To evaluate the initial results of the oesophagogastric cancer registry developed for the Sociedad Valenciana de Cirugía and the Health Department of the Comunidad Valenciana (Spain).
MethodsFourteen of the 24 public hospitals belonging to the Comunidad Valenciana participated. All patients with diagnosis of oesophageal or gastric carcinomas operated from January 2013 to December 2014 were evaluated. Demographic, clinical and pathological data were analysed.
ResultsFour hundred and thirty-four patients (120 oesophageal carcinomas and 314 gastric carcinomas) were included. Only two hospitals operated more than 10 patients with oesophageal cancer per year. Transthoracic oesophagectomy was the most frequent approach (84.2%) in tumours localised within the oesophagus. A total gastrectomy was performed in 50.9% patients with gastroesophageal junction (GOJ) carcinomas. Postoperative 30-day and 90-day mortality were 8% and 11.6% in oesophageal carcinoma and 5.9% and 8.6% in gastric carcinoma.
Before surgery, middle oesophagus carcinomas were treated mostly (76.5%) with chemoradiotherapy. On the contrary, lower oesophagus and GOJ carcinomas were treated preferably with chemotherapy alone (45.5% and 53.4%). Any neoadjuvant treatment was administered to 73.6% of gastric cancer patients. Half patients with oesophageal carcinoma or gastric carcinoma received no adjuvant treatment.
ConclusionsThis registry revealed that half patients with oesophageal cancer were operated in hospitals with less than 10 cases per year at the Comunidad Valenciana. Also, it detected capacity improvement for some clinical outcomes of oesophageal and gastric carcinomas.
Evaluar los resultados iniciales del registro de tumores esófago-gástricos desarrollado conjuntamente por la Sociedad Valenciana de Cirugía y la Consellería de Sanitat de la Comunidad Valenciana.
MétodosParticiparon 14 de los 24 hospitales públicos de la Comunidad Valenciana. Se evaluaron todos los pacientes con diagnóstico de carcinoma de esófago y de estómago operados desde enero 2013 hasta diciembre 2014. Se analizaron variables demográficas, clínicas e histopatológicas.
ResultadosSe incluyeron 434 pacientes, 120 con carcinoma de esófago y 314 con carcinoma gástrico. Solo en 2 centros se operaron a más de 10 pacientes con cáncer de esófago/año. La esofaguectomía transtorácica fue el abordaje más frecuente (84,2%) en los tumores de localización esofágica. En el 50,9% de los carcinomas de la unión esófago-gástrica (UEG) se realizó una gastrectomía total. La mortalidad postoperatoria a los 30 y 90 días fue del 8 y 11,6% en el carcinoma de esófago y del 5,9 y 8,6% en el carcinoma gástrico.
Antes de la cirugía, los tumores esofágicos del tercio medio fueron tratados mayoritariamente (76,5%) con quimiorradioterapia. Por el contrario, los de tercio inferior y los de la UEG fueron tratados preferentemente solo con quimioterapia (45,5 y 53,4%). El 73,6% de los pacientes con carcinoma gástrico no recibió tratamiento neoadyuvante. La mitad de los pacientes con carcinoma esofágico o gástrico no recibió ningún tratamiento adyuvante.
ConclusionesEste registro muestra que en la Comunidad Valenciana, la mitad de los pacientes con cáncer de esófago son operados en hospitales con una casuística menor de 10 casos/año. Asimismo, ha detectado posibilidades de mejora relevantes en indicadores de resultado de los carcinomas esófago-gástricos.
According to data from the EUROCARE-5 study, the long-term 5-year survival rates of European patients with oesophageal and stomach cancer are only 15% and 30%, respectively, although much variability has been observed between the different countries and regions of Europe.1
Curative treatment of oesophageal and stomach cancer is still based on R0 surgery, but there is great variability in the surgical approaches preferred for oesophageal carcinomas (transhiatal or transthoracic) as well as the administration of complementary therapies in stomach cancer. While chemoradiotherapy has been demonstrated to be effective when administered as neoadjuvant therapy in carcinomas of the oesophagus and the oesophagogastric junction (EGJ),2,3 or even alone in squamous cell carcinomas,4 there seem to be fewer benefits of adjuvant5,6 or perioperative7 treatments in gastric carcinoma, even though they are recommended in the ESMO clinical practice guidelines for stages IB or higher.8
The postoperative mortality of these 2 diseases also demonstrates important variability, as observed in several European registries. While mortality diminishes after oesophagectomy, the postoperative mortality rate is higher than 5% after gastrectomy in several western European countries.9,10 The percentages of overall postoperative morbidity for both diseases reported in the literature are wide-ranging and depend a great deal on the detailed collection of data about complications, which has led to the proposal of standardisation.11
Due to the variability in the result indicators for clinically relevant parameters, no standards for quality treatment have been defined for oesophageal and gastric cancer. The objective of our study was to determine the demographic, clinical and histopathologic results for oesophageal and gastric carcinomas at public hospitals in the Spanish autonomous community of Valencia through a recently created register that was developed by the Valencian Society of Surgery (Sociedad Valenciana de Cirugía, SVC) in conjunction with the Healthcare Administration of the Comunidad Valenciana.
