The PICS-AF™ (Curaseal Inc.) device is a new plug made of collagen that has a retention system in the internal orifice. This pilot study was designed to assess both the feasibility and safety of this plug in the treatment of trans-sphincteric anal fístulas.
MethodsA total of 44 patients (34 men), with a mean age of 54.68±7.3, with trans-sphincteric anal fístulas were included in the study; 34 of them were analyzed. All patients were examined according to a strict preoperative protocol and until 6 months after surgery. The feasibility of the procedure and the adverse events were analyzed.
ResultsFinally, 34 patients were operated on, and in 30 of them the plug was used. Therefore, the feasibility was calculated at 88%. There was a total of 16 adverse events, 4 recorded as not related (3 mild and one moderate) and 12 related to the procedure or to the device implanted. Of these, 5 were mild, 5 moderate and 2 severe. The majority of the events reported were related to proctalgia (4 patients) or infection at the implant site (4 patients).
ConclusionsThe present study indicates that the new collagen plug can be placed effectively and with an acceptable complication rate.
El dispositivo PICS-AF ™ es un nuevo tapón hecho de colágeno que tiene un sistema de retención en el orificio fistuloso interno. Este estudio piloto ha sido diseñado para evaluar la factibilidad y seguridad de este dispositivo en el tratamiento de las fístulas anales criptoglandulares transesfinterianas.
MétodosUn total de 44 pacientes (34 hombres) con diagnóstico de fístula transesfinteriana fueron incluidos en el estudio, de los cuales 34 fueron seleccionados. Todos los pacientes fueron examinados según un protocolo estricto antes de la cirugía y hasta 6 meses después. Se analizaron la factibilidad del procedimiento y los acontecimientos adversos.
ResultadosEl dispositivo se colocó sin incidencias en 30 de los 34 pacientes (factibilidad del 88%). Se evidenciaron un total de 16 acontecimientos adversos, 4registrados como no relacionados con el procedimiento (3 leves y uno moderado) y 12 relacionados con el procedimiento o el dispositivo implantado. De ellos, 5fueron leves, 5moderados y 2graves. La mayoría de los efectos adversos reportados fueron proctalgia (4 pacientes) o infección en el sitio del implante (4 pacientes).
ConclusionesEl presente estudio indica que el nuevo tapón de colágeno puede ser colocado de forma efectiva y con una tasa de complicaciones aceptable.
Cryptoglandular anal fistulae are one of the most common anorectal diseases and possibly involve over 30% of coloproctological interventions.1 Today, the treatment of complex fistulae continues to be a real challenge for surgeons, as they must preserve continence while eradicating the suppurative process.2 It is important to mention that neither the results nor the complications after surgery have changed in the last 25 years, regardless of the surgical technique used.3–5
Among the existing procedures for the treatment of anal fistulae, we should highlight the use of plugs to seal the fistulous tract, which are made from lyophilized porcine submucosa. Although initial studies had exceptional results, they have not been reproduced by other groups.6,7
The PICS-AF™ device (CuraSeal Inc.) is a new plug made of collagen that has an internal system to brace it against the internal orifice and a less rigid conformation, designed to resolve the problems of plugs currently available on the market.
The main objective of this study was to evaluate both the feasibility as well as the safety of this device in the treatment of anal fistulae.
MethodsThis is a prospective analysis of a consecutive series of patients, whose inclusion period was May and June 2016. The study was promoted by CuraSeal Inc. in order to obtain FDA approval.
A total of 44 patients (34 men) were evaluated during screening, with a mean age of 54.7±7.3 years, who had complex cryptoglandular fistulae (medium and high transsphincteric fistula, horseshoe fistula, recurrent fistulae, or fistula in a patient with some degree of incontinence). Excluded from the study were those patients with hypersensitivity or allergy to any component of the device, those with 2 or more external fistulous orifices, collections larger than 2cm associated with the fistula, Crohn's disease, pregnancy, or those older than 75 or younger than 18.
