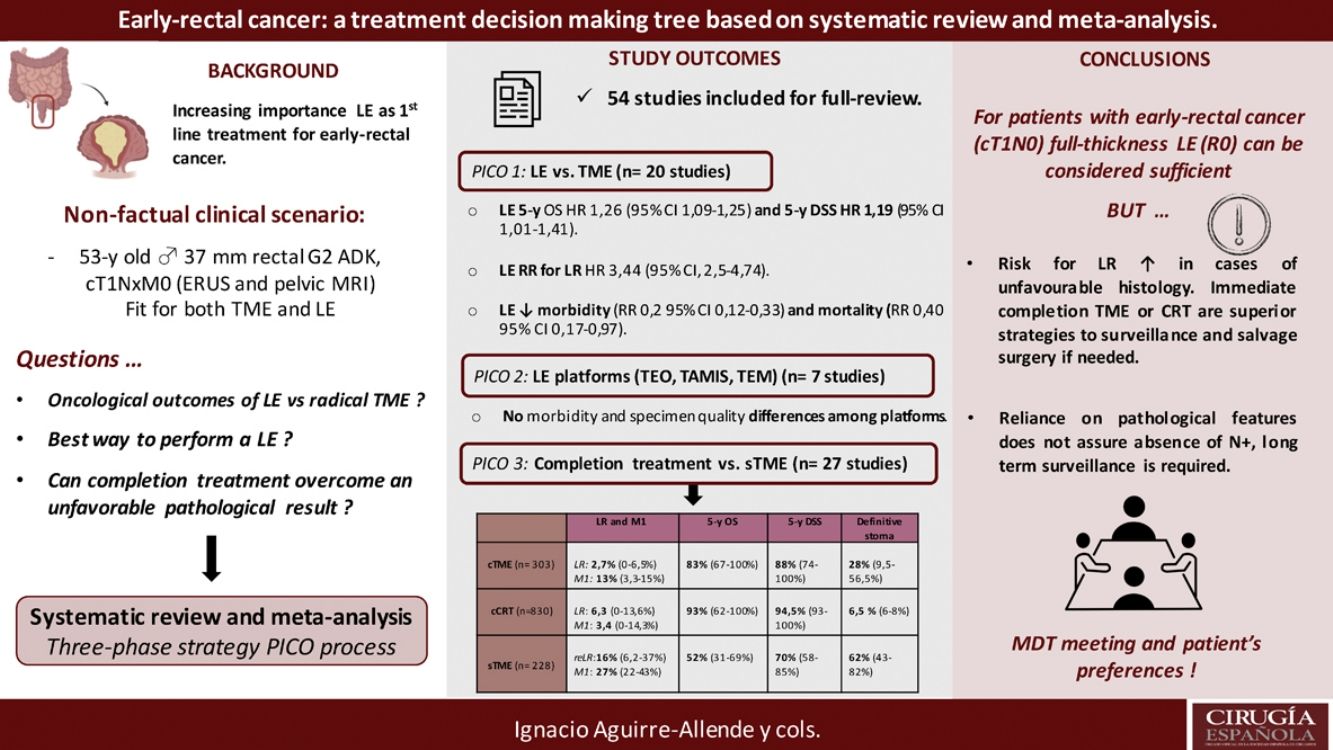
Local excision (LE) has arisen as an alternative to total mesorectal excision for the treatment of early rectal cancer. Despite a decreased morbidity, there are still concerns about LE outcomes.
This systematic-review and meta-analysis design is based on the “PICO” process, aiming to answer to three questions related to LE as primary treatment for early-rectal cancer, the optimal method for LE, and the potential role for completion treatment in high-risk histology tumors and outcomes of salvage surgery.
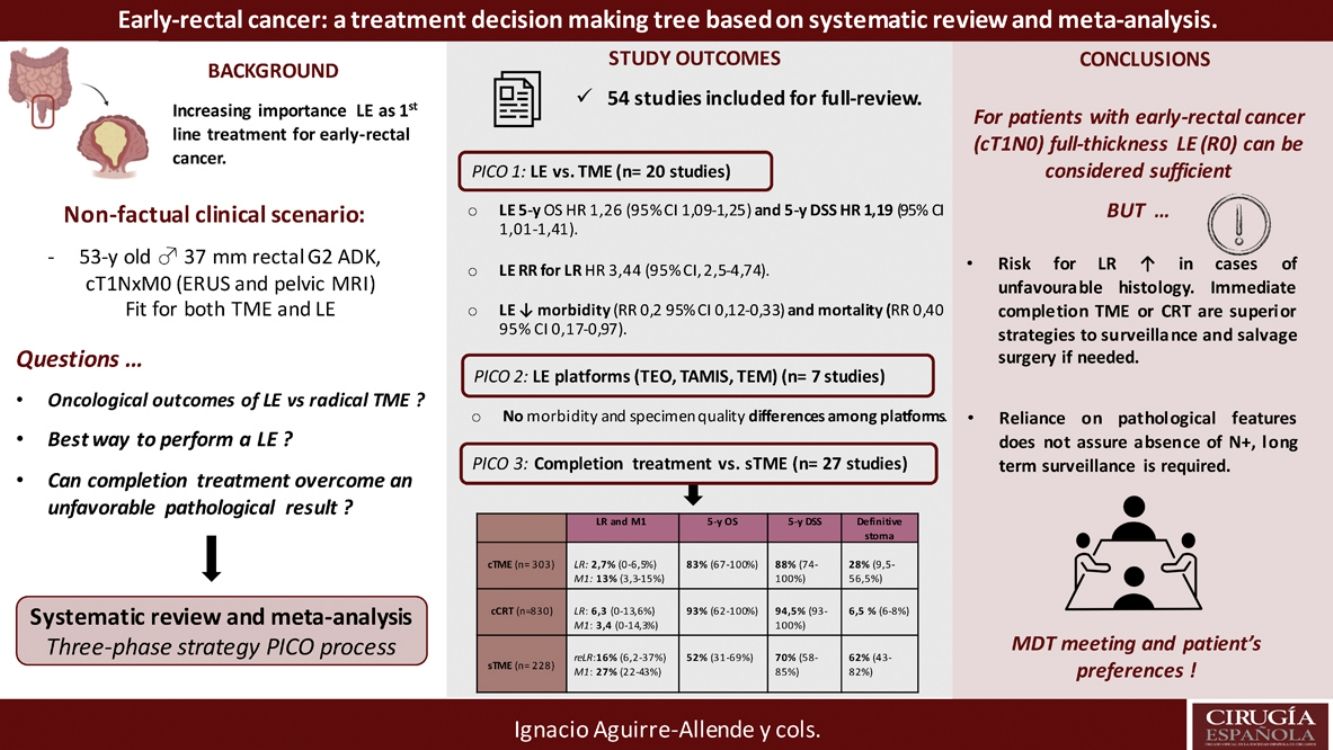
The results revealed that reported overall survival (OS) and disease-specific survival (DSS) were 71%–91.7% and 80%–94% for LE, in contrast to 92.3%–94.3% and 94.4%–97% for radical surgery. Additional analysis of National Database studies revealed lower OS with LE (HR: 1.26; 95%CI, 1.09–1.45) and DSS (HR: 1.19; 95%CI, 1.01–1.41) after LE. Furthermore, patients receiving LE were significantly more prone develop local recurrence (RR: 3.44, 95%CI, 2.50–4.74). Analysis of available transanal surgical platforms was performed, finding no significant differences among them but reduced local recurrence compared to traditional transanal LE (OR:0.24;95%CI, 0.15–0.4). Finally, we found poor survival outcomes for patients undergoing salvage surgery, favoring completion treatment (chemoradiotherapy or surgery) when high-risk histology is present.
In conclusion, LE could be considered adequate provided a full-thickness specimen can be achieved that the patient is informed about risk for potential requirement of completion treatment. Early-rectal cancer cases should be discussed in a multidisciplinary team, and patient's preferences must be considered in the decision-making process.
La escisión local (EL) se ha planteado como una alternativa a la escisión mesorrectal total en el tratamiento del cáncer de recto inicial. A pesar de la reducción de la morbilidad, los resultados de la EL todavía son motivo de preocupación.
Esta revisión sistemática y metaanálisis se basa en el proceso «PICO» con el objetivo de responder a tres preguntas relacionadas con la EL, a saber, como tratamiento principal del cáncer de recto inicial, el método óptimo de EL y su posible papel en el tratamiento completo de tumores histológicos de alto riesgo y complicaciones de la cirugía de rescate.
Los resultados han puesto de manifiesto que la supervivencia general (SG) y la supervivencia específica por enfermedad (SEE) notificadas fueron del 71-91% y del 80-94% en el caso de la EL, en comparación con el 92,3-94,3% y el 94,4-97% en el caso de la cirugía radical, respectivamente. Un análisis complementario de los estudios de la Base de Datos Nacional reveló una SG (HR: 1,26; IC95%: 1,09-1,45) y una SEE inferiores (HR: 1,19; IC95%: 1,01-1,41) después de EL. Además, los pacientes que aceptaron la EL fueron mucho más propensos a presentar una recidiva local (RR: 3,44; IC95%: 2,50-4,74). Se llevó a cabo un análisis de los planteamientos quirúrgicos transanales disponibles. No se encontraron importantes diferencias entre ellos, pero las recidivas locales eran inferiores en comparación con las de la EL transanal tradicional (OR: 0,24; IC95%: 0,15-0,4). Por último, hubo malos resultados de supervivencia entre los pacientes a quienes se les realizó cirugía de rescate, lo que favorece el tratamiento completo (quimiorradioterapia o cirugía) cuando hay histología de alto riesgo.