MethodsThis study was created with the participation of 14 out of 24 hospitals from the public healthcare system in the region of the Comunidad Valenciana (Spain). Included in the study were all patients with a diagnosis of cancer of the oesophagus, EGJ or stomach who were proposed for surgical treatment with curative intent from January 2013 to December 2014. The patient data were input in a computerised data entry form in the NEOS platform of the Cancer Information System (CIS) of the Comunidad Valenciana. This information system allows for compliance with data protection laws, participation of different hospitals, and follow-up by researchers. Once the patient data from the participating hospitals in the CIS had been input, we confirmed the inclusion of all cases that were appropriate for evaluation as a quality control. Throughout the period in which the data entry form was being developed and during data entry, several joint meetings were held between members of the SVC, CIS and Oncologic Plan for the Comunidad Valenciana (OPCV).
The data entry form had 37 variables grouped into the following 5 sections: Preoperative treatment and study, Surgery, Anatomic pathology, Post-op and Follow-up. The study analysed the following clinical variables: age, sex, American Society of Anaesthesiologists (ASA) classification, diagnostic and staging procedures (endoscopy, endoscopic ultrasound, computed tomography [CT], positron emission tomography [PET], staging laparoscopy), tumour location, neoadjuvant therapy, resectability, type of oesophagectomy, type of gastrectomy, postoperative medical complications (pulmonary: distress, pneumonia, pleural effusion requiring drainage, and atelectasis requiring bronchoscopy; and cardiac: arrhythmia requiring treatment, cardiac insufficiency and appearance or decompensation of ischaemic heart disease) and surgical (fistula, haemorrhage, chylothorax, reoperation, etc.), mortality within 30 days post-op, 90-day mortality post-op, and adjuvant therapy. We did not analyse the variables “recurrence” and “long-term survival” because the follow-up time was not sufficient. The histopathological variables analysed included tumour invasion (pT), lymph node invasion (pN), number of lymph nodes analysed, and radicality of the resection (R0, R1, R2). Tumours were staged according to the 7th edition of the Union for International Cancer Control (UICC) TNM pathology classification.12 According to this classification, carcinomas of the EGJ were considered oesophageal carcinomas.
The statistical analysis was carried out with SPSS® v20 software. Values were reported as frequencies and percentages, with the median and interquartile range (as the expression of the most representative values of the distribution) or mean and standard deviation, according to the type of variable. To analyse the association between qualitative variables, the chi-squared test was used; the Mann–Whitney test, Spearman's correlation coefficient and the median test were used for the scale variables. As a multivariate test, we used logistic regression. A P value <.05 was considered statistically significant.
ResultsDemographicsDuring the inclusion period, 434 patients were analysed, 120 with carcinoma of the oesophagus and 314 with gastric carcinoma. The different locations are detailed in Tables 1 and 2. The majority of the oesophageal carcinomas were located in the EGJ (48.3%) and in the distal third (36.7%). In gastric carcinomas, the most frequent location was the distal third (52.2%).
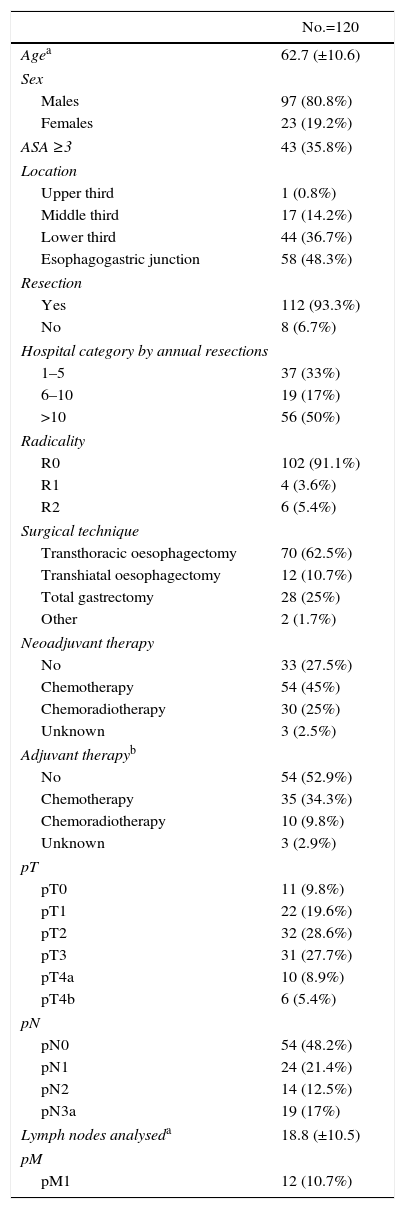
Characteristics and Treatment of Oesophageal Carcinomas of the Esophagogastric Junction.