The study was approved by the Ethics Committee at the Virgen del Rocío Hospital. All patients signed an informed consent form.
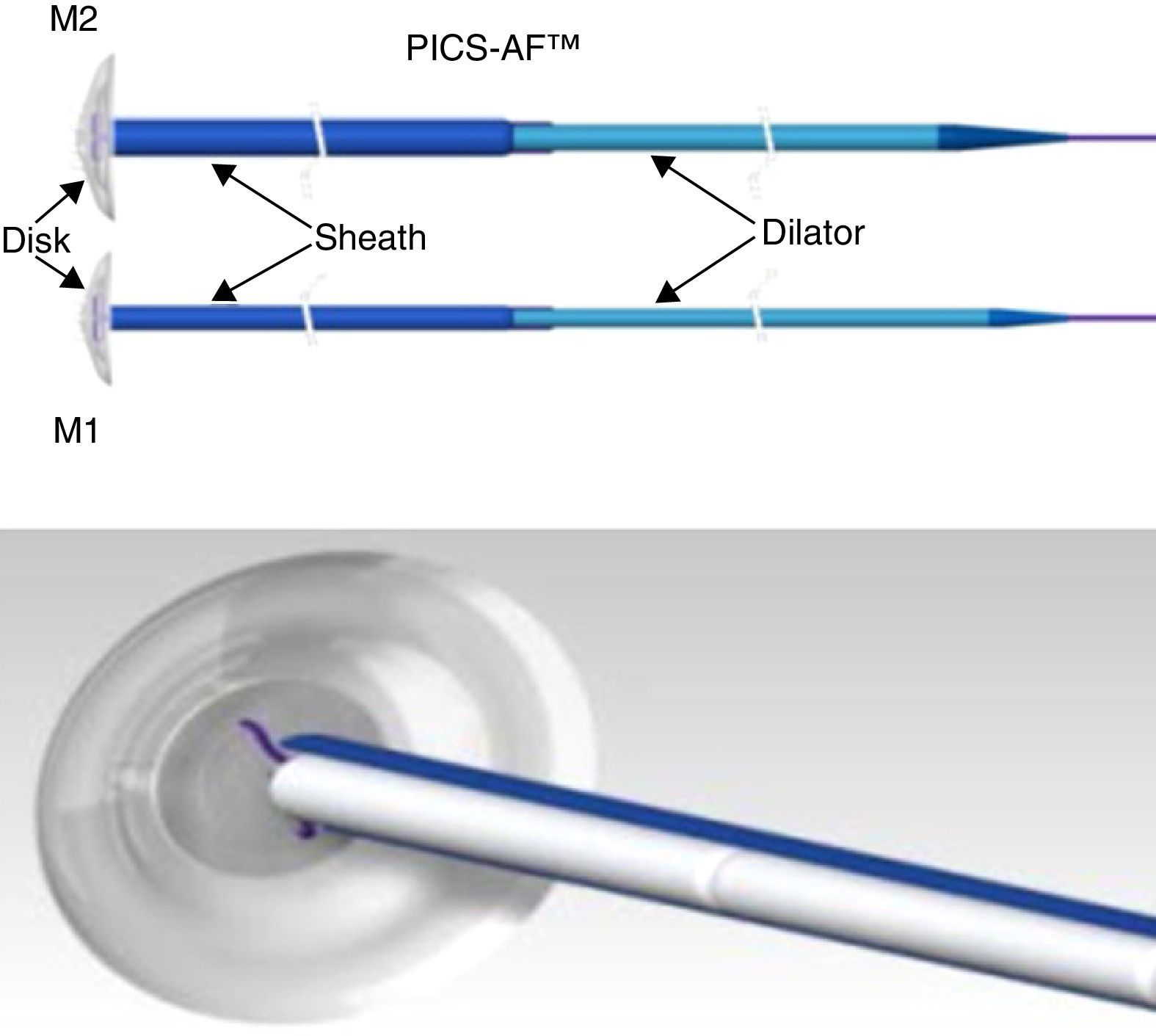
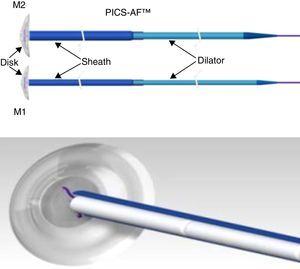
The CuraSeal® Percutaneous Intraluminal Closure System for Anorectal Fistulas (PICS-AF™) consists of a silicone disk and an insertion catheter holding the collagen matrices. There are 2 models (M1 and M2) with different disk diameters. The size of the device selected is based on the size of the internal and external orifices (Fig. 1).