En conclusión, la EL podría considerarse adecuada siempre que se pueda lograr una muestra de espesor completo y el paciente esté informado del riesgo de una posible necesidad de tratamiento completo. Los casos de cáncer de recto inicial deben tratarse en un equipo multidisciplinario y las preferencias del paciente deben tenerse en cuenta en el proceso de toma de decisiones.
Total mesorectal excision (TME) remains the corner stone of curative therapy in rectal cancer of all stages. This approach removes the primary tumor and draining lymph node basin, allowing accurate pathological staging. TME also is also fully curative in patients with node negative and early T-stage cancers. However, radical procedures carry a 2%–3% perioperative mortality rate and 20%–30% overall morbidity rate.1 This should be considered especially in those patients facing a low-rectum anastomosis or an abdominoperineal resection (APR), whom might experience major genitourinary and defecatory dysfunction or require a permanent stoma.2
Local excision (LE) of the rectal tumors avoids common major complications associated with radical operations, but the decreased invasiveness comes at the expense of an oncologically incomplete surgery. Therefore, LE has been reserved for elderly, sicker and frail patients, considered unfit for radical resection procedures.1 However, there are several factors that could explain the increase number of LEs performed in recent years, including: (1) the widespread use of population based screening programs with the consequent increase in the number of early rectal tumors diagnosed; (2) the preoperative staging accuracy of magnifying chromoendoscopy, endorectal ultrasonography (ERUS) and high definition pelvic MRI; and (3) the introduction of new surgical platforms to gain endoscopic access to the rectum with high-definition vision. This has led to consider LE as first line curative option for patients with early-rectal cancer, and no anymore as a second-line treatment for frail patients.2
Nonetheless, LE precludes definitive assessment of nodal involvement. Up to 13%–24% of pT1-2 rectal cancer associate lymph nodes (LN) metastases.2,3 This rate can increase to even 30%–70% when unfavorable histological features are present.3 Consequently, treatment failure could be anticipated in patients with high-risk histological features, and completion treatment advised with either adjuvant completion chemoradiotherapy (cCRT) or TME surgery (cTME).4,5 Additionally, local recurrence (LR) after LE can be effectively treated with salvage surgery (sTME).5,6
Although main clinical practice guidelines (e.g. NCCN, ESMO, etc.) support the use of LE for selected stage I lesions, this is a recommendation based on low-level evidence. Thus, the optimal treatment of early-rectal carcinoma remains debatable.1
In order to answer those question systematically, we create a nonfactual clinical scenario, consisting on a 52 years-old male, with no medical records and good physical status (ECOG 1 point), diagnosed of a 3.6cm rectal adenocarcinoma, G2; 6–7cm above the anal verge. After preoperative extension work-up (including ERUS and pelvic MRI), clinical tumor staging is cT1N0M0; therefore, the patient is fit to undergo either TME or LE.
This systematic review and meta-analysis aim to solve the main therapeutic questions according to the “problem, intervention and comparison (PICO) process”, structured in a three-phase strategy:
- •
PICO phase 1: Compared with patients with early-rectal cancer (cT1, cNo) undergoing primary radical surgery (pTME), do patients undergoing LE experience better long-term oncological outcomes and functional results?
- •
PICO phase 2: Compared with patients with early-rectal cancer undergoing conventional transanal excision (TAE), do patients undergoing transanal endoscopic microsurgery (TEM/TEO) or transanal microinvasive surgery (TAMIS) experience scarcer postoperative complications, obtain a higher-quality specimen and fewer lesion recurrence?
- •
The specimen obtained after performing an LE was adequate, without fragmentation and with negative margins; however, it shows a submucosal invasion depth of 1.3mm. PICO phase 3: Compared with patients with early-rectal cancer undergoing pTME; do patients undergoing cTME or cCRT due to one questionable histopathological feature, or sTME because of LR, experience reduced survival rates and worst functional results?
This systematic review followed a protocol based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines in order to ensure a transparent and complete evidence report.7
Eligibility CriteriaThe defined time frame for literature search was January 2000 to February 2020. The literature selection was focused on each of the defined 3 PICOS questions. Inclusion criteria were limited to the English language and peer-reviewed studies of adult humans.
Search StrategyA comprehensive systematic review of the published literature in the MEDLINE, Embase, SCOPUS and Cochrane database was conducted. The inclusion and exclusion criteria were predetermined according to the PRISMA guidelines. Database were searched from 1 January 2000 to 29 February 2020, in other to cover modern contemporary management of early-rectal cancer; including LR techniques such as TEM or TAMIS. The search strategy terms for all question captured: rectal cancer, radical resection, LE techniques, completion treatment and salvage surgery for local recurrence; using Boolean operators “OR” et “AND”. This systematic review was complemented by hand research of the reference list of included studies in selected reviews.
Study SelectionInclusion criteria included studies reporting both, oncological, major morbidity and mortality outcomes, of patients undergoing LE and radical surgery for early-rectal adenocarcinoma. Studies analyzing oncological outcomes of patients after completion treatment, both surgical or chemo-radiotherapy (CRT), and salvage surgery for local recurrence were also included.
Studies were excluded if they were case reports, case series of less than 10 patients and non-systematic review articles. Papers including outdated LE procedures were also excluded. Additional exclusion criteria comprised studies with patients receiving neoadjuvant treatment and those with fewer than 3-years follow-up evaluation in PICO questions 1 and 3. In the case of multiple publications reporting on the same cohort of patients, the most recently published analysis was included; unless additional relevant data were acquirable only in previous publications.
Data ExtractionData were extracted by three independent reviewers (IAA, GEE and AEL). Disagreement was resolved by discussion with the senior authors (JME and CPG). A data extraction form was developed to capture detailed data regarding study design, methodologic rigor, results and conclusions. The number of patients in each study, the study design, tumor characteristics, local resection techniques, adjuvant completion treatments, salvage surgery and treatment associated complications were recorded. After screening the abstracts and application of the inclusion and exclusion criteria, articles that fulfilled eligibility criteria were selected for full-text review.
Quality and Risk of Bias Assessment in Individual StudiesThe assessment of the methodological quality of the selected studies included the Cochrane Collaboration's tool for assessment of risk of bias in randomized controlled trial,8 the Newcastle-Ottawa scale (NOS) for quality assessment of nonrandomized observational studies,9 and the AMSTAR2 (MeaSurement Tool to Assess systematic Reviews)10 criteria for systematic reviews.
Data Synthesis and Statistical AnalysisThe following end-points were identified: overall survival (OS), disease-free survival (DFS), disease specific survival (DSS), distant and LR, mortality, morbidity and stoma rate.
Where it was not appropriate to pool data, the results were presented narratively or reported as median and ranges. For survival estimates, only figures derived from Kaplan–Meier survival analysis with adequate follow-up were documented. For dichotomous outcomes the relative risk (RR) was used as a measure of treatment effect and for continuous outcomes the standardized mean differences (SMD) were calculated, when appropriate. For time-to-event data, the log of the hazard ratio (log (HR)) and its standard error was used.
Statistical analysis was performed with Review Manager (RevMan) v. 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen Denmark, 2014). Heterogeneity was explored using the chi-squared test, with significance set at P>.05, and was quantified using I2, with a maximum value of 50% identified low heterogeneity. The Mantel-Haenszel method was used for the calculation of the RR in the random effects model. All pooled outcome measures were determined by using a random-effects model described by DerSimonian and Laird, and the RR was estimated with its variance and 95% CI. The analysis of publication bias was obtained using a funnel plot and the Beg and Egger tests.
