Upper gastrointestinal (UGI) bleeding is a very common medical emergency with an annual incidence that ranges between 50 and 150 cases per 100000 inhabitants. Most UGI hemorrhages (80%–90%) are non-varicose. Gastroduodenal peptic ulcer is the most frequent disease involved (40%–50%), but bleeding may also be due to acute gastric mucosal lesions, esophagitis, Mallory-Weiss syndrome, tumors or vascular lesions. Aortoenteric fistulae (AEF) are a rare and potentially fatal cause of upper GI bleeding. Proper treatment requires early diagnosis, which depends on a high rate of clinical suspicion.
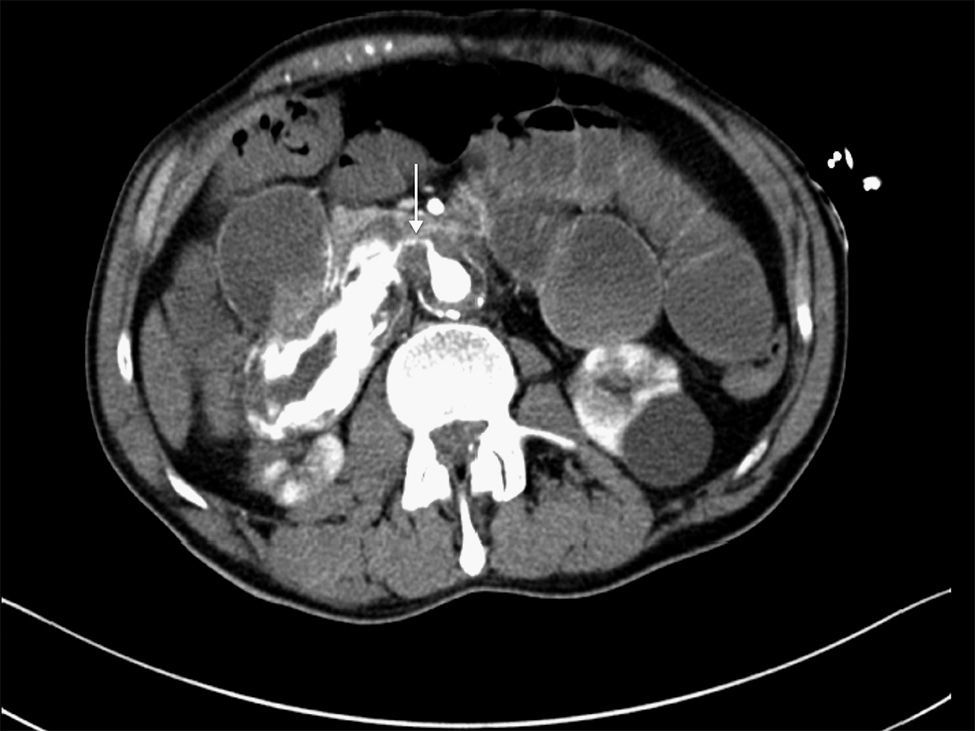
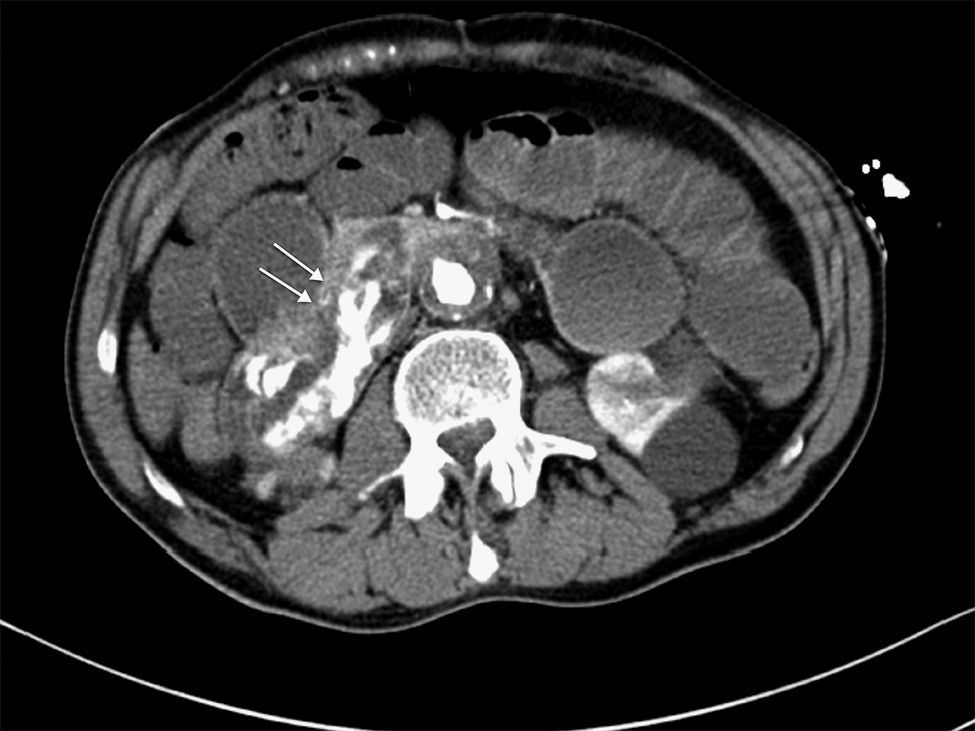
We present the case of a 63-year-old male with a prior history of arterial hypertension and dyslipidemia who came to our Emergency Department due to epigastralgia and upper GI hemorrhage with melena for several hours. Upon arrival, the patient was hemodynamically stable, with hemoglobin (Hb) 10g/dL on admittance. Endoscopy was performed up to the second portion of the duodenum, where no hemorrhage or peptic lesions were observed. The patient remained in the Emergency Department while waiting to be admitted to the Gastroenterology Unit for complementary studies. Twelve hours after admittance, the patient's general condition started to deteriorate and he became hemodynamically unstable. Hb had dropped to 6.8g/dL, arterial blood gas showed a pH of 6.8, and lactate was 17. After initiating patient stabilization with serum therapy and transfusion of 2 units of red blood cells, CT scan detected an infrarenal abdominal aortic aneurysm measuring 6cm×3.2cm with fistula to the duodenum and active bleeding (Figs. 1 and 2). With these findings, laparotomy was performed, which revealed a thin fistulous tract from the infrarenal aorta to the