Surgical treatment of hilar cholangiocarcinoma remains a challenge. Multiple prognostic factors have been proposed. The number of positive nodes and the ratio between positive lymph node and total lymph node (G+/Gt) are considered by some authors as the most important factor.
Materials and methodsWe analysed a series of 58 patients with Klatskin tumours. We evaluated the prognostic factors and survival with emphasis on the prognostic impact of the number of positive nodes and its relation to total lymph nodes.
ResultsResectability was 78% with a 5-year survival of 32%. The median number of nodes examined was 9.5. No significant differences were found in several of the proposed prognostic factors. The presence of 2 or more positive nodes or a ratio G+/Gt≥0.2 were found to be poor prognostic factors.
ConclusionThe relationship between positive lymph nodes and total lymph nodes and the number of positive lymph nodes are important prognostic factors.
El tratamiento quirúrgico del colangiocarcinoma hiliar representa un verdadero desafío. Múltiples factores pronósticos han sido propuestos. El número de ganglios positivos y la relación ganglios positivos y ganglios totales (G+/Gt) son considerados por algunos autores como los más importantes.
Material y métodoSe analiza una serie de 58 pacientes con tumores de Klatskin. Se evalúan los factores pronósticos y la supervivencia con especial interés en el impacto pronóstico del número de ganglios positivos y su relación con los ganglios totales.
ResultadosLa resecabilidad fue de 78% con una sobrevida a 5 años del 32%. La mediana de ganglios estudiados fue de 9,5. No se encontraron diferencias significativas en varios de los factores pronósticos analizados. La presencia de 2 o más ganglios positivos o una relación G+/Gt≥0,2 resultaron ser factores de mal pronóstico.
ConclusiónLa relación entre ganglios positivos sobre los ganglios totales y el número de ganglios positivos son factores pronósticos importantes.
Hilar cholangiocarcinoma or Klatskin's tumour is a rare neoplasia and its surgical treatment poses a major challenge. Radical resection is the only chance of cure1,2 for these patients. A great many prognostic factors have been assessed and proposed with regard to survival.
Lymph node involvement in tumours of the digestive system (pancreas, colon, stomach, etc.) is a significant predictive factor for survival.3–9 Lymphatic involvement in Klatskin tumours is also a significant prognostic factor.10–13 Furthermore, the ratio between positive and total lymph nodes (G+/Gt) has been mentioned as a strong survival predictor by some authors, although there are few studies in this regard.14,15
The objective of this work was to assess the lymph node status of patients who were treated for hilar cholangiocarcinoma by resection, and to corroborate in our study whether the ratio G+/Gt is indeed a prognostic factor for survival.
Materials and MethodsA series of patients with diagnosed Klatskin tumour were studied jointly (Spain and Argentina), operated on in 2 hospitals by only 2 surgeons (JF and GAN) and treated between 1998 and 2012.
The general data assessed were, age, whether or not they presented diabetes, liver disease or cholangitis, whether they had undergone preoperative biliary drainage or whether they had required embolisation, and the resectability rate. Biliary drainage was indicated in patients who exceeded 15mg bilirubinaemia. Preoperative portal embolisation was indicated in patients with a future remnant liver volume of less than 30% measured by liver volume calculation using CT.
The Bismuth-Corlette16 classification was used for locating the tumour, the different surgical techniques used and whether resection of the caudate lobe was included in the major hepatectomies. The same surgical indication criteria were used in both centres. In the case of the liver resections, the nomenclature used was as suggested and published on the website of the International Hepato-Pancreato-Biliary Association in Brisbane.17 Lymphadenectomy included the lymph nodes of the common hepatic artery and of the liver pedicle, including the retroportal and retropancreatic lymph nodes. Neither Kocher's manoeuvre or interaortocaval lymphadenectomy were performed. The tendency in recent years has been to include the lymph nodes of the coeliac trunk.
Vascular clamping, associated venous or arterial resection and transfusion requirements were also assessed. Morbidity was classified according to the Dindo-Clavien18 scale.
Death within 90 days following surgery was considered perioperative mortality. The hospital stay in days, the reoperation rate and readmittance to hospital were also studied. In resected patients the type of resection performed was assessed (R0, R1, R2). Tumours were staged according to the UICC TNM classification.19 The level of differentiation of the tumour and whether there was any perineural spread were analysed. All the patients were followed up by the attending surgeon and blood tests and ultrasound scans were performed every 3 months in the first year and subsequently every 6 months up to five years. Recurrence and survival were assessed in months.
StatisticsThe data were analysed using SPSS software (version 13, SPSS, Inc, Chicago, IL, USA). The survival time was calculated from the time of surgery until death or until the date of the patient's last follow-up check.