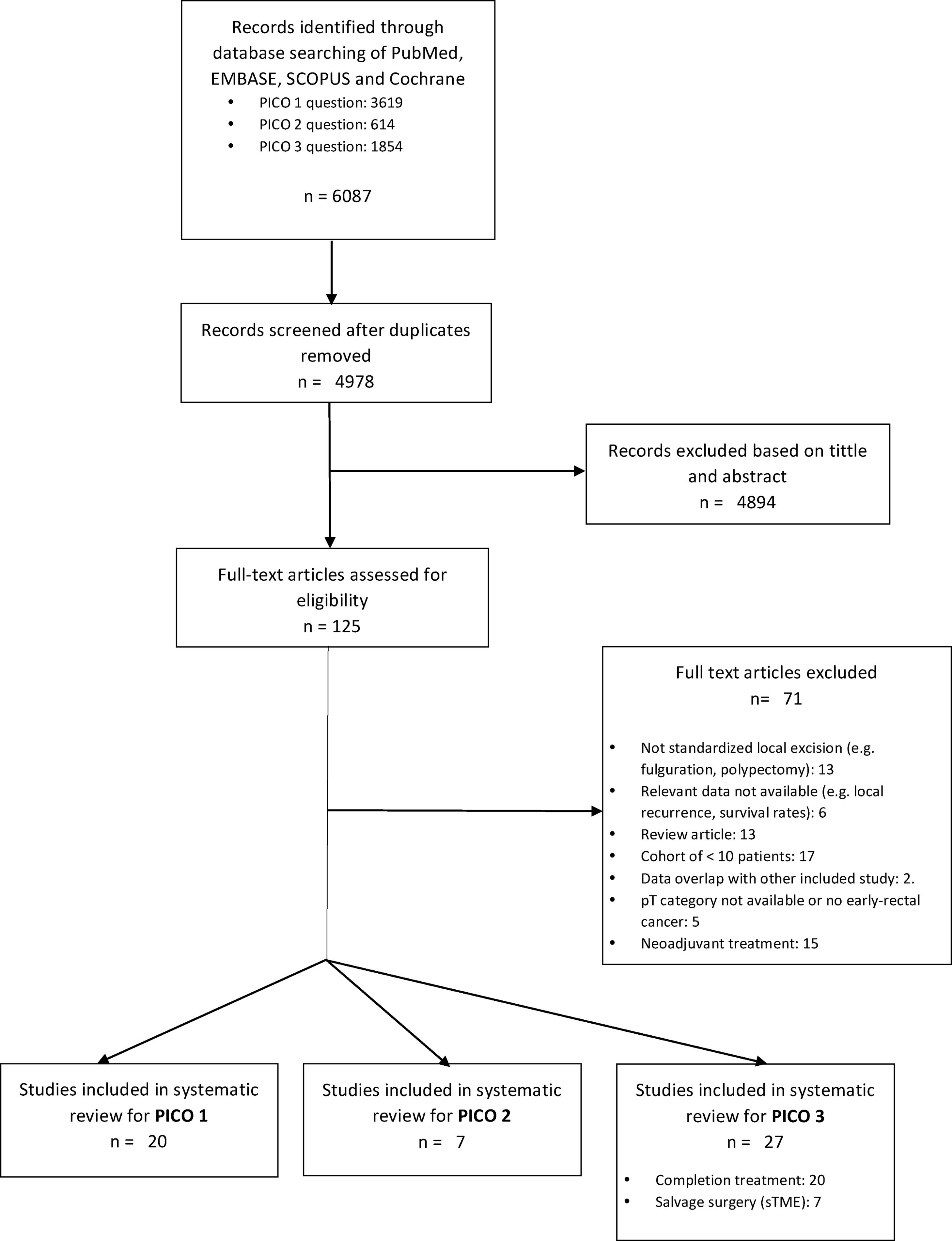
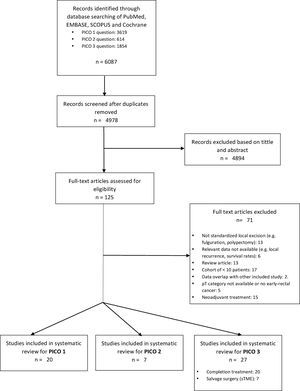
ResultsA total of 125 full-text articles were reviewed against eligibility criteria. Finally, 54 articles met all the criteria for full review and analysis. The PRISMA flow-diagram in Fig. 1 describes the inclusion and exclusion process.
PICO 1Search Results and Study Selection: After title and abstract review, 8 systematic reviews (SR)2,3,5,6,11–14 (5 SRs with meta-analysis-Mas-)3,5,6,11,12 and 8 large National database studies15–22 comparing LE vs. TME for T1 rectal cancer were included for full-text analysis. Of the National databases, one study included early rectal cancer,15 without discriminating data between T1 and T2, and another of them compared the results of LE vs. APR22 exclusively. Of the 8 SRs, 55,11–14 analyzed studies including patients with tumors T2, “initial” T3, benign lesions, or patients undergoing neoadjuvant or adjuvant CRT, all of which were out of scope of the present study. Among the three remaining SRs,2,3,6 there were 9 different observational studies,23–30 which met the inclusion criteria for the present review. Three observational studies31–33 and one randomized clinical trial (RCT)34 were newly retrieved for the present review after performing additional search until February 2020.
In conclusion, the selection process yielded a total of 20 different studies: 19 observational studies (including 7 large population database studies) and one RCT.
Characteristics of the included studies: The characteristics of the selected studies for qualitative and quantitative analysis are presented in Appendix 1.
Quality assessment of the included studies: The assessment of the risk of bias of included studies is presented in Appendix 1.
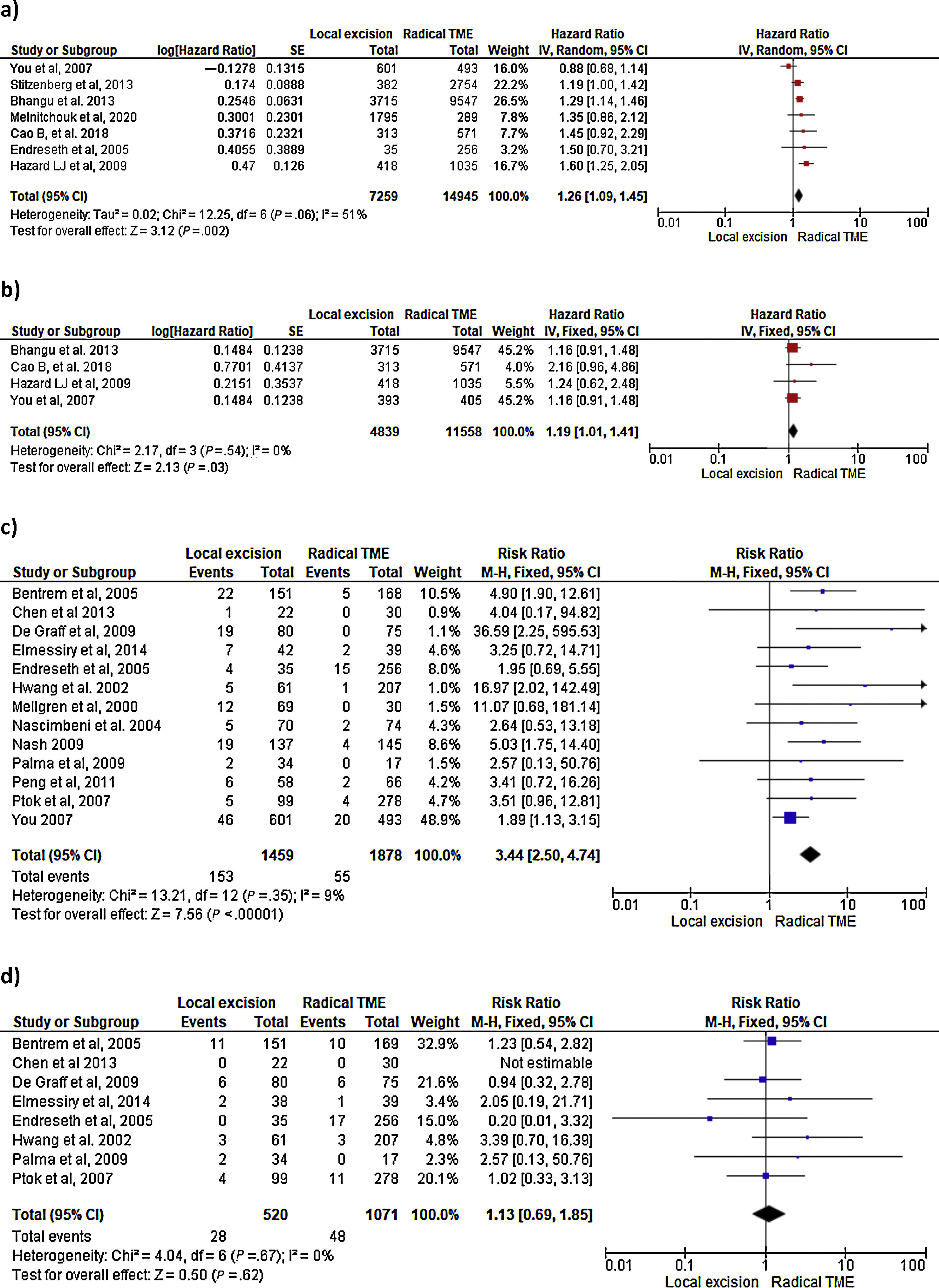
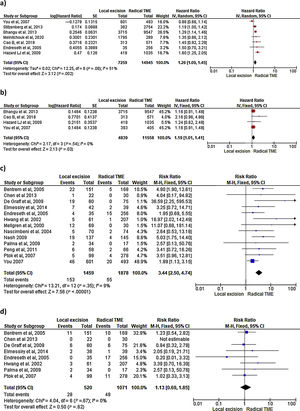
Survival outcomes (i.e. OS, DFS and DSS): Time-to-event outcomes such as survival are ideally pooled using meta-analysis of hazard ratios (HR). However, HR was not reported in any of the selected observational studies or in the RCT. Two SRs perform MAs3,6 from pooled survival data by visually estimating the results of the Kaplan–Meier plots. However, this approach does not take into account censorship and loss of patients in the follow-up that occurs, and does not allow the HR estimation. We therefore reported the 5-y OS and 5-y DSS of the observational studies23,25–29,31–33,35 as ranges: from 71%–91.7% to 80%–94% and from 92.3%–94.3% to 94.4%–97% for LE and RS respectively. However, seven National database studies16–22 did provide survival and HR data, after adjusting for patient and tumor characteristics, with fair homogeneity among these studies. As shown in Fig. 2 there was a small but significantly lower 5-y OS with LE in comparison with RS (HR: 1.26; 95%CI, 1.09–1.45). LR results also in small but significantly decreased 5-y DSS (HR: 1.19; 95%CI, 1.01–1.41).
- -
LR rate and distant metastasis rate: Thirteen studies16,17,23,26–35 reported postoperative local recurrences rates, with fair homogeneity among the studies (P: .35; I2: 9%). The results showed a significant difference between the LE and TME (RR: 3.44, 95%CI, 2.50–4.74). Eight studies16,26–29,31–34 reported postoperative distant metastasis rates, with no significant heterogeneity among studies (P: .67; I2: 0%). The results showed that the differences between the two groups were not statistically significant (RR: 1.13, 95%CI 0.60–1.85). Forest and funnel plots are shown in Fig. 2.