| No.=120 | |
|---|---|
| Agea | 62.7 (±10.6) |
| Sex | |
| Males | 97 (80.8%) |
| Females | 23 (19.2%) |
| ASA ≥3 | 43 (35.8%) |
| Location | |
| Upper third | 1 (0.8%) |
| Middle third | 17 (14.2%) |
| Lower third | 44 (36.7%) |
| Esophagogastric junction | 58 (48.3%) |
| Resection | |
| Yes | 112 (93.3%) |
| No | 8 (6.7%) |
| Hospital category by annual resections | |
| 1–5 | 37 (33%) |
| 6–10 | 19 (17%) |
| >10 | 56 (50%) |
| Radicality | |
| R0 | 102 (91.1%) |
| R1 | 4 (3.6%) |
| R2 | 6 (5.4%) |
| Surgical technique | |
| Transthoracic oesophagectomy | 70 (62.5%) |
| Transhiatal oesophagectomy | 12 (10.7%) |
| Total gastrectomy | 28 (25%) |
| Other | 2 (1.7%) |
| Neoadjuvant therapy | |
| No | 33 (27.5%) |
| Chemotherapy | 54 (45%) |
| Chemoradiotherapy | 30 (25%) |
| Unknown | 3 (2.5%) |
| Adjuvant therapyb | |
| No | 54 (52.9%) |
| Chemotherapy | 35 (34.3%) |
| Chemoradiotherapy | 10 (9.8%) |
| Unknown | 3 (2.9%) |
| pT | |
| pT0 | 11 (9.8%) |
| pT1 | 22 (19.6%) |
| pT2 | 32 (28.6%) |
| pT3 | 31 (27.7%) |
| pT4a | 10 (8.9%) |
| pT4b | 6 (5.4%) |
| pN | |
| pN0 | 54 (48.2%) |
| pN1 | 24 (21.4%) |
| pN2 | 14 (12.5%) |
| pN3a | 19 (17%) |
| Lymph nodes analyseda | 18.8 (±10.5) |
| pM | |
| pM1 | 12 (10.7%) |
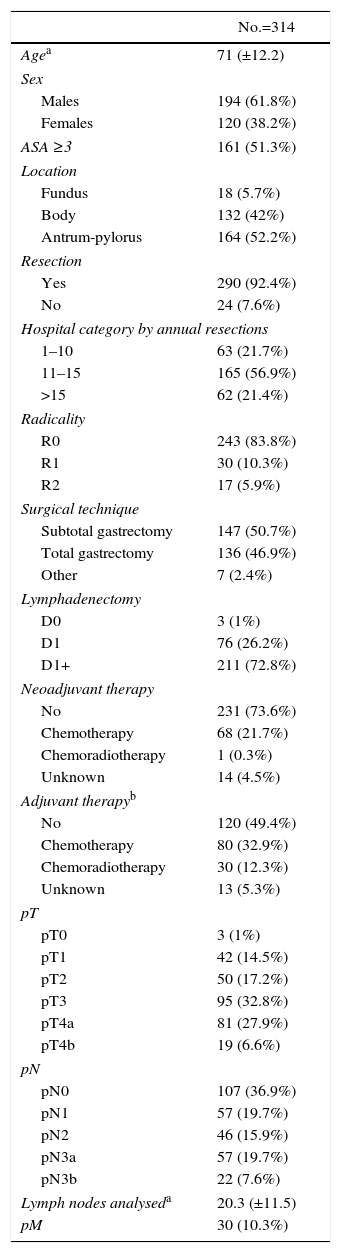
Characteristics and Treatment of Stomach Carcinoma.
| No.=314 | |
|---|---|
| Agea | 71 (±12.2) |
| Sex | |
| Males | 194 (61.8%) |
| Females | 120 (38.2%) |
| ASA ≥3 | 161 (51.3%) |
| Location | |
| Fundus | 18 (5.7%) |
| Body | 132 (42%) |
| Antrum-pylorus | 164 (52.2%) |
| Resection | |
| Yes | 290 (92.4%) |
| No | 24 (7.6%) |
| Hospital category by annual resections | |
| 1–10 | 63 (21.7%) |
| 11–15 | 165 (56.9%) |
| >15 | 62 (21.4%) |
| Radicality | |
| R0 | 243 (83.8%) |
| R1 | 30 (10.3%) |
| R2 | 17 (5.9%) |
| Surgical technique | |
| Subtotal gastrectomy | 147 (50.7%) |
| Total gastrectomy | 136 (46.9%) |
| Other | 7 (2.4%) |
| Lymphadenectomy | |
| D0 | 3 (1%) |
| D1 | 76 (26.2%) |
| D1+ | 211 (72.8%) |
| Neoadjuvant therapy | |
| No | 231 (73.6%) |
| Chemotherapy | 68 (21.7%) |
| Chemoradiotherapy | 1 (0.3%) |
| Unknown | 14 (4.5%) |
| Adjuvant therapyb | |
| No | 120 (49.4%) |
| Chemotherapy | 80 (32.9%) |
| Chemoradiotherapy | 30 (12.3%) |
| Unknown | 13 (5.3%) |
| pT | |
| pT0 | 3 (1%) |
| pT1 | 42 (14.5%) |
| pT2 | 50 (17.2%) |
| pT3 | 95 (32.8%) |
| pT4a | 81 (27.9%) |
| pT4b | 19 (6.6%) |
| pN | |
| pN0 | 107 (36.9%) |
| pN1 | 57 (19.7%) |
| pN2 | 46 (15.9%) |
| pN3a | 57 (19.7%) |
| pN3b | 22 (7.6%) |
| Lymph nodes analyseda | 20.3 (±11.5) |
| pM | 30 (10.3%) |
The median and interquartile range for age was 63 (55–70)years in carcinomas of the oesophagus and 74 (64–81)years in gastric carcinomas (P<.001); 72.5% of the patients with oesophageal carcinoma and 39.2% with gastric carcinoma were younger than 70. The prevalence of males was greater in carcinomas of the oesophagus (81%) than of the stomach (62%).