The quantitative variables are expressed as: mean (SD) if they presented a “normal” distribution or otherwise as median and range. Qualitative variables are presented in absolute numbers and percentages. Survival was calculated using the Kaplan–Meier method and the differences in survival curves were compared using the log-rank test. Patients with perioperative mortality were excluded from the analysis of prognostic factors. The level of differentiation (poor, moderate and non-differentiated), hepatectomy vs resection of the isolated biliary tract, transfused vs non-transfused, number of lymph nodes resected (more or less than 5 and more or less than 7), number of positive lymph nodes, and the G+/Gt ratio were assessed as prognostic factors. P<.05 was considered significant. An analysis was made of the predictive factors of survival to distinguish between those which were explanatory or causal and of the likelihood of confusion factors using Cox regression analysis. Factors such as P<.1, or any that we considered significant as researchers were included in the multivariate analysis.
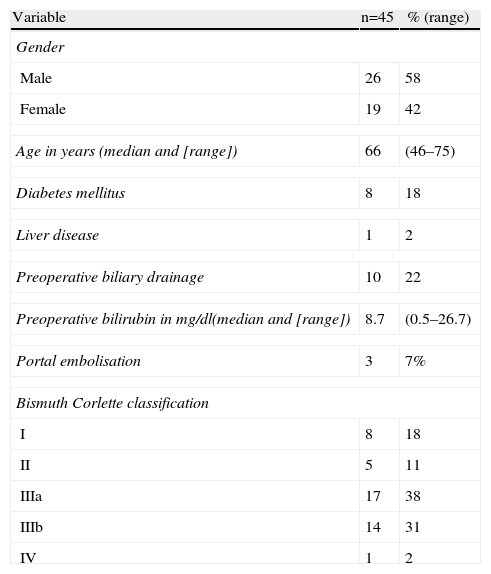
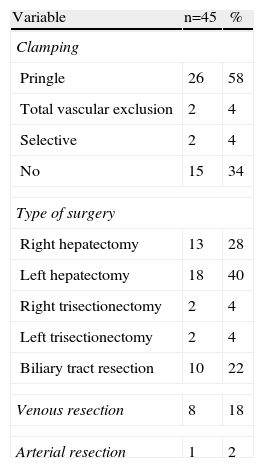
ResultsA total of 58 patients with hilar cholangiocarcinoma were included in this study between 1998 and 2012. Of the 58 patients, 13 were considered unresectable or inoperable preoperatively (n=9) or during surgery (n=4); in the latter a transtumoral intubation technique was used. The remaining 45 were resected, which represents a 78% resectability rate. The epidemiological data of the 45 patients are shown in Table 1. The majority were males and the median age was 65. Preoperative biliary drainage was performed on a fourth of the patients because of jaundice. Portal embolisation was performed on 3 patients. Preoperative staging using the Bismuth–Corlette classification showed that the majority were stage III, similarly distributed between the right and left biliary tracts.
Epidemiological Data, and Preoperative Study.
| Variable | n=45 | % (range) |
| Gender | ||
| Male | 26 | 58 |
| Female | 19 | 42 |
| Age in years (median and [range]) | 66 | (46–75) |
| Diabetes mellitus | 8 | 18 |
| Liver disease | 1 | 2 |
| Preoperative biliary drainage | 10 | 22 |
| Preoperative bilirubin in mg/dl(median and [range]) | 8.7 | (0.5–26.7) |
| Portal embolisation | 3 | 7% |
| Bismuth Corlette classification | ||
| I | 8 | 18 |
| II | 5 | 11 |
| IIIa | 17 | 38 |
| IIIb | 14 | 31 |
| IV | 1 | 2 |
The intra-operative results can be seen in Table 2. Most of the patients underwent hepatectomy (78%). Ten patients underwent biliary tract resection, lymphadenectomy and reconstruction using hepaticojejunostomy alone. It was necessary to perform hilar clamping or total vascular exclusion on most of the patients. Resection of the portal vein and anastomosis were associated in 8 cases. The right hepatic artery was resected in only one patient with T–T anastomosis.
Operative Data.