- -
Mortality, morbility and final stoma rate: Eight studies16,22,24–29 reported mortality, seven16,24–29,31,32,35 reported morbidity and eight the stoma rate.16,23,25,26,28,29,31,32,35 The results showed that LE was associated with significantly less mortality (RR: 0.40, 95%IC 0.17–0.97), fewer major postoperative complications (RR:0.20, 95%CI 0.12–0.33) and a much lower need for permanent colostomy (RR: 0.09, 95%CI 0.05–0.17). Forest and funnel plots are shown in Appendix 1.
- -
Search Results and Study Selection: A total of 7 articles met all the criteria for full-review, with data abstraction in the systemic review, including one SR and MA,36 one RCT37 and 538–42 observational comparative studies.
- -
Characteristics and outcome results of the included studies: One systematic review and meta-analysis36 containing comparative data of six non-randomized studies comparing TAE and TEM clearly stated that TEM had a higher rate of negative microscopic margins, reduced specimen fragmentation and lesion recurrence compared with TAE.
A prospective randomized clinical trial comparing TEM vs. TEO was found,37 showing no technical or clinical differences between the results (morbidity and specimen) obtained with the two systems, except lower cost with TEO. Five observational studies compare the surgical platforms to the rectum with each other (TEM vs. TAMIS38–40 and TEO vs. TAMIS41–42) without finding clinical (morbidity) or specimen high-quality procurement differences among them.
- -
Quality assessment of the included studies: The data related to the qualitative assessment of the selected studies are shown in Appendix 2.
Search Results and Study Selection:
- -
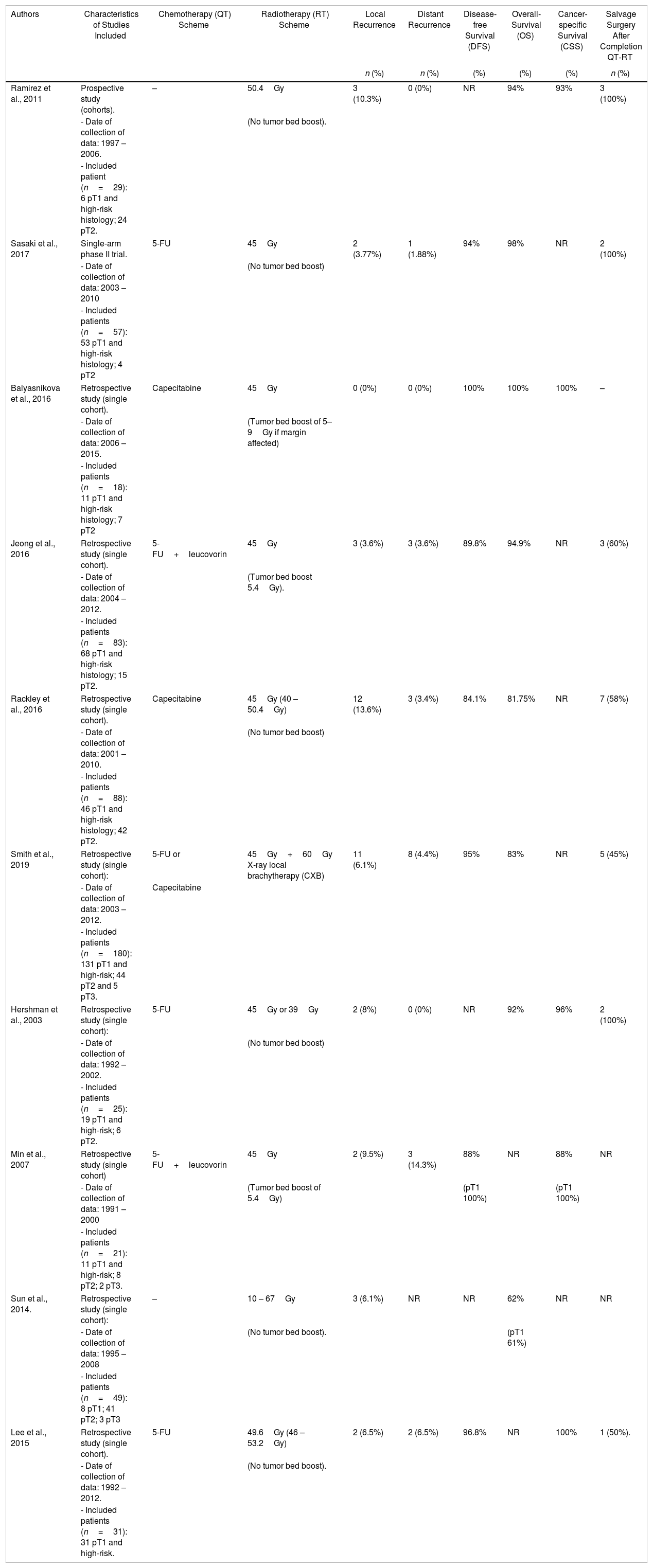
cCRT: Overall, 10 studies43–52 (8 retrospective, 1 prospective cohort study43 and 1 single-arm phase II multicentre randomized trial44) including 830 patients met the inclusion criteria and were analyzed in this SR. Indications for cCRT were specified in nine studies43,49,50,52 most considering high-risk histological features for LR as core indication. Patient's preferences along with comorbidities were appointed as further indication for cCRT in 3 studies.45,50,52 Most patients received long-course radiotherapy43,48,50–52 with additional local X-ray brachytherapy in one single study.47 Concomitant chemotherapy administration was reported in eight studies,40,52 most frequently based on 5-FU.44,47,48,50
- -
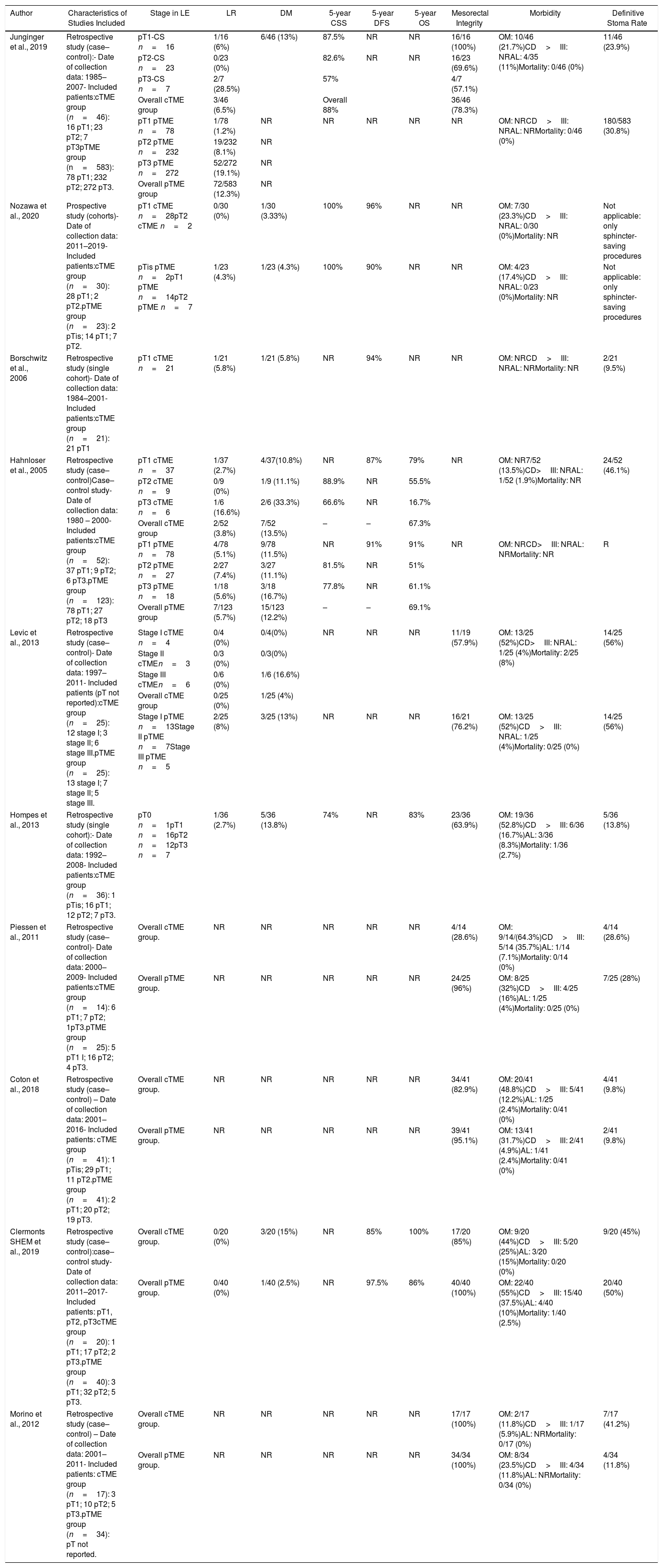
cTME: Overall, ten studies focusing cTME including 308 patients were considered eligible for inclusion.53–62 All comprised studies reported similar indications for cTME, including previously defined high-risk histological features on margin compromise. Three studies included patients undergoing radical cTME after previous full-thickness LE59,61,62; six studies53,55–58,60 included cases treated with either full or partial thickness, and one cohort study54 only considered patients with previous endoscopic mucosal resection (EMR) or previous submucosal endoscopic dissection (ESD).