duodenum. Axillofemoral bypass was carried out with closure of the duodenal defect. During the operation, the patient continued to be hemodynamically unstable, requiring perfusion of noradrenaline and transfusion of 12 units of packed red blood cells, 4 units of plasma and 2 pools of platelets. After the procedure, the patient remained in the ICU, although his situation of hemodynamic instability never improved and he died within 4h of surgery.
AEF are communications between the aorta and the digestive tract that have an incidence between 0.1 and 0.8%.1 They were described for the first time in 1829 by Sir Astley Cooper.2 These fistulae are classified as either primary AEF (the result of arteriosclerotic, inflammatory or infectious aneurysms) or AEF secondary to aorta repair surgery either with or without vascular stent placement (0.5%–2.3% of cases).3 Secondary AEF are 10 times more frequent. They are usually late complications that appear between a few months and up to 15 years after surgery. AEF typically affect proximal sutures, although they may also arise as a complication associated with the infection of stents used in the endovascular treatment of aortic and iliac disease.
Primary AEF are less common; in large autopsy series, their frequency is 0.04%–0.07%.3,4 The most frequent causes of primary AEF are atherosclerosis and aortic aneurysm; less frequent causes include syphilis, TBC, infections, neoplasms, foreign bodies or any other disease that may cause the aorta to erode the wall of the intestine.3
Most AEF communicate the aorta and the duodenum, especially the third portion of the duodenum4–6 given the position of this segment in the retroperitoneum and in proximity to the descending aorta. There have also been reports of fistulae to the esophagus, small bowel, colon and stomach.