| Variable | n=45 | % |
| Clamping | ||
| Pringle | 26 | 58 |
| Total vascular exclusion | 2 | 4 |
| Selective | 2 | 4 |
| No | 15 | 34 |
| Type of surgery | ||
| Right hepatectomy | 13 | 28 |
| Left hepatectomy | 18 | 40 |
| Right trisectionectomy | 2 | 4 |
| Left trisectionectomy | 2 | 4 |
| Biliary tract resection | 10 | 22 |
| Venous resection | 8 | 18 |
| Arterial resection | 1 | 2 |
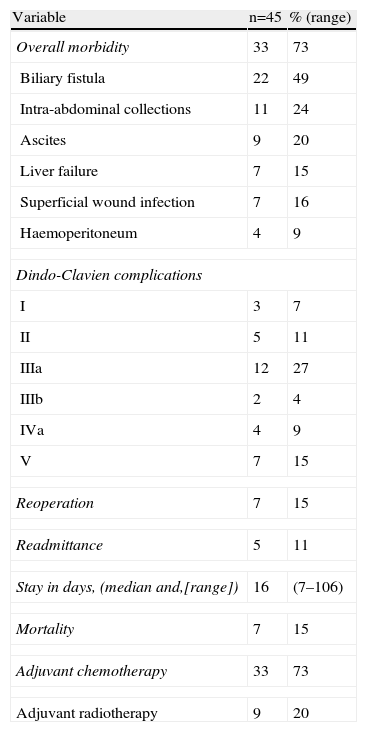
The postoperative results can be seen in Table 3. The most frequent complication was biliary fistula followed by intra-abdominal collection. Eight patients presented mild complications according to the Dindo-Clavien classification. Some form of intervention was necessary to solve complications in 14 patients. The complications were severe in 4 patients and they had to be admitted to ICU, and 7 patients died. All the patients with advanced tumour ≥pT3 or with positive lymph nodes received chemotherapy and adjuvant radiotherapy.
Postoperative Evolution.
| Variable | n=45 | % (range) |
| Overall morbidity | 33 | 73 |
| Biliary fistula | 22 | 49 |
| Intra-abdominal collections | 11 | 24 |
| Ascites | 9 | 20 |
| Liver failure | 7 | 15 |
| Superficial wound infection | 7 | 16 |
| Haemoperitoneum | 4 | 9 |
| Dindo-Clavien complications | ||
| I | 3 | 7 |
| II | 5 | 11 |
| IIIa | 12 | 27 |
| IIIb | 2 | 4 |
| IVa | 4 | 9 |
| V | 7 | 15 |
| Reoperation | 7 | 15 |
| Readmittance | 5 | 11 |
| Stay in days, (median and,[range]) | 16 | (7–106) |
| Mortality | 7 | 15 |
| Adjuvant chemotherapy | 33 | 73 |
| Adjuvant radiotherapy | 9 | 20 |
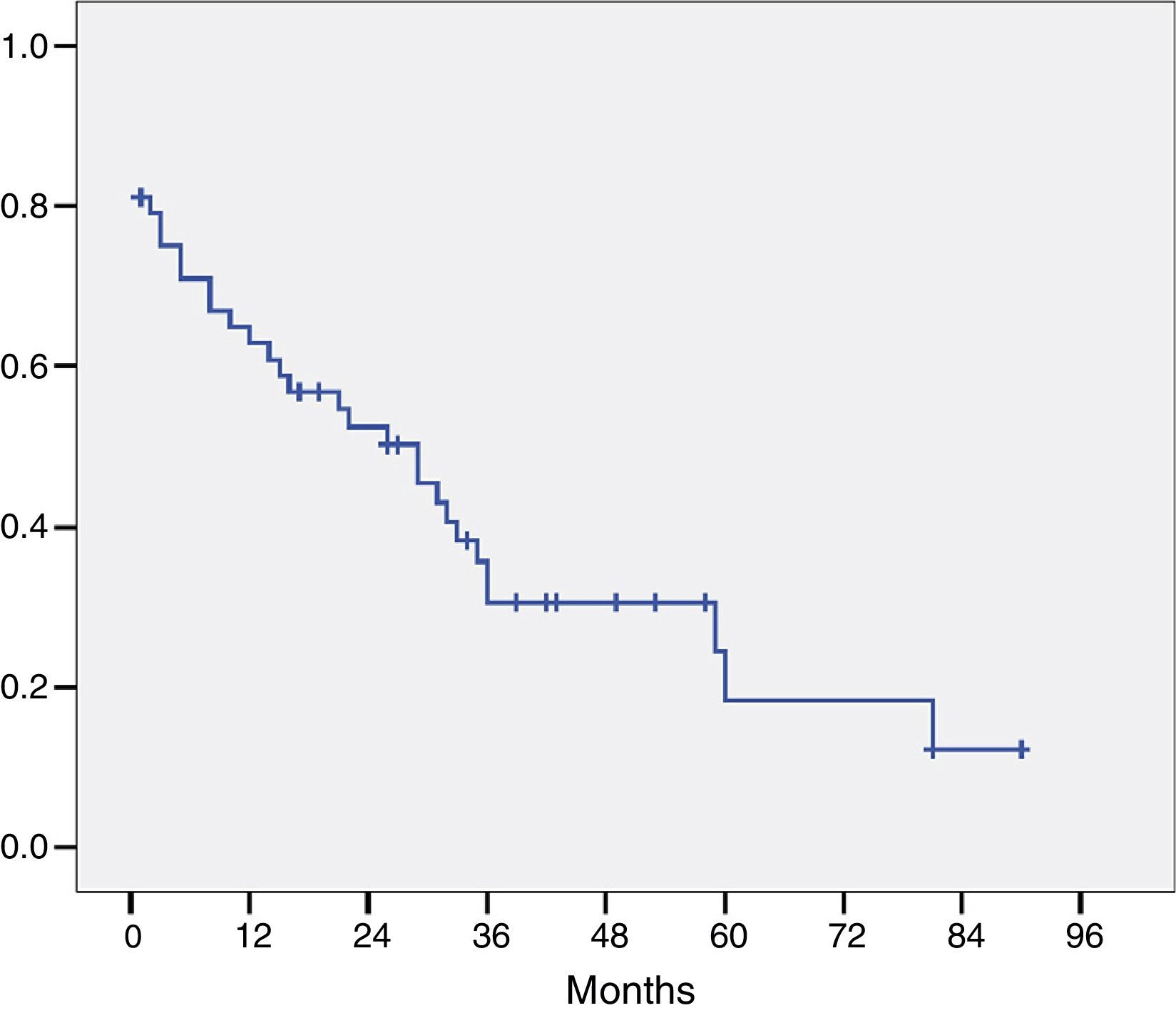
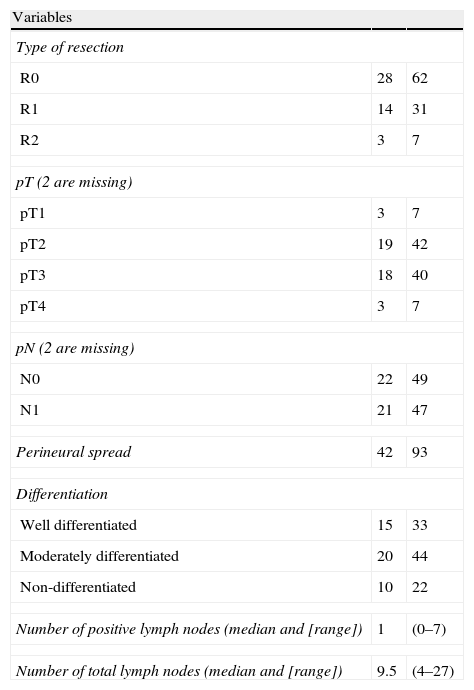
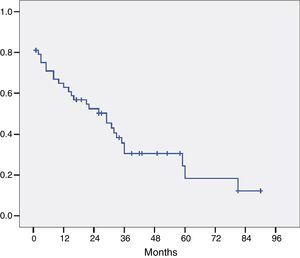