- -
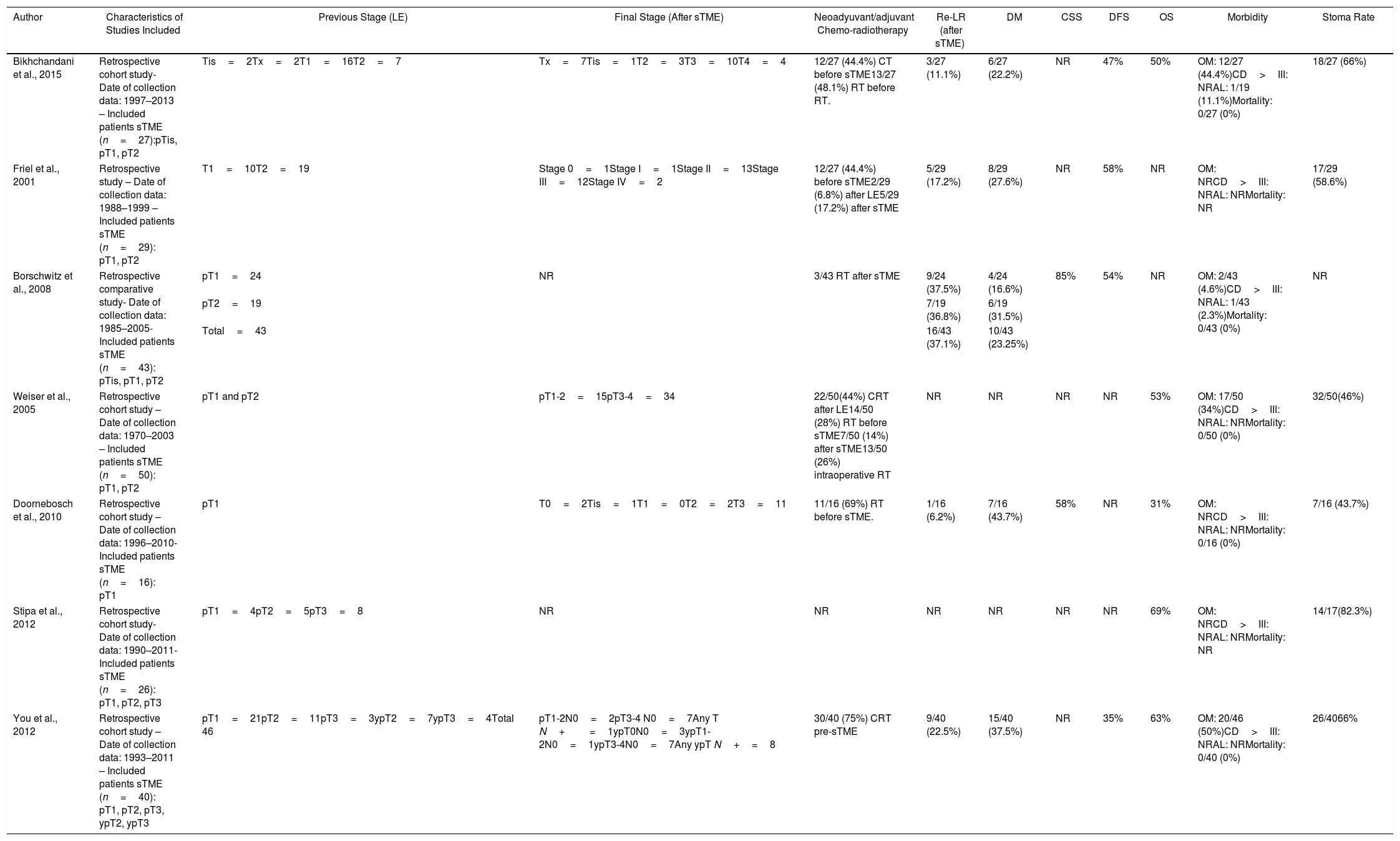
sTME: Radical salvage surgery with curative intent was performed in patients with a LR after LE. The recurrences included in the studies were luminal, nodal, or extrarectal (pelvic). Seven studies focusing in sTME including 228 patients met the inclusion criteria and were analyzed.63–69 One study included only patients with LR after LE for pT1,67 the rest included pT1-3.
Characteristics of the included studies:Tables 1a–1c presents the characteristics, interventions and outcomes of the studies included within each of the three strategies.
Summary of Completion Chemo-radiotherapy (cCRT) Treatment for High-risk Histology Tumors and Patient Oncological Outcomes.
| Authors | Characteristics of Studies Included | Chemotherapy (QT) Scheme | Radiotherapy (RT) Scheme | Local Recurrence | Distant Recurrence | Disease-free Survival (DFS) | Overall-Survival (OS) | Cancer-specific Survival (CSS) | Salvage Surgery After Completion QT-RT |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | (%) | (%) | (%) | n (%) | ||||
| Ramirez et al., 2011 | Prospective study (cohorts). | – | 50.4Gy | 3 (10.3%) | 0 (0%) | NR | 94% | 93% | 3 (100%) |
| - Date of collection of data: 1997 – 2006. | (No tumor bed boost). | ||||||||
| - Included patient (n=29): 6 pT1 and high-risk histology; 24 pT2. | |||||||||
| Sasaki et al., 2017 | Single-arm phase II trial. | 5-FU | 45Gy | 2 (3.77%) | 1 (1.88%) | 94% | 98% | NR | 2 (100%) |
| - Date of collection of data: 2003 – 2010 | (No tumor bed boost) | ||||||||
| - Included patients (n=57): 53 pT1 and high-risk histology; 4 pT2 | |||||||||
| Balyasnikova et al., 2016 | Retrospective study (single cohort). | Capecitabine | 45Gy | 0 (0%) | 0 (0%) | 100% | 100% | 100% | – |
| - Date of collection of data: 2006 – 2015. | (Tumor bed boost of 5–9Gy if margin affected) | ||||||||
| - Included patients (n=18): 11 pT1 and high-risk histology; 7 pT2 | |||||||||
| Jeong et al., 2016 | Retrospective study (single cohort). | 5-FU+leucovorin | 45Gy | 3 (3.6%) | 3 (3.6%) | 89.8% | 94.9% | NR | 3 (60%) |
| - Date of collection of data: 2004 – 2012. | (Tumor bed boost 5.4Gy). | ||||||||
| - Included patients (n=83): 68 pT1 and high-risk histology; 15 pT2. | |||||||||
| Rackley et al., 2016 | Retrospective study (single cohort). | Capecitabine | 45Gy (40 – 50.4Gy) | 12 (13.6%) | 3 (3.4%) | 84.1% | 81.75% | NR | 7 (58%) |
| - Date of collection of data: 2001 – 2010. | (No tumor bed boost) | ||||||||
| - Included patients (n=88): 46 pT1 and high-risk histology; 42 pT2. | |||||||||
| Smith et al., 2019 | Retrospective study (single cohort): | 5-FU or | 45Gy+60Gy X-ray local brachytherapy (CXB) | 11 (6.1%) | 8 (4.4%) | 95% | 83% | NR | 5 (45%) |
| - Date of collection of data: 2003 – 2012. | Capecitabine | ||||||||
| - Included patients (n=180): 131 pT1 and high-risk; 44 pT2 and 5 pT3. | |||||||||
| Hershman et al., 2003 | Retrospective study (single cohort): | 5-FU | 45Gy or 39Gy | 2 (8%) | 0 (0%) | NR | 92% | 96% | 2 (100%) |
| - Date of collection of data: 1992 – 2002. | (No tumor bed boost) | ||||||||
| - Included patients (n=25): 19 pT1 and high-risk; 6 pT2. | |||||||||
| Min et al., 2007 | Retrospective study (single cohort) | 5-FU+leucovorin | 45Gy | 2 (9.5%) | 3 (14.3%) | 88% | NR | 88% | NR |
| - Date of collection of data: 1991 – 2000 | (Tumor bed boost of 5.4Gy) | (pT1 100%) | (pT1 100%) | ||||||
| - Included patients (n=21): 11 pT1 and high-risk; 8 pT2; 2 pT3. | |||||||||
| Sun et al., 2014. | Retrospective study (single cohort): | – | 10 – 67Gy | 3 (6.1%) | NR | NR | 62% | NR | NR |
| - Date of collection of data: 1995 – 2008 | (No tumor bed boost). | (pT1 61%) | |||||||
| - Included patients (n=49): 8 pT1; 41 pT2; 3 pT3 | |||||||||
| Lee et al., 2015 | Retrospective study (single cohort). | 5-FU | 49.6Gy (46 – 53.2Gy) | 2 (6.5%) | 2 (6.5%) | 96.8% | NR | 100% | 1 (50%). |
| - Date of collection of data: 1992 – 2012. | (No tumor bed boost). | ||||||||
| - Included patients (n=31): 31 pT1 and high-risk. | |||||||||
Summary of Salvage Surgery (cTME) Treatment for High-risk Histology Tumors and Patient Oncological Outcomes.