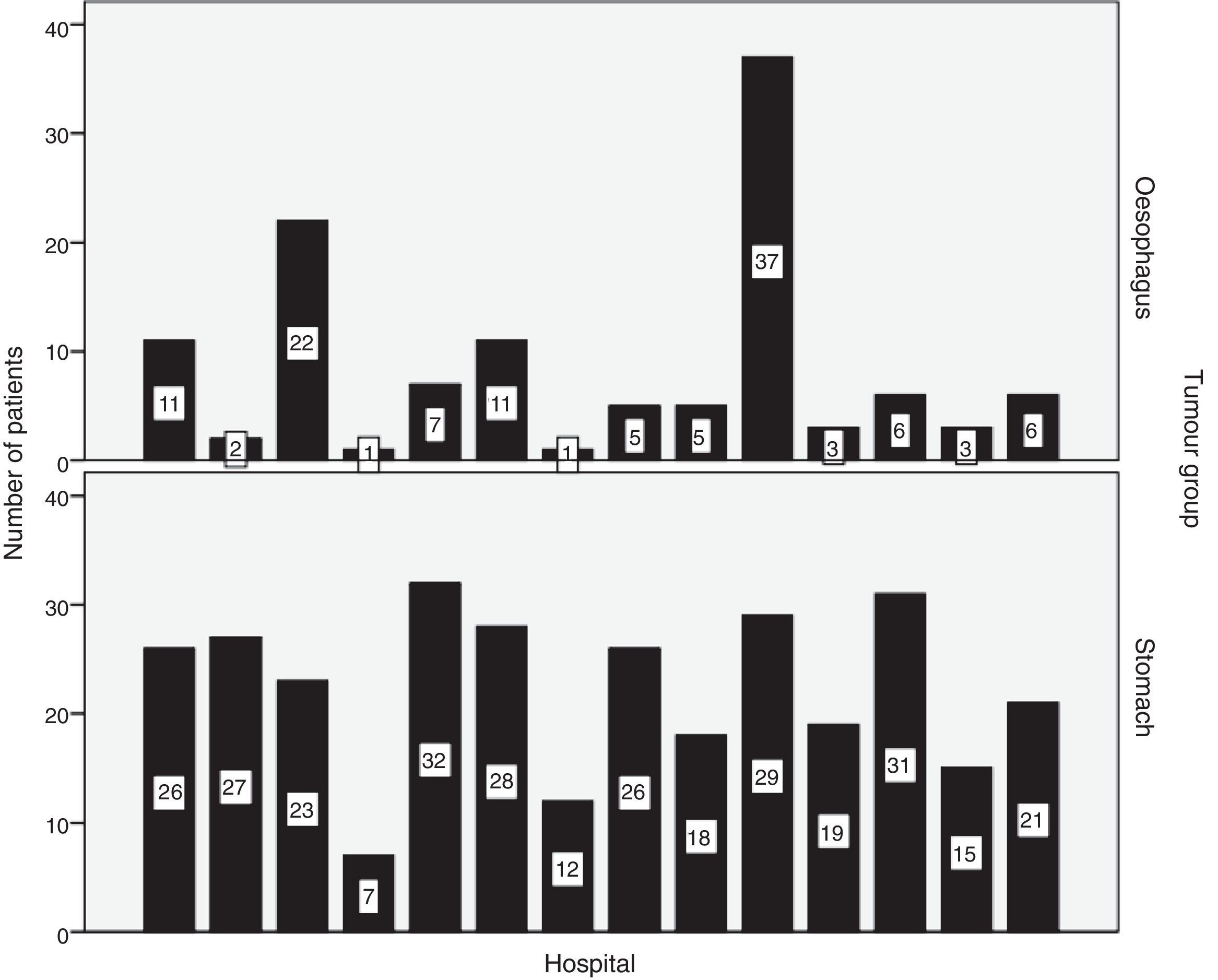
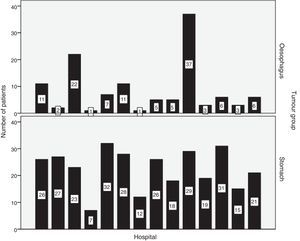
Hospital distribution of the number of patients with oesophageal and stomach carcinoma treated surgically is shown in Fig. 1. 50.8% of patients with oesophageal cancer were treated at hospitals with less than 10 cases per year. Only 2 hospitals surgically treated more than 10 patients with oesophageal cancer each year (Fig. 1). Tables 1 and 2 show the number of patients with oesophageal and stomach carcinomas resected at Valencian hospitals according to their annual caseload.
Preoperative Study and TreatmentThe ASA classification of the patients with gastric carcinoma was higher than those with oesophageal carcinoma (P=.007).