The histological study of the resection specimens is shown in Table 4. Three patients presented with macroscopic invasion of the margin (R2) and in 15 patients the microscopic margin was less than 1mm and they were considered R1 for the prognostic factor analysis. The most common histological stage was pT2 or pT3 and in 21 patients the extracted lymph nodes were positive for adenocarcinoma. Most of the tumours were moderately differentiated. The median of positive lymph nodes was 1 (range 0–7). The median lymph nodes studied per patient was 9.5 (range 4–27). Overall survival, including postoperative mortality was 77%, 35%, and 21% at 12, 36 and 60 months, respectively, with a median survival of 31 months (Fig. 1).
Histological Data.
| Variables | ||
| Type of resection | ||
| R0 | 28 | 62 |
| R1 | 14 | 31 |
| R2 | 3 | 7 |
| pT (2 are missing) | ||
| pT1 | 3 | 7 |
| pT2 | 19 | 42 |
| pT3 | 18 | 40 |
| pT4 | 3 | 7 |
| pN (2 are missing) | ||
| N0 | 22 | 49 |
| N1 | 21 | 47 |
| Perineural spread | 42 | 93 |
| Differentiation | ||
| Well differentiated | 15 | 33 |
| Moderately differentiated | 20 | 44 |
| Non-differentiated | 10 | 22 |
| Number of positive lymph nodes (median and [range]) | 1 | (0–7) |
| Number of total lymph nodes (median and [range]) | 9.5 | (4–27) |
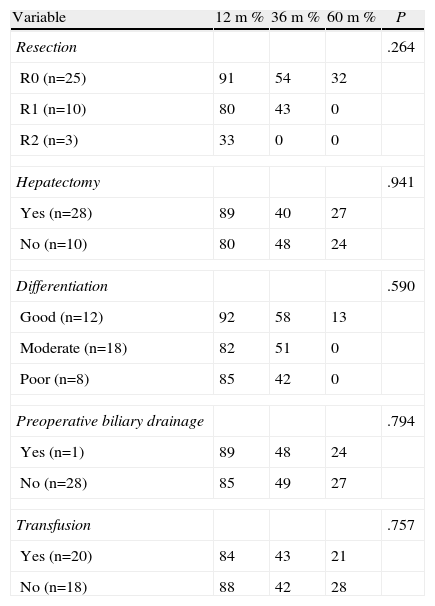
With respect to the assessment of factors which might influence survival, when the patients were compared according to resection type (R0, R1, R2), survival at 1, 3 and 5 years was 91%, 54%, and 32% respectively for the R0 patients; 80%, 43%, and 0% for R1, and 33%, 0%, and 0% for R2, respectively. There was no survival at 5 years for patients with R1 and R2 resections, even though the difference was not statistically significant (P=.264) (Table 5).