| Author | Characteristics of Studies Included | Stage in LE | LR | DM | 5-year CSS | 5-year DFS | 5-year OS | Mesorectal Integrity | Morbidity | Definitive Stoma Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Junginger et al., 2019 | Retrospective study (case–control):- Date of collection data: 1985–2007- Included patients:cTME group (n=46): 16 pT1; 23 pT2; 7 pT3pTME group (n=583): 78 pT1; 232 pT2; 272 pT3. | pT1-CS n=16 | 1/16 (6%) | 6/46 (13%) | 87.5% | NR | NR | 16/16 (100%) | OM: 10/46 (21.7%)CD>III: NRAL: 4/35 (11%)Mortality: 0/46 (0%) | 11/46 (23.9%) |
| pT2-CS n=23 | 0/23 (0%) | 82.6% | NR | NR | 16/23 (69.6%) | |||||
| pT3-CS n=7 | 2/7 (28.5%) | 57% | 4/7 (57.1%) | |||||||
| Overall cTME group | 3/46 (6.5%) | Overall 88% | 36/46 (78.3%) | |||||||
| pT1 pTME n=78 | 1/78 (1.2%) | NR | NR | NR | NR | NR | OM: NRCD>III: NRAL: NRMortality: 0/46 (0%) | 180/583 (30.8%) | ||
| pT2 pTME n=232 | 19/232 (8.1%) | NR | ||||||||
| pT3 pTME n=272 | 52/272 (19.1%) | NR | ||||||||
| Overall pTME group | 72/583 (12.3%) | NR | ||||||||
| Nozawa et al., 2020 | Prospective study (cohorts)- Date of collection data: 2011–2019- Included patients:cTME group (n=30): 28 pT1; 2 pT2.pTME group (n=23): 2 pTis; 14 pT1; 7 pT2. | pT1 cTME n=28pT2 cTME n=2 | 0/30 (0%) | 1/30 (3.33%) | 100% | 96% | NR | NR | OM: 7/30 (23.3%)CD>III: NRAL: 0/30 (0%)Mortality: NR | Not applicable: only sphincter-saving procedures |
| pTis pTME n=2pT1 pTME n=14pT2 pTME n=7 | 1/23 (4.3%) | 1/23 (4.3%) | 100% | 90% | NR | NR | OM: 4/23 (17.4%)CD>III: NRAL: 0/23 (0%)Mortality: NR | Not applicable: only sphincter-saving procedures | ||
| Borschwitz et al., 2006 | Retrospective study (single cohort)- Date of collection data: 1984–2001- Included patients:cTME group (n=21): 21 pT1 | pT1 cTME n=21 | 1/21 (5.8%) | 1/21 (5.8%) | NR | 94% | NR | NR | OM: NRCD>III: NRAL: NRMortality: NR | 2/21 (9.5%) |
| Hahnloser et al., 2005 | Retrospective study (case–control)Case–control study- Date of collection data: 1980 – 2000- Included patients:cTME group (n=52): 37 pT1; 9 pT2; 6 pT3.pTME group (n=123): 78 pT1; 27 pT2; 18 pT3 | pT1 cTME n=37 | 1/37 (2.7%) | 4/37(10.8%) | NR | 87% | 79% | NR | OM: NR7/52 (13.5%)CD>III: NRAL: 1/52 (1.9%)Mortality: NR | 24/52 (46.1%) |
| pT2 cTME n=9 | 0/9 (0%) | 1/9 (11.1%) | 88.9% | NR | 55.5% | |||||
| pT3 cTME n=6 | 1/6 (16.6%) | 2/6 (33.3%) | 66.6% | NR | 16.7% | |||||
| Overall cTME group | 2/52 (3.8%) | 7/52 (13.5%) | – | – | 67.3% | |||||
| pT1 pTME n=78 | 4/78 (5.1%) | 9/78 (11.5%) | NR | 91% | 91% | NR | OM: NRCD>III: NRAL: NRMortality: NR | R | ||
| pT2 pTME n=27 | 2/27 (7.4%) | 3/27 (11.1%) | 81.5% | NR | 51% | |||||
| pT3 pTME n=18 | 1/18 (5.6%) | 3/18 (16.7%) | 77.8% | NR | 61.1% | |||||
| Overall pTME group | 7/123 (5.7%) | 15/123 (12.2%) | – | – | 69.1% | |||||
| Levic et al., 2013 | Retrospective study (case–control)- Date of collection data: 1997–2011- Included patients (pT not reported):cTME group (n=25): 12 stage I; 3 stage II; 6 stage III.pTME group (n=25): 13 stage I; 7 stage II; 5 stage III. | Stage I cTME n=4 | 0/4 (0%) | 0/4(0%) | NR | NR | NR | 11/19 (57.9%) | OM: 13/25 (52%)CD>III: NRAL: 1/25 (4%)Mortality: 2/25 (8%) | 14/25 (56%) |
| Stage II cTMEn=3 | 0/3 (0%) | 0/3(0%) | ||||||||
| Stage III cTMEn=6 | 0/6 (0%) | 1/6 (16.6%) | ||||||||
| Overall cTME group | 0/25 (0%) | 1/25 (4%) | ||||||||
| Stage I pTME n=13Stage II pTME n=7Stage III pTME n=5 | 2/25 (8%) | 3/25 (13%) | NR | NR | NR | 16/21 (76.2%) | OM: 13/25 (52%)CD>III: NRAL: 1/25 (4%)Mortality: 0/25 (0%) | 14/25 (56%) | ||
| Hompes et al., 2013 | Retrospective study (single cohort):- Date of collection data: 1992–2008- Included patients:cTME group (n=36): 1 pTis; 16 pT1; 12 pT2; 7 pT3. | pT0 n=1pT1 n=16pT2 n=12pT3 n=7 | 1/36 (2.7%) | 5/36 (13.8%) | 74% | NR | 83% | 23/36 (63.9%) | OM: 19/36 (52.8%)CD>III: 6/36 (16.7%)AL: 3/36 (8.3%)Mortality: 1/36 (2.7%) | 5/36 (13.8%) |
| Piessen et al., 2011 | Retrospective study (case–control)- Date of collection data: 2000–2009- Included patients:cTME group (n=14): 6 pT1; 7 pT2; 1pT3.pTME group (n=25): 5 pT1 I; 16 pT2; 4 pT3. | Overall cTME group. | NR | NR | NR | NR | NR | 4/14 (28.6%) | OM: 9/14/(64.3%)CD>III: 5/14 (35.7%)AL: 1/14 (7.1%)Mortality: 0/14 (0%) | 4/14 (28.6%) |
| Overall pTME group. | NR | NR | NR | NR | NR | 24/25 (96%) | OM: 8/25 (32%)CD>III: 4/25 (16%)AL: 1/25 (4%)Mortality: 0/25 (0%) | 7/25 (28%) | ||
| Coton et al., 2018 | Retrospective study (case–control) – Date of collection data: 2001–2016- Included patients: cTME group (n=41): 1 pTis; 29 pT1; 11 pT2.pTME group (n=41): 2 pT1; 20 pT2; 19 pT3. | Overall cTME group. | NR | NR | NR | NR | NR | 34/41 (82.9%) | OM: 20/41 (48.8%)CD>III: 5/41 (12.2%)AL: 1/25 (2.4%)Mortality: 0/41 (0%) | 4/41 (9.8%) |
| Overall pTME group. | NR | NR | NR | NR | NR | 39/41 (95.1%) | OM: 13/41 (31.7%)CD>III: 2/41 (4.9%)AL: 1/41 (2.4%)Mortality: 0/41 (0%) | 2/41 (9.8%) | ||
| Clermonts SHEM et al., 2019 | Retrospective study (case–control):case–control study- Date of collection data: 2011–2017- Included patients: pT1, pT2, pT3cTME group (n=20): 1 pT1; 17 pT2; 2 pT3.pTME group (n=40): 3 pT1; 32 pT2; 5 pT3. | Overall cTME group. | 0/20 (0%) | 3/20 (15%) | NR | 85% | 100% | 17/20 (85%) | OM: 9/20 (44%)CD>III: 5/20 (25%)AL: 3/20 (15%)Mortality: 0/20 (0%) | 9/20 (45%) |
| Overall pTME group. | 0/40 (0%) | 1/40 (2.5%) | NR | 97.5% | 86% | 40/40 (100%) | OM: 22/40 (55%)CD>III: 15/40 (37.5%)AL: 4/40 (10%)Mortality: 1/40 (2.5%) | 20/40 (50%) | ||
| Morino et al., 2012 | Retrospective study (case–control) – Date of collection data: 2001–2011- Included patients: cTME group (n=17): 3 pT1; 10 pT2; 5 pT3.pTME group (n=34): pT not reported. | Overall cTME group. | NR | NR | NR | NR | NR | 17/17 (100%) | OM: 2/17 (11.8%)CD>III: 1/17 (5.9%)AL: NRMortality: 0/17 (0%) | 7/17 (41.2%) |
| Overall pTME group. | NR | NR | NR | NR | NR | 34/34 (100%) | OM: 8/34 (23.5%)CD>III: 4/34 (11.8%)AL: NRMortality: 0/34 (0%) | 4/34 (11.8%) | ||
Summary of Salvage Surgery (sTME) Treatment for Local Recurrence Characteristics and Patient Oncological Outcomes.