Image of the PICS-AF™ device, models 1 and 2. Model 1 has a diameter of 1.3cm and a thickness of 2.1mm; the sheath measures 2.5mm, which means that the collagen strands measure up to 6.4mm each. Meanwhile, model 2 has a diameter of 1.6cm, a thickness of 2.3mm; the sheath measures 3.6mm and each collagen strand measures up to 9.4mm.
The disk stays in the internal fistulous orifice, while the collagen matrices remain in the fistulous tract. The disk is expelled with defecation after a few days, or it can be easily removed if necessary. The collagen matrix acts as scaffolding and promotes the sealing of the fistula. The complete absorption process takes from 3 to 6 months.
As for the surgical procedure, all patients underwent bowel preparation 24h prior to the procedure (requirement established by the study sponsor) with disodium phosphate dodecahydrate and monosodium phosphate dihydrate (Fosfoevac oral solution, Laboratorios Bohm, Madrid, Spain).
Preoperative antibiotics were administered, specifically one iv dose of 2g of amoxicillin-clavulanic acid. Also, in the operating room rectal lavage was performed with saline and povidone-iodine solution. Spinal anesthesia was used in all patients.
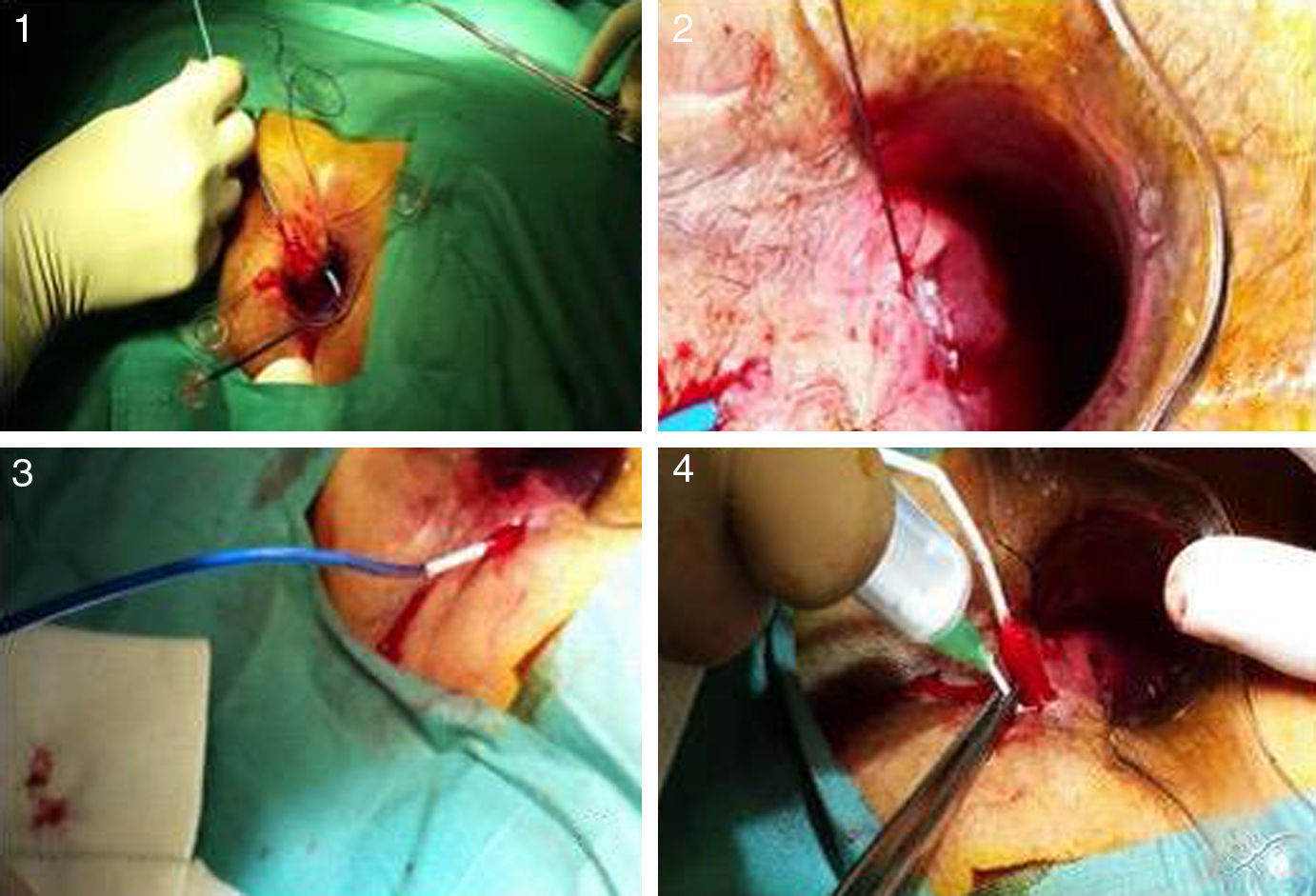
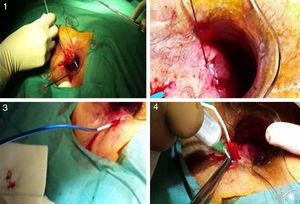
After catheterization of the tract, intense debridement was performed with a brush and saline. The catheter with the matrices was then inserted from the internal to the external orifice, and the silicone disk was anchored to the internal orifice with two 3.0 absorbable sutures. Afterwards, the device was hydrated with gentamycin and the procedure was completed with the elimination of the external orifice (Fig. 2).