Survival Prognostic Factors Excluding Operative Mortality.
| Variable | 12m % | 36m % | 60m % | P |
| Resection | .264 | |||
| R0 (n=25) | 91 | 54 | 32 | |
| R1 (n=10) | 80 | 43 | 0 | |
| R2 (n=3) | 33 | 0 | 0 | |
| Hepatectomy | .941 | |||
| Yes (n=28) | 89 | 40 | 27 | |
| No (n=10) | 80 | 48 | 24 | |
| Differentiation | .590 | |||
| Good (n=12) | 92 | 58 | 13 | |
| Moderate (n=18) | 82 | 51 | 0 | |
| Poor (n=8) | 85 | 42 | 0 | |
| Preoperative biliary drainage | .794 | |||
| Yes (n=1) | 89 | 48 | 24 | |
| No (n=28) | 85 | 49 | 27 | |
| Transfusion | .757 | |||
| Yes (n=20) | 84 | 43 | 21 | |
| No (n=18) | 88 | 42 | 28 | |
Furthermore, when the survival rates of patients with R1 vs R2 were compared there were no significant differences (P=.906).
Survival according to level of differentiation was high and no statistically significant differences were found (P=.590) (Table 5).
When patients who had undergone hepatic resection as part of the operation were compared with those who had undergone resection of the biliary tract alone, no significant differences in terms of survival were found (P=.941).