The diagnosis and clinical staging of oesophageal and stomach carcinomas were almost always performed with upper gastrointestinal endoscopy (100% and 99%) and CT scan (98.3% and 98.7%). Endoscopic ultrasound and PET were performed more frequently in oesophageal carcinomas (56.7% and 36.7%) than in gastric carcinomas (19.4% and 2.5%). Staging laparoscopy was performed in a minority of patients with both oesophageal carcinoma (3.3%) and stomach carcinoma (6.7%).
27.5% of patients with oesophageal carcinomas did not receive any neoadjuvant therapy. 76.5% of the tumours located in the middle third followed a chemoradiotherapy regimen, while the lower third and the EGJ group either followed a chemotherapy regimen (45.5% and 53.4%) or they received no type of neoadjuvant therapy (29.5% and 32.8%). In contrast, 73.6% of the patients with gastric carcinoma did not receive any type of neoadjuvant treatment. The details of the neoadjuvant therapies administered are shown in Tables 1 and 2.
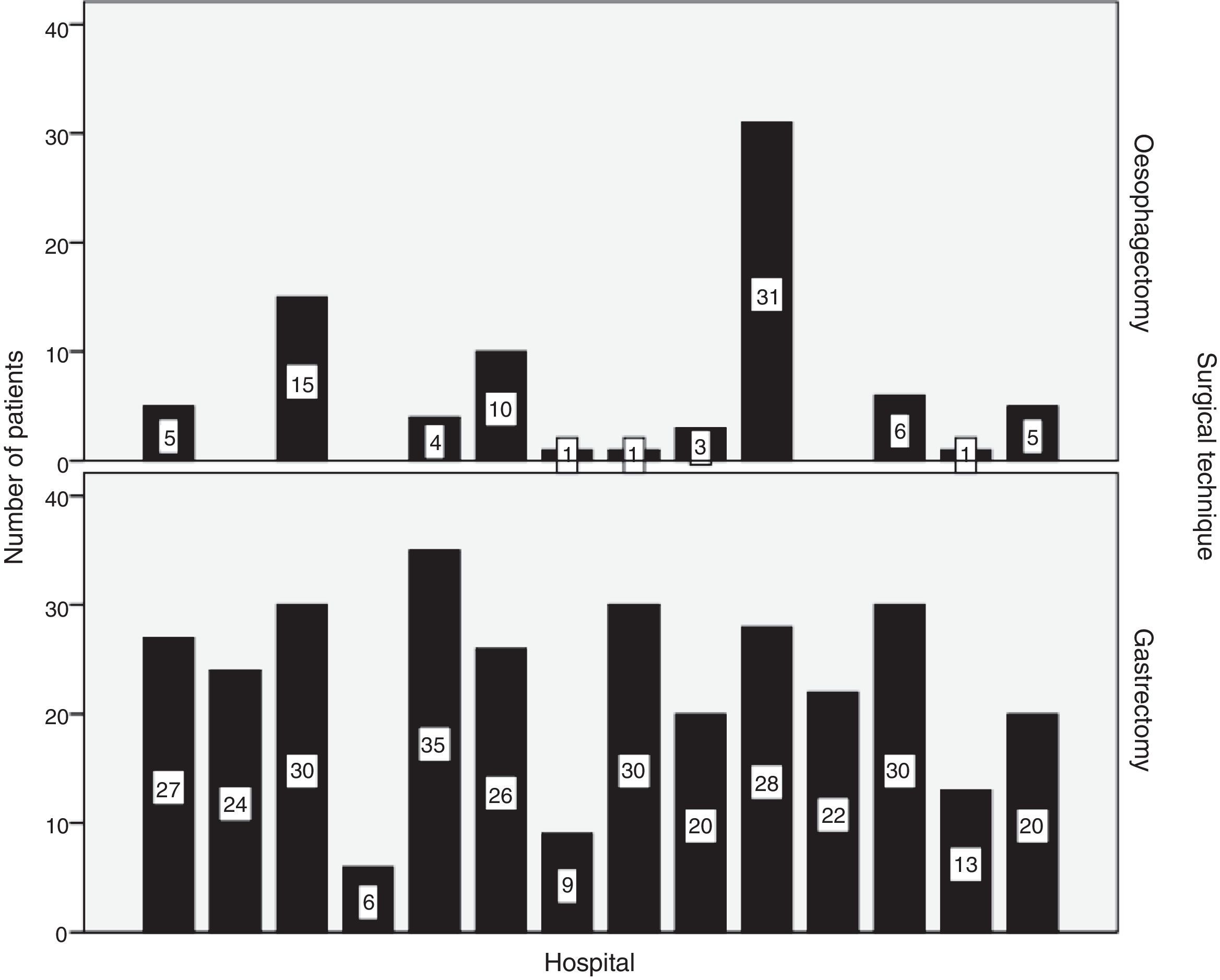
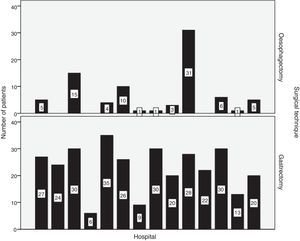
SurgeryResection was performed in 93.3% of patients with oesophageal carcinomas and in 92.4% of patients with gastric carcinoma. R0 resection was achieved in 91.1% and 83.8%, respectively. The distribution by hospitals of the number of oesophagectomies and gastrectomies performed is shown in Fig. 2.