Photographs of the insertion procedure: (1) insertion of the complete device through the internal orifice, which comes out the external orifice; (2) anchoring of the silicone button to the internal orifice; (3) withdrawal of the sheath, allowing the collagen to remain in the tract; (4) instillation of gentamicin in the collagen, which expands with hydration.
All patients received antibiotic treatment with oral ciprofloxacin and metronidazole during the first week post-op, as well as analgesia with paracetamol.
For later assessment, we preoperatively recorded the associated comorbidities and clinical characteristics of the fistulae treated, as well as the progression timeline and previous treatments. A quality-of-life test (Jorge-Wexner's score8) was also used to evaluate the degree of continence, and pain was assessed using the Visual Analogue Scale (VAS). In all patients, endoanal ultrasound and nuclear magnetic resonance studies were carried out before the procedure in order to determine the type of fistula and rule out associated abscesses.
In the postoperative period, in addition to the previous data, we assessed the ability to perform the procedure (feasibility) and all adverse events (AE) related to the device or the placement process (safety data), as well as unrelated events.
All AE were coded and classified as mild, moderate or severe. The following classification criteria were used: mild AE are noticed by the patient, but do not interfere with his or her life; moderate AE cause discomfort and interfere with normal life; severe AE severely limit the patient's ability to perform routine tasks and require treatment of symptoms. Furthermore, severe AE may directly endanger the patient's life (Spanish Royal Decree 223/2004 from 6 February regulating clinical drug trials).