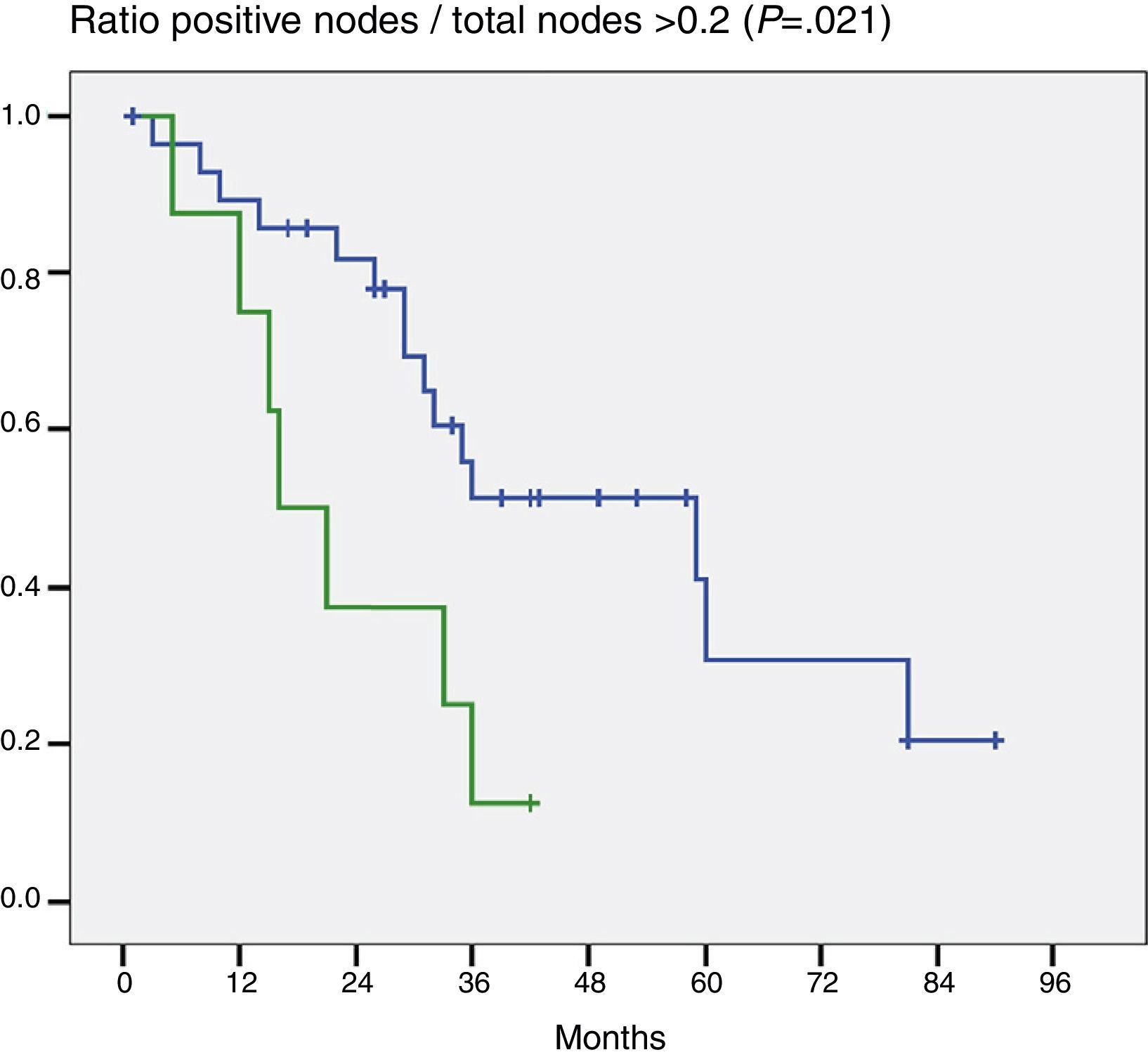
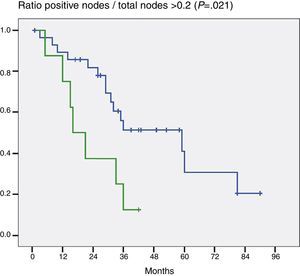
When the impact that the number of resected lymph nodes had on survival (more or less than 5 and more or less than 7) was assessed the number of lymph nodes was not shown to be a prognostic factor in either of the 2 groups (P=.132 and P=.427). On the other hand, when the ratio G+/Gt≥0.2 was assessed, the difference was statistically significant (P=.021) (Fig. 2).
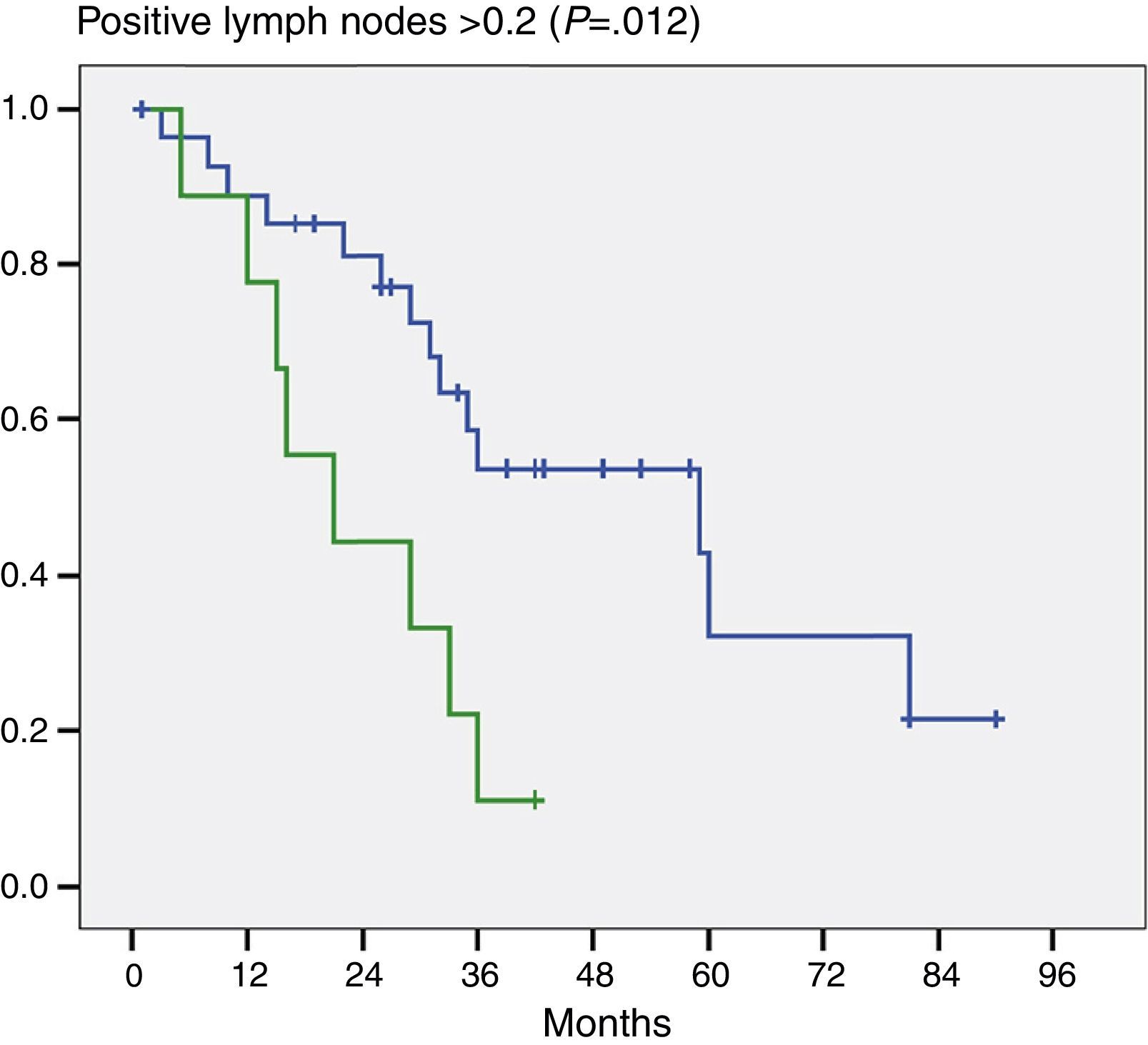
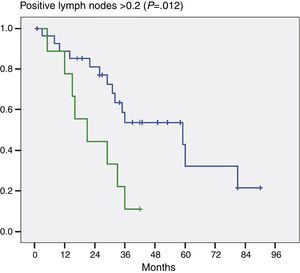
Moreover, and this was important data, when we evaluated the number of positive lymph nodes we noted a significant difference in patients who had 2 or more positive lymph nodes (P=.012) (Fig. 3).
When evaluating other factors considered prognostic such as transfusions and the preoperative placement of a biliary drain, no statistically significant differences were found (P=.726 and P=.585 respectively) (Table 5).
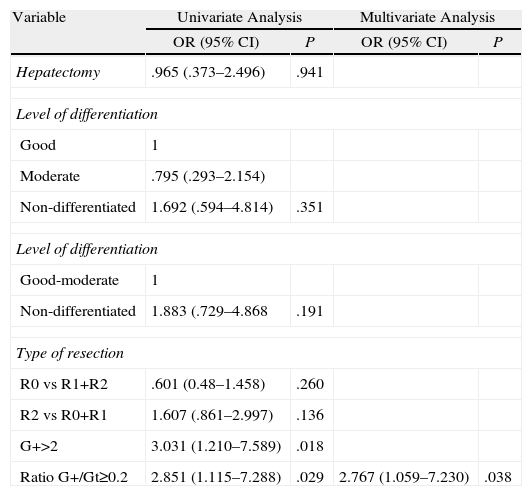