The classic triad includes UGI bleeding, abdominal pain, and a pulsating abdominal mass, although it is present in less than 25% of patients.1,4,7 Despite the communication between the aorta and the digestive tract, fever and other signs of systemic infection are rare. There is usually an initial self-limiting hemorrhage (sentinel hemorrhage), which in most cases is followed by a massive hemorrhage that occurs some hours or even a month later.2–4 In up to one-third of patients, massive hemorrhage occurs within 6h after the initial bleeding, as happened in our patient. This time period is the ideal window of opportunity to make an early diagnosis, although it requires a high index of clinical suspicion. UGI hemorrhage in a patient with known aortic aneurysm or the classic triad can guide us toward the diagnosis, but, as mentioned, it is only present in a small percentage of cases. In more than half of patients, diagnosis is reached in the operating room.5
CT angiography is the main diagnostic method in these cases. It has a diagnostic sensitivity of 40%–90% and specificity of 33%–100%.8,9 The most frequent findings are bubbles observed around the aorta, lack of a fat plane between the aorta and digestive tract, edema of the intestinal wall around the aorta, and extravasation of contrast medium from the aorta to the intestinal lumen (Fig. 2). Some authors consider this latter finding the only definitive sign of AEF, but it is uncommon. Angiography has a limited ability to demonstrate fistulae (30%) because it requires losses of more than 0.5ml/min to be able to visualize the lesion.8 It should be reserved for patients who are stable or patients with no underlying aneurysmatic disease in order to plan the type of surgery or percutaneous embolization.5
Gastroscopy is the method of choice for the initial assessment of patients with UGI hemorrhage. Nonetheless, in these cases the lesion is rarely observed because of the difficulty to reach the third portion of the duodenum or, in cases of massive active hemorrhage, to locate the origin. Despite everything, gastroscopy is able to exclude other causes of UGI hemorrhage and narrows the possibilities to other much less frequent conditions.7
Treatment is surgical with an associated mortality of 30%–40%. Four main factors should be considered during surgery: bleeding control, intestinal defect repair, selective restoration of distal circulation, and control of associated infection.1 The type of intestinal repair for each case depends on the size of the defect and local conditions. If technically possible, primary suture is sufficient, although resection of the duodenum may be necessary.10 According to the latest review of the literature by Rodrigues dos Santos et al.,11 primary sutures are associated with a higher rate of fistula recurrence, so omental interposition and even duodenal switch are recommended in cases with large defects.
In order to reestablish distal circulation, in situ aorta repair or extra-anatomic bypass are usually used, as in our case. In situ stent repair (PTFE, dacron-silver) has provided the best results in terms of survival: 65% in patients with stents, versus 53% in patients who underwent extra-anatomical revascularization.5 Nonetheless, this technique should be reserved for situations in which the infection is limited and there is no systemic sepsis. In patients with systemic or extensive local sepsis or severe peritonitis, the technique of choice is aneurysm resection with revascularization of the extremities by means of bypass revascularization (generally axillofemoral bypass).2
During the postoperative period, wide-spectrum antibiotic coverage is essential, which should be maintained for one week if the cultures are negative and 4–6 weeks if they are positive.1,6 In spite of antibiotic treatment, infectious complications are not uncommon, and these may lead to recurrent fistulae.
In recent years, endovascular aneurysm repair (EVAR) has been the treatment of choice in hemodynamically unstable patients or those with high surgical risk. Although it provides effective short-term treatment for bleeding control, in patients with AEF the risk of infection remains. Thus, it is best to consider EVAR a transitional treatment used before a definitive surgical procedure in hemodynamically unstable patients with active bleeding.12 In any event, these techniques should be accompanied by intensive antibiotic therapy for infection control and, if necessary, intraabdominal collections should be drained.
The prognosis of AEF is discouraging. Without surgery, mortality is 100%, mostly due to hypovolemic shock secondary to gastrointestinal bleeding. According to several published series, the mortality rate with treatment varies between 18 and 93%.3,4,6 A high percentage of patients die during surgery or during the immediate postoperative period due to hypovolemia,5 which occurred with our patient.
In conclusion, AEF are a rare cause of UGI bleeding that have a high mortality rate. Early diagnosis and treatment can improve the prognosis, but a high index of clinical suspicion is required. Aortoenteric fistulae should be considered a possible cause in patients with gastrointestinal bleeding of uncertain etiology and especially in patients with known abdominal aortic aneurysm.
Please cite this article as: Romera Barba E, Sánchez Pérez A, Bertelli Puche J, Duque Pérez C, Vazquez Rojas JL. Fístula aortoduodenal primaria: una causa rara y potencialmente fatal de hemorragia digestiva. Cir Esp. 2015;93:121–123.