Transthoracic oesophagectomy was the most frequent approach (84.2%) in carcinomas of the middle and lower thirds of the oesophagus. In 50.9% of EGJ tumours, total gastrectomy was performed.
In gastric carcinomas, subtotal and total gastrectomies were performed in similar percentages (50.7% and 46.9%). A D1+ lymphadenectomy or higher was performed in 72.8% of the cases. Older patient age and a greater extent of lymphadenectomy correlated negatively with moderate intensity (Spearman's Rho: −0.20; P<.001), so that D1+ or higher lymphadenectomy was preferably performed in younger patients (median age: 69 years) in contrast to D1 lymphadenectomy (median age: 74 years), as well as D0 lymphadenectomy (median age: 82 years) (median test: P=.004).
Anatomical PathologyThe pT and pN classification of the carcinomas of the oesophagus and stomach are shown in Tables 1 and 2.
In oesophageal carcinomas, the prevalence of pT3/pT4 was 42%. The median number of lymph nodes analysed per patient was 17 (interquartile range: 11–25). Less than 10 lymph nodes were analysed in only 16.2% of the patients. The prevalence of tumours with positive lymph nodes was 50.9%.
In gastric carcinomas, the prevalence of pT3/pT4 was 67.2%. The median number of lymph nodes analysed per patient was 18 (interquartile range: 12–25). In 60%, 16 or more nodes were analysed, and less than 10 nodes were analysed in 13.7% of the patients. Positive lymph nodes were detected in 62.8% of the cases.
Postoperative PeriodMedical complications were reported in the postoperative period of 34 (30.4%) patients resected with carcinoma of the oesophagus and in 69 (24%) with gastric cancer. Cardiac or respiratory complications appeared in 3 (2.7%) and 28 (25%) patients with oesophageal cancer and in 20 (6.9%) and 41 (14.1%) patients with gastric carcinoma.
Regarding surgical complications, these appeared in 38 (34.5%) patients with oesophageal carcinomas and in 85 (29.4%) patients with gastric carcinoma. Nine (8%) patients with oesophageal carcinoma and 25 (8.6%) patients with carcinoma of the stomach required surgical reoperation. Twenty-one (18.8%) patients with oesophageal carcinoma and 40 (13.8%) patients with carcinoma of the stomach developed fistulae.
The 30- and 90-day postoperative mortality rates of patients with oesophageal carcinoma who underwent resection were 8% and 11.6%. Six of the 13 (46%) patients who died within 90 days had been operated on at hospitals with a caseload of less than 5 patients per year. Patients with tumours located in the EGJ had the highest 90-day postoperative mortality rate (15.5%). The 30- and 90-day postoperative mortality rates of patients with resected gastric carcinoma were 5.9% and 8.6%. Seventeen of the 25 (68%) patients who died within 90 days had been operated on at hospitals with caseloads of 11–15 patients per year.
When considering only the surgical procedure regardless of tumour location, the 90-day mortality rates after oesophagectomy and gastrectomy were 7.3% and 10%, respectively.
In oesophageal carcinomas, 25% of respiratory complications and 19% of gastrointestinal fistulae were related with patient death. In gastric carcinomas, 36.6% of respiratory complications and 35% of gastrointestinal fistulae were related with mortality.
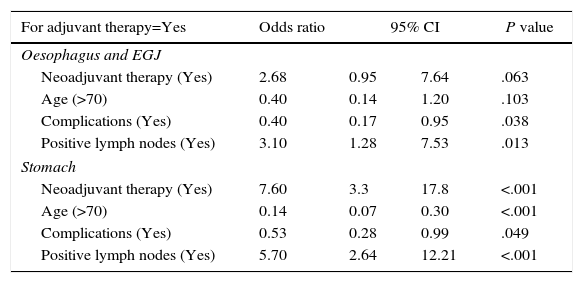
Half of the patients with both oesophageal carcinoma (52.9%) and gastric carcinoma (49.4%) did not receive any type of adjuvant treatment. The regimen of adjuvant treatments administered is shown in Tables 1 and 2. Following the logistic regression analysis, previous administration of neoadjuvant chemotherapy was most frequently related with the administration of adjuvant chemotherapy in gastric carcinoma. The next major factor in the decision to administer adjuvant therapy was the presence of positive lymph nodes (Table 3). There was also a significant association between not administering adjuvant treatment with the presence of postoperative complications and older age (Table 3). In oesophageal carcinoma, the factors most frequently associated with the administration of adjuvant therapy were the presence of positive lymph nodes and the absence of postoperative complications. The administration of neoadjuvant therapy and age >70 years only showed a tendency to be associated with adjuvant therapy (Table 3).