AE requiring hospitalization for more than 24h was reported to the promoter immediately. All patients were followed up systematically in the outpatient consultation one week, 2 weeks and 1, 2, 3 and 6 months after surgery.
Mild proctalgia was defined as that which could be adequately controlled with the administration of analgesia and lasted less than 2 weeks. Moderate proctalgia required analgesia for more than 2 weeks. Severe proctalgia could not be controlled with analgesics and required additional procedures (such as removal of part of the device).
Infection was determined by suppuration through the anus or the external wound, in association with the presence of cellulitis in the implant area, within 30 days after the device was implanted. Abscess of the tract was identified when the external orifice was closed, but there was hardening or fluctuation in the implant area, possibly requiring surgical drainage or spontaneous drainage.
Statistical AnalysisThe statistical analysis was performed with IBM® SPSS® Statistics 19 software. Continuous variables have been reported using standard statistical measurements, including the number of observations, number of missing observations, average, standard deviation, minimum and maximum value, mean and median. Categorical variables have been summarized in frequency tables.
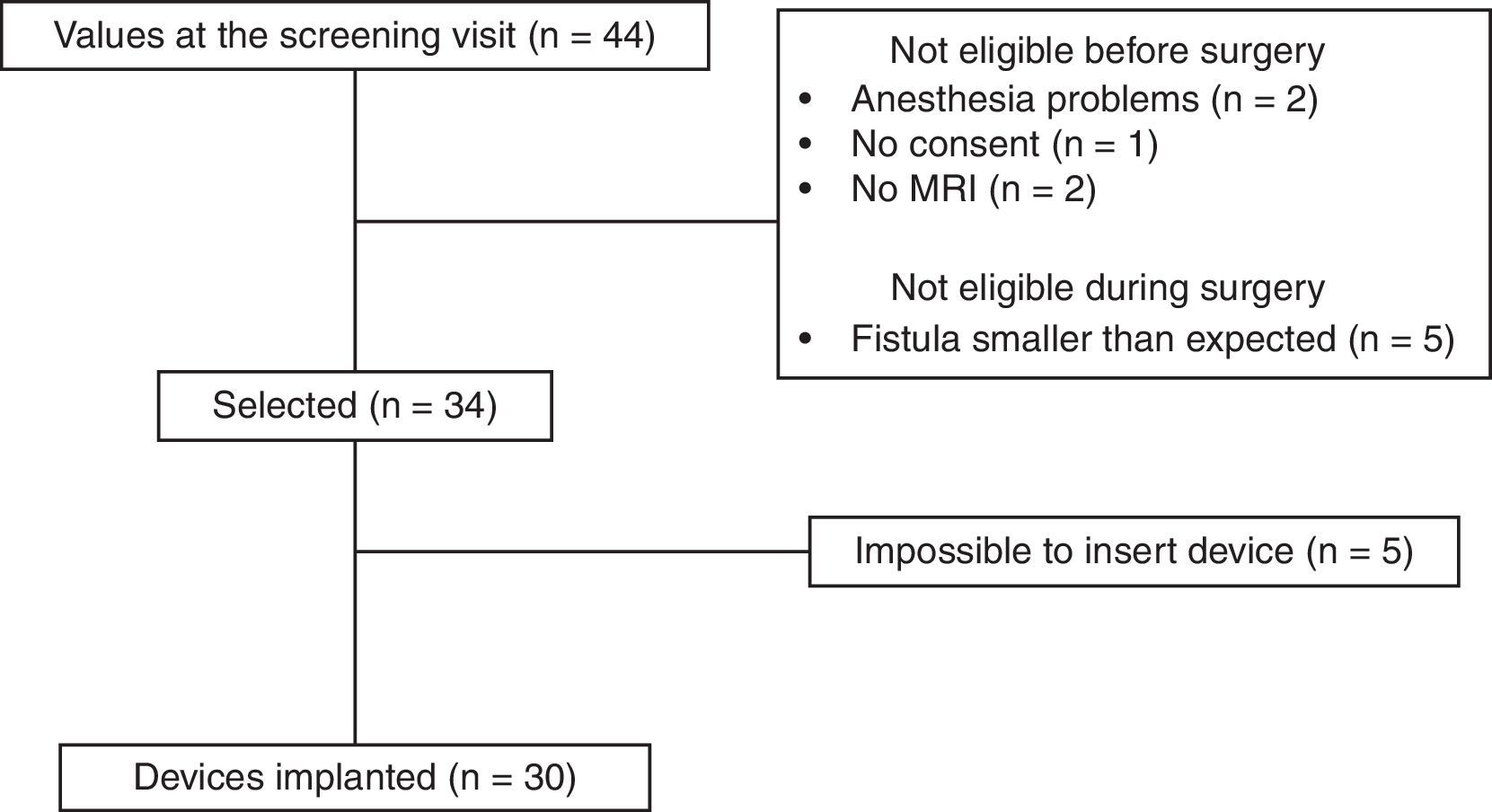
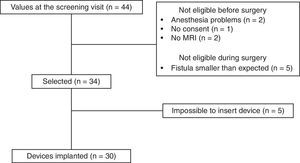
ResultsAfter applying the inclusion and exclusion criteria, 34 patients were finally included in the study, as 10 patients were classified as ineligible. Five were excluded before surgery (2 due to contraindication for anesthesia, 2 for inability to perform a magnetic resonance imaging study, and one for withdrawal of consent). Five patients were also excluded in the operating room because the fistula was less complex than observed during the previous clinical examination and complementary studies (Fig. 3).