| Author | Characteristics of Studies Included | Previous Stage (LE) | Final Stage (After sTME) | Neoadyuvant/adjuvant Chemo-radiotherapy | Re-LR (after sTME) | DM | CSS | DFS | OS | Morbidity | Stoma Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bikhchandani et al., 2015 | Retrospective cohort study- Date of collection data: 1997–2013 – Included patients sTME (n=27):pTis, pT1, pT2 | Tis=2Tx=2T1=16T2=7 | Tx=7Tis=1T2=3T3=10T4=4 | 12/27 (44.4%) CT before sTME13/27 (48.1%) RT before RT. | 3/27 (11.1%) | 6/27 (22.2%) | NR | 47% | 50% | OM: 12/27 (44.4%)CD>III: NRAL: 1/19 (11.1%)Mortality: 0/27 (0%) | 18/27 (66%) |
| Friel et al., 2001 | Retrospective study – Date of collection data: 1988–1999 – Included patients sTME (n=29): pT1, pT2 | T1=10T2=19 | Stage 0=1Stage I=1Stage II=13Stage III=12Stage IV=2 | 12/27 (44.4%) before sTME2/29 (6.8%) after LE5/29 (17.2%) after sTME | 5/29 (17.2%) | 8/29 (27.6%) | NR | 58% | NR | OM: NRCD>III: NRAL: NRMortality: NR | 17/29 (58.6%) |
| Borschwitz et al., 2008 | Retrospective comparative study- Date of collection data: 1985–2005- Included patients sTME (n=43): pTis, pT1, pT2 | pT1=24 | NR | 3/43 RT after sTME | 9/24 (37.5%) | 4/24 (16.6%) | 85% | 54% | NR | OM: 2/43 (4.6%)CD>III: NRAL: 1/43 (2.3%)Mortality: 0/43 (0%) | NR |
| pT2=19 | 7/19 (36.8%) | 6/19 (31.5%) | |||||||||
| Total=43 | 16/43 (37.1%) | 10/43 (23.25%) | |||||||||
| Weiser et al., 2005 | Retrospective cohort study – Date of collection data: 1970–2003 – Included patients sTME (n=50): pT1, pT2 | pT1 and pT2 | pT1-2=15pT3-4=34 | 22/50(44%) CRT after LE14/50 (28%) RT before sTME7/50 (14%) after sTME13/50 (26%) intraoperative RT | NR | NR | NR | NR | 53% | OM: 17/50 (34%)CD>III: NRAL: NRMortality: 0/50 (0%) | 32/50(46%) |
| Doornebosch et al., 2010 | Retrospective cohort study – Date of collection data: 1996–2010- Included patients sTME (n=16): pT1 | pT1 | T0=2Tis=1T1=0T2=2T3=11 | 11/16 (69%) RT before sTME. | 1/16 (6.2%) | 7/16 (43.7%) | 58% | NR | 31% | OM: NRCD>III: NRAL: NRMortality: 0/16 (0%) | 7/16 (43.7%) |
| Stipa et al., 2012 | Retrospective cohort study- Date of collection data: 1990–2011- Included patients sTME (n=26): pT1, pT2, pT3 | pT1=4pT2=5pT3=8 | NR | NR | NR | NR | NR | NR | 69% | OM: NRCD>III: NRAL: NRMortality: NR | 14/17(82.3%) |
| You et al., 2012 | Retrospective cohort study – Date of collection data: 1993–2011 – Included patients sTME (n=40): pT1, pT2, pT3, ypT2, ypT3 | pT1=21pT2=11pT3=3ypT2=7ypT3=4Total 46 | pT1-2N0=2pT3-4 N0=7Any T N+=1ypT0N0=3ypT1-2N0=1ypT3-4N0=7Any ypT N+=8 | 30/40 (75%) CRT pre-sTME | 9/40 (22.5%) | 15/40 (37.5%) | NR | 35% | 63% | OM: 20/46 (50%)CD>III: NRAL: NRMortality: 0/40 (0%) | 26/4066% |
cCRT: completion chemo-radiotherapy; CT: chemotherapy; RT; radiotherapy; NR: not reported; LR: local recurrence after cTME; DM: distant metastases; DSS: disease-specific survival; DFS: disease-free survival.
sTME: salvage surgery; CT: chemotherapy; RT; radiotherapy; NR: not reported; Re-LR: re-local recurrence after sTME; DM: distant metastases; DSS: disease-specific survival; DFS: disease-free survival; OS: overall survival; Morbidity: overall morbidity; CD> III: major postoperative morbidity Clavien-Dindo>III; AL: anastomotic leak; Mortality: considered as early-postoperative mortality,<30 days.
sTME: salvage surgery; CT: chemotherapy; RT; radiotherapy; NR: not reported; Re-LR: re-local recurrence after sTME; DM: distant metastases; DSS: disease-specific survival; DFS: disease-free survival; OS: overall survival; Morbidity: overall morbidity; CD> III: major postoperative morbidity Clavien-Dindo>III; AL: anastomotic leak; Mortality: considered as early-postoperative mortality,<30 days.
Quality assessment of the included studies: The data related to the qualitative assessment of the selected studies for PICO 3 are shown in the Appendix 3.
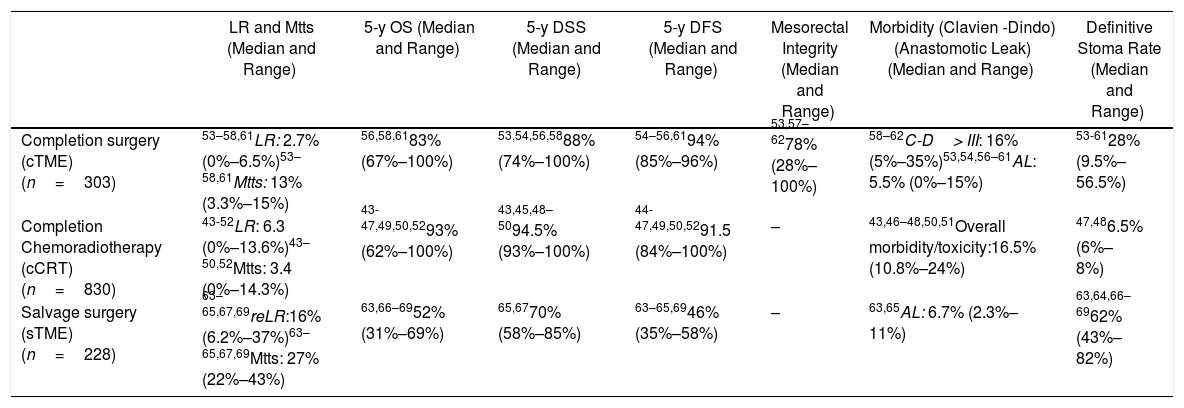
Primary outcome results: There were no comparative studies among cCRT vs. cTME vs. sTME; neither were comparative studies analysing cCRT and pTME. Nine studies compared cTME with historical series of pTME,53,59–62 but we did not consider it appropriate to pool the data due to their heterogeneity. In Table 2 we present a quantitative summary of the main outcomes of the analyzed studies for each of the three strategies.
Main Outcomes of the Studies Included in Each of the Three Strategies to Be Evaluated in the PICO 3 Question.
| LR and Mtts (Median and Range) | 5-y OS (Median and Range) | 5-y DSS (Median and Range) | 5-y DFS (Median and Range) | Mesorectal Integrity (Median and Range) | Morbidity (Clavien -Dindo) (Anastomotic Leak) (Median and Range) | Definitive Stoma Rate (Median and Range) | |
|---|---|---|---|---|---|---|---|
| Completion surgery (cTME) (n=303) | 53–58,61LR: 2.7% (0%–6.5%)53–58,61Mtts: 13% (3.3%–15%) | 56,58,6183% (67%–100%) | 53,54,56,5888% (74%–100%) | 54–56,6194% (85%–96%) | 53,57–6278% (28%–100%) | 58–62C-D> III: 16% (5%–35%)53,54,56–61AL: 5.5% (0%–15%) | 53-6128% (9.5%–56.5%) |
| Completion Chemoradiotherapy (cCRT) (n=830) | 43-52LR: 6.3 (0%–13.6%)43–50,52Mtts: 3.4 (0%–14.3%) | 43-47,49,50,5293% (62%–100%) | 43,45,48–5094.5% (93%–100%) | 44-47,49,50,5291.5 (84%–100%) | – | 43,46–48,50,51Overall morbidity/toxicity:16.5% (10.8%–24%) | 47,486.5% (6%–8%) |
| Salvage surgery (sTME) (n=228) | 63–65,67,69reLR:16% (6.2%–37%)63–65,67,69Mtts: 27% (22%–43%) | 63,66–6952% (31%–69%) | 65,6770% (58%–85%) | 63–65,6946% (35%–58%) | – | 63,65AL: 6.7% (2.3%–11%) | 63,64,66–6962% (43%–82%) |
n: total number of patients included in the studies; LR: local recurrence; Mtts: metastases; cTME: completion TME; *: The LR rate increases according to pT-stage; cCRT: completion chemoradiotherapy; sTME: Salvage radical TME for LR after local excision; re-LR: local re-recurrence; C-D: Claviend-Dindo classification for postoperative surgical complications; AL: anastomotic leakage.