Logistic Regression; Administration of Adjuvant Treatment After R0 Surgical Resection.
| For adjuvant therapy=Yes | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Oesophagus and EGJ | ||||
| Neoadjuvant therapy (Yes) | 2.68 | 0.95 | 7.64 | .063 |
| Age (>70) | 0.40 | 0.14 | 1.20 | .103 |
| Complications (Yes) | 0.40 | 0.17 | 0.95 | .038 |
| Positive lymph nodes (Yes) | 3.10 | 1.28 | 7.53 | .013 |
| Stomach | ||||
| Neoadjuvant therapy (Yes) | 7.60 | 3.3 | 17.8 | <.001 |
| Age (>70) | 0.14 | 0.07 | 0.30 | <.001 |
| Complications (Yes) | 0.53 | 0.28 | 0.99 | .049 |
| Positive lymph nodes (Yes) | 5.70 | 2.64 | 12.21 | <.001 |
CI: confidence interval; EGJ: esophagogastric junction.
In gastric carcinoma with positive lymph nodes, the probability of receiving adjuvant treatment at an age ≥70 years and having suffered postoperative complications was 30%, whereas at <70 years and no postoperative complications this probability was situated at 90%. For carcinomas located in the oesophagus or EGJ with positive lymph nodes, reported probabilities were 27% and 74%, respectively.
DiscussionThe registry of oesophageal-gastric cancer that we present has been created and developed jointly by the SVC and the autonomic healthcare administration (CIS and OPCV). This collaboration provides for the collection, storage and protection of data and guarantees the completeness of the study after comparing the cases included with the total number of cases registered in the CIS of the Comunidad Valenciana, thereby avoiding selection bias. In Spain, another registry of oesophageal-gastric carcinomas is being created by surgeons at public hospitals in Catalonia and Navarra as part of the EURECCA project13 to evaluate the differences and improve the quality of the results, taking advantage of the centralisation that has been initiated for the treatment of the oesophageal-gastric cancers in these autonomous communities, although without the participation of the administrations of these autonomous communities.
This study shows that, in the Comunidad Valenciana, hospitals with an annual minimum caseload of patients undergoing surgery for oesophageal cancer, as recommended by the literature in order to obtain excellent results, are the exception.14–16 Half of the patients with oesophageal cancer were operated on at hospitals with a caseload of less than 10 cases per year. Only 2 hospitals surgically treated more than 10 patients with oesophageal cancer per year, and only one performed more than 10oesophagectomies/year. EGJ carcinomas were considered tumours of the oesophagus, in accordance with the 7th classification of the UICC,12 although half of them were treated with total gastrectomy and reconstructed with a Roux-en-Y oesophagojejunostomy. This fact is relevant when evaluating postoperative mortality, since the tumours located in the EGJ were associated with a high postoperative mortality and, consequently, this indicator varied in the analysis by carcinoma type (oesophageal or gastric) or procedure (oesophagectomy or gastrectomy). Thus, the mortality of patients with oesophageal carcinoma who underwent esophagectomy (7.3%) was slightly elevated when compared to other European registries (2.9%–6.3%),9,10 but it increased considerably when analysing carcinomas of the oesophagus together with those of the EGJ, which in half of cases were treated by total gastrectomy. There is still great variability in the treatment of EGJ carcinomas, as observed in a recent study comparing the results of registries or working groups in 5 western European countries, where gastrectomy was hardly performed in France and the Netherlands but was the surgical technique chosen in approximately 1/3 of patients in the UK and Ireland and up to 2/3 of the patients operated on at hospitals in the Catalonian region of Spain.10 The controversy about carcinomas of the EGJ lies largely in the type of surgical resection to be done and its classification, which generates difficulties especially between Siewert types I and II, depending on whether it should be performed after endoscopy findings or after the histopathological study.17–21
The postoperative mortality due to gastric carcinoma was remarkably high (8.6%), although in our study it was calculated up to 90 days post-op. This high mortality in gastric carcinoma was also observed in the Dutch cancer registry9 and in the Spanish EURECCA project,10 at around 7% in both. Unlike oesophageal cancer, in gastric carcinomas there is no unanimity in the conclusions of the studies on the reduction of postoperative mortality and its correlation with the volume of annual surgical cases at each hospital. However, the mortality rates over 5% reported by several registries or multicentre studies in western European countries,9,22 which are far different from the rates less than 1% described in Japanese studies,23 indicate that there is room for improvement. In the United Kingdom and Denmark, where this disease has been centralised, postoperative mortality due to gastric carcinoma has dropped significantly to 2.2% and 2.4%.9,24,25
Median age and ASA were significantly lower in patients with oesophageal carcinoma or those who underwent esophagectomy, indicating a greater selection in the indication of a surgical treatment, with its possible influence on postoperative mortality rates. The comorbidity of the patients included during this period was not analysed, although a later update now includes the Charlson index26 because of its correlation with postoperative complications.27
Regarding the quality of the surgery, both in patients with oesophageal and stomach carcinomas, the percentage of patients with R0 resection and the number of lymph nodes analysed (≥10) were within the range recently published by the EURECCA project.10 In carcinomas of the stomach, the percentage of patients with more than 15 lymph nodes analysed or with positive lymph nodes has not changed from what was observed in a joint study of 3 hospitals in the same autonomous community in the period from 2004 to 2010.28 The percentage of reoperations in both carcinomas was similar in oesophageal and stomach carcinomas and clearly higher than the percentage reported by the FREGAT,27 and it was decided to include the Clavien–Dindo classification29 in the new updated form.