Fifty percent of patients (15/30) had required prior abscess drainage; each (15/30) had had a seton placed for more than 6 months. Previous treatment for fistula had been used in 90% of patients (27/30), including fistulotomy 33.3% (10/30), fistulectomy 13.3% (4/30), sealing of the tract 36.7% (11/30) and flap 6.7% (2/30).
In 30 of the 34 patients, the procedure established by the protocol was able to be performed, so the feasibility rate was 88%. No intraoperative complications occurred in any of the patients. All patients were treated with an M1 model device and were discharged within 24h of the procedure. No patients were lost to follow-up.
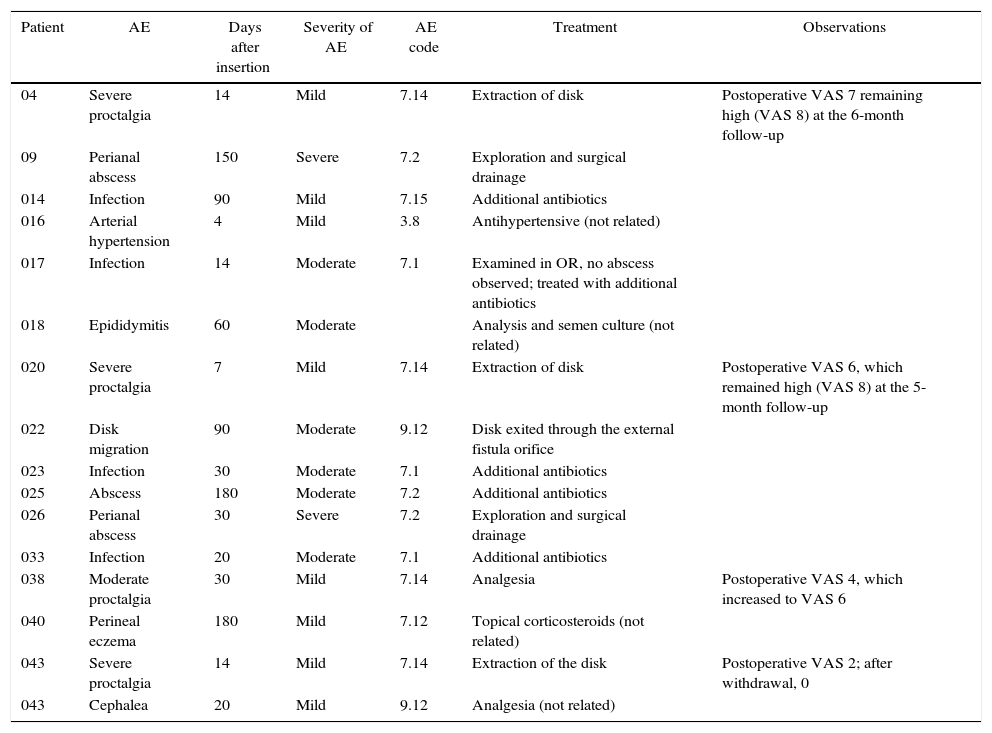
There were a total of 16 AE in 15 patients: 4 were classified as unrelated (hypertension, headache, epididymitis and perineal eczema) and 12 were related to the procedure or to the implanted device. Of these, 5 were mild, 5 moderate and 2 severe. The most frequent AE were proctalgia (4 AE) and infection of the implant site (4 AE), which were controlled with medical treatment. Two patients required drainage of an abscess in the operating room, followed by hospitalizations of less than 24h. Table 1 shows the AE that occurred.
Adverse Events.
| Patient | AE | Days after insertion | Severity of AE | AE code | Treatment | Observations |
|---|---|---|---|---|---|---|
| 04 | Severe proctalgia | 14 | Mild | 7.14 | Extraction of disk | Postoperative VAS 7 remaining high (VAS 8) at the 6-month follow-up |
| 09 | Perianal abscess | 150 | Severe | 7.2 | Exploration and surgical drainage | |