Uni- and Multivariate Cox AnalysisAll the factors which are cited in most publications were entered into the univariate analysis: hepatectomy, level of differentiation, resection and vascular spread margin. Only the ratio ≥2 G+/Gt with a risk of 2.851 and for patients with ≥2 positive lymph nodes with a risk of 3 were significant. In the multivariate analysis only the ratio ≥2 G+/Gt was significant with a P=.038 (Table 6).
Cox Regression.
| Variable | Univariate Analysis | Multivariate Analysis | ||
| OR (95% CI) | P | OR (95% CI) | P | |
| Hepatectomy | .965 (.373–2.496) | .941 | ||
| Level of differentiation | ||||
| Good | 1 | |||
| Moderate | .795 (.293–2.154) | |||
| Non-differentiated | 1.692 (.594–4.814) | .351 | ||
| Level of differentiation | ||||
| Good-moderate | 1 | |||
| Non-differentiated | 1.883 (.729–4.868 | .191 | ||
| Type of resection | ||||
| R0 vs R1+R2 | .601 (0.48–1.458) | .260 | ||
| R2 vs R0+R1 | 1.607 (.861–2.997) | .136 | ||
| G+>2 | 3.031 (1.210–7.589) | .018 | ||
| Ratio G+/Gt≥0.2 | 2.851 (1.115–7.288) | .029 | 2.767 (1.059–7.230) | .038 |
G+2=2 or more invaded lymph nodes; Ratio G+/Gt≥0.2: ratio positive lymph nodes/total lymph nodes; type of resection: R0: margin not invaded; R1: microscopic margin invaded, macroscopic margin invaded.