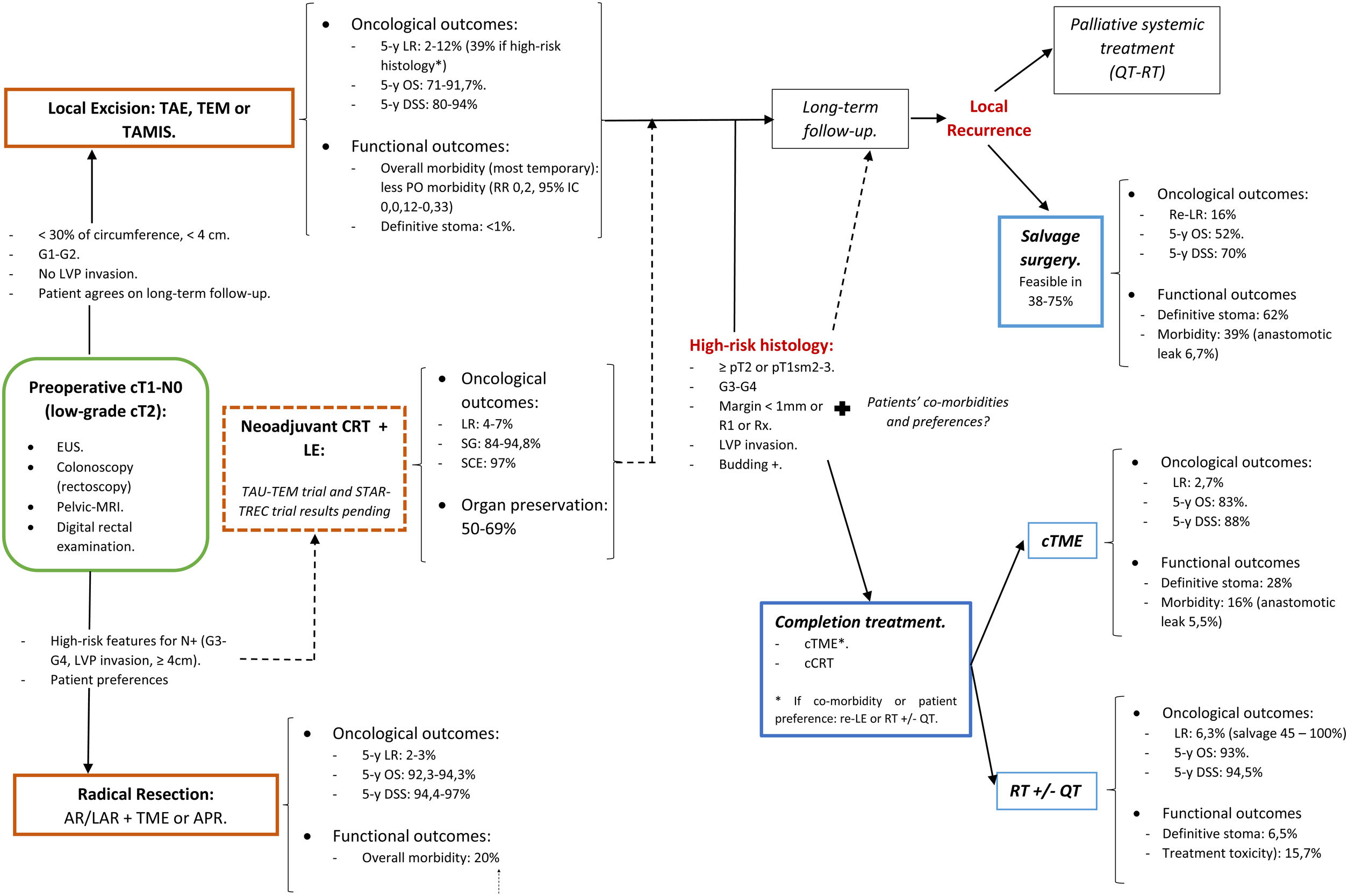
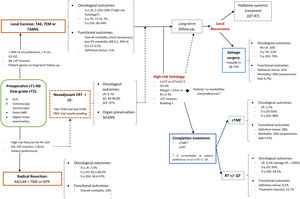
A structured model of the decision tree and the main outcomes is shown in Fig. 3.
DiscussionThis study provides an understanding of the key factors influencing therapeutic decision-making for early rectal cancer initially amenable to both TME surgery and LE.
The relative incidence of early rectal cancer has increased in recent years as a result of the extension of population screening programs for colorectal cancer. This early stage accounts for approximately 25% of all new diagnoses.70 As a consequence, a steady climb in rates of LE as a single modality therapy is being observed.22
Overall, evidence showed that LE has benefits in terms of less postoperative morbidity and fewer functional sequelae than radical surgery, in particular for distal rectal tumors. No studies have provided quality of life (QoL) data for meta-analysis, however, we have considered the “need for a permanent stoma” as an acceptable surrogate for QoL.
Although some observational studies showed mixed and almost non-significant differences in long term survival and mortality rate between LE and radical resection patients,3,6 this meta-analysis considering large population databases shows that LE is inferior to radical resection from an oncological standpoint. The greater difference in OS compared with DSS suggest the increased patient frailty in LE group may have contributed to mortality. This type of selection bias is common in population-based studies. Thus, when the study population is restricted to those under 45 years of age, as in the propensity matching study by Cao et al.,21 the difference in OS and DSS for pT1 lesions does not reach statistical significance. However, that population database (SEER) does not include record of LR, and because LR may be amenable to salvage surgery, differences in LR rates do not translate into differences in OS.
This meta-analysis confirms that LE results in significantly higher 5-year LR rate but similar distant metastasis rate in comparison with radical TME. Such local recurrence may be due to undiagnosed LN, vascular invasion, involved deep surgical margin or implantation of cancer cells at the time of LE. Therefore, LE risks missing occult disease due to both incomplete LN resection and missed opportunity for adjuvant treatment when involved LN or vascular invasion are present.
We found that fragmented specimens and LR appear to be lower among the more contemporary cohort of patients operated on with new surgical platforms than with traditional TAE. Thus, the superiority of endoscopic assistance (TEMS, TEO, TAMIS) compared with standard TAE is well established. Therefore, LE should be performed in an environment where any of the three analyzed surgical platforms are available. However, the availability of these platforms does not justify pushing the limits of indications for this approach. Without definitive predictors of high risk T1lesions who need TME, we may be doing some of our patients is disservice by offering a LE for a T1 lesion.71
Outlining the clinicopathological criteria that correlate with the incidence of LN spread should help determine whether LE should be accompanied by additional therapy. LN's are difficult to diagnose accurately before the operation based on their size, since at least 25% are reported to be 3mm or less in diameter.72 Several histological risk factors are associated with LN involvement. Unfortunately, many of those findings are not standardized analyzed or routinely reported in clinical practice. While tumor size alone is not an indicator of LN involvement, the rate of LN metastases does increases in parallel to the depth of invasion. A “1mm rule” of submucosal invasion is recommended as a criterion for further radical surgery by some guidelines.73 In addition to technical difficulties in measurement,74 no single pathological feature reliably predicts locoregional spread in isolation. And so, low-risk T2 tumors may have a lower rate of LN metastasis compared to T1 with poor histology.74,75 The relative influence of these risk factors is not well quantified and there is a need to develop a validated model incorporating a combination of histological and molecular features for the prediction of locoregional spread.75
Since patients with poor histology have even higher rates of LN metastases than previously thought,74 careful consideration of further therapy needs to be given to patients with these finding.
We found poor results for patients undergoing sTME for LR after LE, showing a clear decline in survival. Development of LR probably indicates the presence of an intrinsically more aggressive biology; this concept is also supported by the high incidence of metastatic disease after sTME. It is crucial to remind that development of LR seriously compromises patient’ survival even if sTME is feasible.
In contrast, we confirmed that main oncological outcomes and LR rate after cTME within 30 days interval, were similar to those after pTME. This may negate sphincter preservation and patients may instead opt for cCRT owing to the possibility of an organ-preserving strategy with no survival detriment and without incurring an unreasonable risk of LR. CRT after LE is associated with a trend toward a reduced rate of LR, even for high-risk disease.76 The toxicity of CRT needs to be taken into account and also communicated to the patient prior to any treatment decision.
There is a remarkable absence of studies focusing on functional outcomes after cCRT or cTME. Decision between cCRT and cTME in those cases is still under debate. Result of TESAR trial (NCT 02371304), a non-inferiority RCT investigating outcomes of cCRT vs cTME in patients with intermediate risk pT1-pT2 rectal carcinoma are expected to clarify these controversies.77
Of course, a review is only as reliable as the literature upon which it is based. So, our systematic review is limited by the quality of the existing studies, few of which were randomized. The vast majority were cohort studies, most of them retrospective in nature and heterogeneous. We appreciate that the methodology of meta-analysis with inclusion of many non-randomized studies may carry a degree of bias.
In summary, for patients with cT1, cN0, cases should be discussed by multidisciplinary team to determine optimal management with respect to the risk of LR, avoidance of a stoma, and fitness for surgery. LE can be considered sufficient provided that the tumor can be removed as a single full-thickness specimen with clear margins and that the treating surgeon counsels the patient that: (1): The risk of LR increased as the depth of tumor invasion increases, although the optimum risk stratification has yet to be defined; (2) Further completion surgery or CRT may be required after histopathological review of the LE specimen; so, patients initially managed with LE might not avert functional disorders or permanent stoma creation; and (3) Reliance on pathological features alone may not reliable assure the absence of lymph node metastases, therefore, a subsequent surveillance program is required.72
Decision-making is more complex than considering tumor stage alone. The location of the cancer within the rectum is also a critical determinant. Early rectal cancer cannot be considered as a homogeneous group. So, this decision analysis cannot prescribe a radical TME or a LE for each patient with low T1 rectal cancer. Moreover, the patient's values must be included in decision-making. Again, a statistical difference does not equal to clinical relevance. Different patients will value weighing between the loss of QoL and the risk of developing a LR differently. In this sense, a multicriteria decision-making tool able to classify and select alternatives based on a hierarchical structure might be explored.78
Conflict of InterestsThe authors declare that they have no conflict of interest.