| 014 | Infection | 90 | Mild | 7.15 | Additional antibiotics | |
| 016 | Arterial hypertension | 4 | Mild | 3.8 | Antihypertensive (not related) | |
| 017 | Infection | 14 | Moderate | 7.1 | Examined in OR, no abscess observed; treated with additional antibiotics | |
| 018 | Epididymitis | 60 | Moderate | Analysis and semen culture (not related) | ||
| 020 | Severe proctalgia | 7 | Mild | 7.14 | Extraction of disk | Postoperative VAS 6, which remained high (VAS 8) at the 5-month follow-up |
| 022 | Disk migration | 90 | Moderate | 9.12 | Disk exited through the external fistula orifice | |
| 023 | Infection | 30 | Moderate | 7.1 | Additional antibiotics | |
| 025 | Abscess | 180 | Moderate | 7.2 | Additional antibiotics | |
| 026 | Perianal abscess | 30 | Severe | 7.2 | Exploration and surgical drainage | |
| 033 | Infection | 20 | Moderate | 7.1 | Additional antibiotics | |
| 038 | Moderate proctalgia | 30 | Mild | 7.14 | Analgesia | Postoperative VAS 4, which increased to VAS 6 |
| 040 | Perineal eczema | 180 | Mild | 7.12 | Topical corticosteroids (not related) | |
| 043 | Severe proctalgia | 14 | Mild | 7.14 | Extraction of the disk | Postoperative VAS 2; after withdrawal, 0 |
| 043 | Cephalea | 20 | Mild | 9.12 | Analgesia (not related) |
AE: adverse events.
In one patient, the silicone disk migrated into the fistulous tract and exited through the external fistulous orifice. The disk had to be removed due to pain in one patient 7 days after surgery and in 2 patients after 14 days. The device removal was conducted in the outpatient consultation by means of digital rectal examination. The rest of the patients reported disk expulsion with defecation prior to the 3-month follow-up visit.