An increase in the incidence of hilar cholangiocarcinoma has been described by some authors.13 At present, surgery is the only curative treatment which can be offered to these patients, by complete resection (R0) of the tumour.1,2,20–24 On the one hand, improved complementary diagnostic methods have enabled these patients to be better staged preoperatively, and surgery can be avoided in patients with non-resectability criteria25,26 and on the other hand, the development of surgical technique has meant that an increased number of vascularly compromised patients can be resected because it is possible to perform venous and arterial excisions.27–29 The use of portal embolisation proposed by Makuuchi was another factor leading to an increased number of resected patients as it allows hypertrophy of the future remnant and reduces the likelihood of postoperative liver failure.30,31
All in all, the percentage of resected patients in the majority of series is around 75%, this figure is similar to that obtained in our case study (78%). Of this figure, 62% were R0, which is comparable to other publications.21,32 Hepatectomy was associated in 35 patients (78%) and resection of the biliary tract was performed on 10 with lymphadenectomy and hepatic-jejunal anastomosis. There were no significant differences in terms of survival between those who had undergone hepatectomy and those who had not. These results coincide with those published by some authors and differ from others, remaining a point of controversy for Bismuth type I and some Bismuth type II tumours.33–36 It is important to bear in mind that the majority of our patients who were only treated by resection of the biliary tract and lymphadenectomy had type I Bismuth-Corlette tumours.
Morbidity was 73%, biliary fistulas and intra-abdominal collections were the most common; this figure is a little higher than in other series and is likely due to the effort made to achieve a higher rate of resectability. The same applies to mortality, which occurred in 7 patients (15%). On this point it is important to take into account that survival at 3 and 5 years, without discounting operative mortality and with mortality, was 45% and 32% respectively, similar to that published in other series.10,37
Several prognostic factors have been proposed in the literature.11,38–40 R0 resections have been associated with greater survival. In our series there were no significant differences when they were compared with R1 and R2 resections, although those for whom an R0 was achieved were the only survivors at 5 years. Survival of R0 patients at 5 years was 32%, this figure coincides with most published series.10,37 Other authors41–43 have proposed that patients who required blood product transfusions or those who underwent preoperative biliary drainage had a poorer prognosis than those who did not. When we analysed these 2 points we did not find any significant differences to suggest it would be an advantage to avoid transfusions or biliary drainage.
The level of differentiation is also mentioned in the literature as another prognostic factor to keep in mind.10,44 In our experience, cellular differentiation had no significant differences in terms of survival at 1, 3 and 5 years. 93% of our patients presented with perineural spread, which has also been associated with a poor prognosis, but this was not significant in our series. On this point, and given the high percentage of spread in our case studies, we tend to believe that it is a factor which depends on the interest on the part of the pathologist in finding it.
Nagino et al.12 suggest that lymph node metastases are the most significant predictive factor. When we assessed our results the mean total lymph nodes reviewed by pathology was 9.5; this figure is slightly lower than that referred to by Aoba et al.13 which was 12.9, but higher than that of the Sloan-Kettering Cancer Center group which was 3.13,45 In the seventh edition of the UICC TNM classification it is recommended that 15 lymph nodes should be pathologically analysed for hilar cholangiocarcinoma, coinciding with some authors; few patients in our series and others achieve this figure.13
When we study the impact on the survival of patients in whom more or less than 5 and 7 lymph nodes were resected, we found that there was no significant difference. On this point we coincide with Aoba et al.13 in that the resection of 5 or more lymph nodes is, on the one hand, an acceptable figure, and on the other, that it is likely that the total number of lymph nodes resected and their location has no significant prognostic impact. Moreover, the number of positive lymph nodes would be an important prognostic factor. Aoba et al.13 state that there is a major difference between patients who have one positive lymph node and those with more, and that this is the most important prognostic factor. In our series, we found a statistically significant difference between patients who had 2 or more positive lymph nodes and those who had fewer.
In the same regard, some authors referring to other tumours (stomach, colon, pancreas, etc.) have suggested that the ratio G+/Gt3–9 is a significant prognostic factor. Guglielmi et al.14 and Oshiro et al.15 have reported that a ratio above .25 for the first and .2 for the second have had far poorer survival that those with less. In our patients, a ratio above .2 had poorer survival than those with a lower ratio and the difference was statistically significant in the Cox multivariate analysis.
However, some authors13 do not report similar results and state that the ratio would be affected by the total number of lymph nodes and that it would also be difficult to obtain comparable homogeneous data as there are series with a higher number of lymph nodes and other series13,45 with very few in their lymphadenectomies or where the pathologists would only have studied very few out of the total.
In conclusion, although there are few studies which assess the ratio of positive lymph nodes in patients resected for hilar cholangiocarcinoma, we consider that the number of positive lymph nodes resected and the ratio G+/Gt are probably the 2 most significant survival prognosis factors. These results should be important when making a decision on adjuvant therapy after surgery.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Nari GA, Palacios OG, Lopez-Ben S, Albiol M, Falgueras L, Castro-Gutierrez E, et al. Colangiocarcinoma hiliar: el número de ganglios positivos y la relación ganglios positivos/ganglios totales son un factor pronóstico importante de supervivencia. Cir Esp. 2014;92:247–253.