It is noteworthy that neoadjuvant treatment using a chemoradiotherapy regimen was administered predominantly in oesophageal carcinomas of the middle third. Meanwhile, the option of exclusive chemotherapy was most frequently used in the lower third, similarly to what occurred in carcinomas of the EGJ. It is likely that the gradual implementation of the CROSS2 scheme will change this trend in subsequent years. It is also remarkable that less than one-quarter of the patients with gastric carcinoma received neoadjuvant chemotherapy, although their significant association with the administration of adjuvant chemotherapy suggests a certain implementation in our setting of the MAGIC perioperative regimen.7 Similarly, the administration of Macdonald's adjuvant regimen5 to only 12% of patients with gastric carcinoma appears to indicate a downward trend in the preference for this treatment compared to an exclusive postoperative chemotherapy regimen either alone or within a perioperative treatment regimen. In a later period, this situation could vary depending on the implementation of chemotherapy or chemoradiotherapy regimens from the CLASCIS30 or ARTIST31 studies. Finally, almost half of the patients with gastric carcinoma did not receive any adjuvant treatment, and our analysis suggests that its administration was mainly based on the existence of positive lymph nodes, but also due to the advanced age and the presence of complications in the postoperative period.
The limitations of this study are that data from all public hospitals are not included, although it is a very representative sample. In addition, only a 2-year period is analysed, with a follow-up that does not provide for a survival analysis. Finally, there are limitations inherent to this type of patient registries in which only a limited number of variables are included by consensus, considered the most important and feasible to supply by all participants.
In conclusion, this registry of oesophageal-gastric cancer of the Comunidad Valenciana in Spain contributes the global results of a very representative number of hospitals from an entire region of our country and not just a surgical unit or reference hospital. In this autonomous community, half of the patients with oesophageal cancer are operated on at hospitals with a caseload of less than 10 cases per year. Likewise, this study has detected relevant possibilities for improvement in outcome indicators for oesophageal and gastric carcinomas.
Authorship- –
Study design: Francisco Javier Lacueva, Javier Escrig, Dolores Salas and Carmen Alberich
- –
Article composition: Francisco Javier Lacueva and Javier Escrig
- –
Data collection: Javier Escrig, Fernando Mingol, Roberto Martí, José Puche, Ramón Trullenque, José Antonio Barreras, Francisco Asencio, Javier Aguiló, José Manuel Navarro, Francisco Javier Lacueva, Carmen Zaragoza, Gonzalo Todolí, Andrés García-Marín, Enrique Canelles, Javier Vaqué, Fernando López-Mozos, Miguel Oviedo, Nuria Peris, Joaquín Civera, Miguel Ángel Morcillo, Amparo Roig, Pedro Sansó, Mario Mella, Alicia Calero and Xavier Peñalver
- –
Analysis and interpretation of the results: Javier Escrig, Fernando Mingol, Roberto Martí, José Puche, Ramón Trullenque, José Antonio Barreras, Francisco Asencio, Javier Aguiló, Gonzalo Todolí, Francisco Javier Lacueva, Dolores Salas and Carmen Alberich
- –
Critical review and approval of the final version: Javier Escrig, Fernando Mingol, Roberto Martí, José Puche, Ramón Trullenque, José Antonio Barreras, Francisco Asencio, Javier Aguiló, José Manuel Navarro, Francisco Javier Lacueva, Dolores Salas, Carmen Alberich, Carmen Zaragoza, Gonzalo Todolí, Andrés García-Marín, Enrique Canelles, Javier Vaqué, Fernando López-Mozos, Miguel Oviedo, Nuria Peris, Joaquín Civera, Miguel Ángel Morcillo, Amparo Roig, Pedro Sansó, Mario Mella, Alicia Calero and Xavier Peñalver
The authors have no conflict of interests to declare.
Carmen Zaragoza (Hospital General Universitario de Alicante), Gonzalo Todolí (Hospital General La Plana), Andrés García-Marín (Hospital Universitario San Juan), Enrique Canelles (Hospital de Requena), Javier Vaqué (Hospital Universitario Politécnico La Fé de Valencia), Fernando López-Mozos (Hospital Clínico Universitario de Valencia), Miguel Oviedo (Hospital General Universitario de Valencia), Nuria Peris (Hospital Doctor Peset de Valencia), Joaquín Civera (Hospital Arnau de Vilanova de Valencia), Miguel Ángel Morcillo (Hospital Vega Baja de Orihuela), Amparo Roig (Hospital Lluís Alcanyís de Xàtiva), Pedro Sansó (Hospital General Universitario de Alicante), Mario Mella (Hospital Universitario San Juan), Alicia Calero (Hospital General Universitario de Elche) and Xavier Peñalver (Servicio de Estudios Epidemiológicos y Estadísticas Sanitarias de la Comunidad Valenciana).
More information on the components of the RECEG-CV group is available in Annex 1.
Please cite this article as: Escrig J, Mingol F, Martí R, Puche J, Trullenque R, Barreras JA, et al. Resultados iniciales del registro de carcinomas esófago-gástricos de la Comunidad Valenciana. Cir Esp. 2017;95:428–436.