DiscussionSince 2006, when the idea of using collagen plugs to occlude the fistula tract without compromising continence was first introduced, not many changes have been made to these devices.6,7
The first device created was the Surgisis (COOK Biotech, West Lafayette, IN), made out of collagen from lyophilized porcine intestinal submucosa (which was, a priori, resistant to infection) did not generate a foreign-object reaction and led to cell repopulation approximately 3 months after implantation.9 This compact device was conical in shape, and its insertion in the fistulous tract of the anal canal theoretically provided stability and avoided migration.
Nonetheless, the surprisingly high cure results could not be reproduced in subsequent studies. Therefore, current cure rates average around 30%.10 Moreover, the possibility of migration of these devices forced them to be redesigned or the placement technique to be modified, requiring internal fixation or even a recommended mucosal flap performed concomitantly.11,12
What is striking is that there are no feasibility and safety studies for these plugs available on the market, in spite of the years passed since their possible efficacy was first reported.6,7,9
The PICS-AF™ collagen device provides the ability to be anchored to the internal orifice by means of a silicone disk, which facilitates closure and complete blockage of the internal orifice, thereby impeding the passage of fecal content to the sealed fistula tract. Furthermore, it is not a compact device; instead, it is fragmented in order to better adapt to the fistulous tract, even if it is not rectilinear. In addition, it has absorbable threads that facilitate anchoring the device to the outside in order to avoid displacement.
The feasibility analysis performed in our study shows that it is possible to place it without difficulty in the majority of patients (88%), with no intraoperative or immediate postoperative complications, and hospital discharge is achieved in all patients in less than 24h. If we look closely at those in which it could not be implanted, we see that it was not due to problems with the device or the placement technique, but to the impossibility of locating the internal orifice, which can occur in up to 7% of cases.13
Most of the AE described are mild to moderate and are related to pain or infection of the device, both of which can be controlled with medical treatment. We have attributed this pain to the anchoring of the disk in the internal orifice, since most patients reported it inside the anus, and also because the pain ceased after withdrawal or spontaneous expulsion.
Early infection of the device may be due to filtration of feces through the internal orifice or to contamination due to poor tract curettage, despite have been done vigorously, and to the concomitant use of a gentamicin solution.
We should recommend the removal of the disk between the second and third months, if the patient has not already expelled it, in order to avoid migration inside the fistulous tract. Keep in mind that if it is removed before this time, when there is still insufficient cellular colonization of the device, it can become loosened and expelled.
Another of the observed AE (although only in one patient) was the existence of an ulcer in the anal canal, which we think may be due to the erosion and subsequent ulceration caused by the disk. This fact can also be prevented by avoiding leaving the disk in the anal canal for a prolonged period.
In conclusion, the placement of the PICS-AF™ plug is a simple and feasible procedure. However, its use is not free of complications, although most related complications were mild. Considering the limitations imposed by the type of study and the relatively small number of patients, more data are needed to more exactly determine the efficacy and safety of this device.
FundingThis study was sponsored by CuraSeal Inc. (clinicaltrials.gov ID NCT02456324).
Authorship/CollaborationsF. de la Portilla was responsible for the study concept and design, as well as the interpretation and design of the article.
M.L. Reyes and M.V. Maestre were responsible for the data collection and analysis. M.L. Reyes also actively participated in the composition of the article.
A.M. García-Cabrera, J.M. Díaz-Pavón, J.M. Vázquez-Monchul and R.M. Jiménez-Rodríguez have provided essential contributions to the development of the study.
J.A. Villanueva has actively participated in the systematic compilation of adverse events and their classification. He also participated in the study design, in representation of the sponsor.
Conflict of InterestsThe team of researchers has no conflict of interests to declare.
Please cite this article as: de la Portilla F, Reyes-Díaz ML, Maestre MV, Jiménez-Rodríguez RM, García-Cabrera AM, Díaz-Pavón JM, et al. Estudio de factibilidad y seguridad del plug de colágeno (PICS-AF™) en el tratamiento de la fístula anal criptoglandular. Cir Esp. 2017;95:208–213.
This study was previously presented at the National Coloproctology Conference in Elche, Spain, May 2016.