Papillary thyroid microcarcinoma (PTMC) usually has an indolent course, but some have worse prognostic factors, such as the presence of central (6.9%–51.5%) and lateral (3%–49.6%) lymph node metastases. The aim of this study is to analyze the factors associated with PTMC with metastatic lymph nodes and its long-term prognosis.
MethodsRetrospective study whose study population consists of patients with PTMC (size ≤1cm). Patients with previous thyroid surgery, other synchronous malignancies and ectopic location of the PTMC were excluded. Two groups were compared: PTMC without metastatic lymph nodes (group 1) and PTMC with metastatic lymph nodes (group 2). A multivariate analysis using a logistic regression model and a Kaplan–Meier survival analysis with log-rank test were performed.
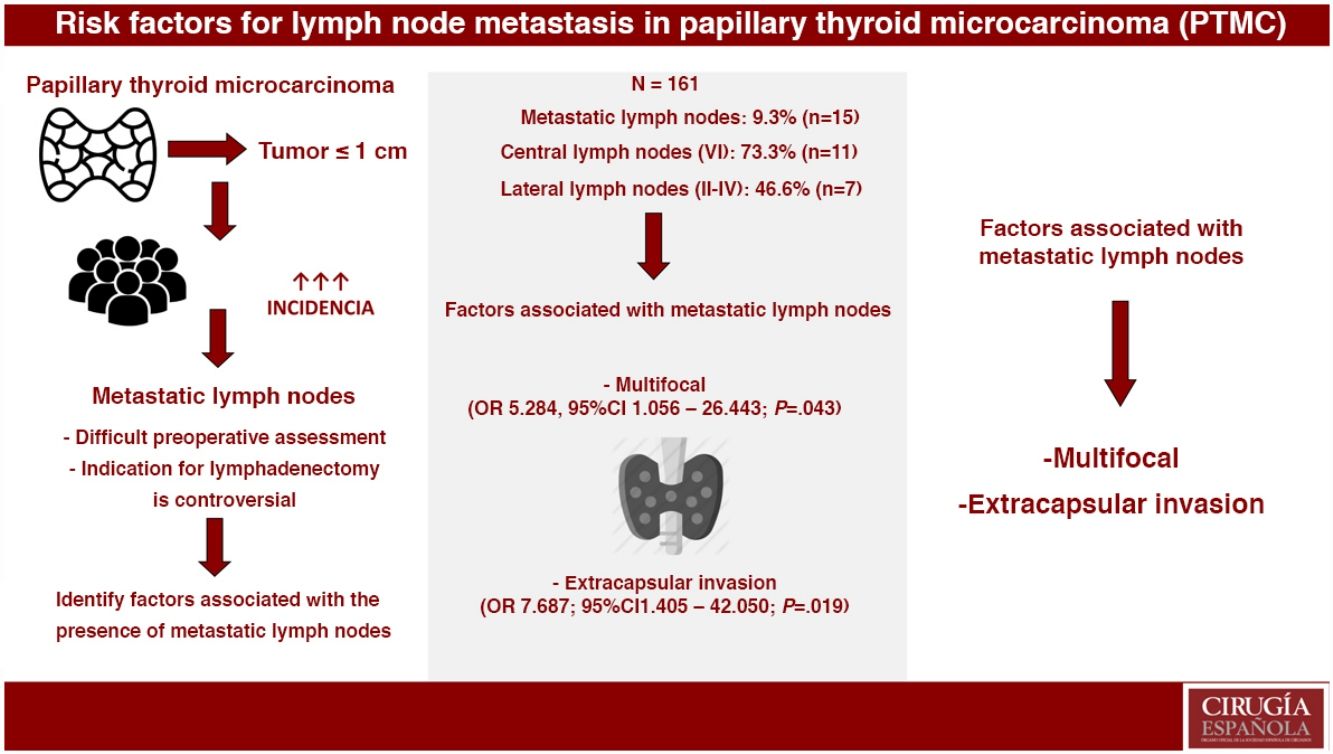
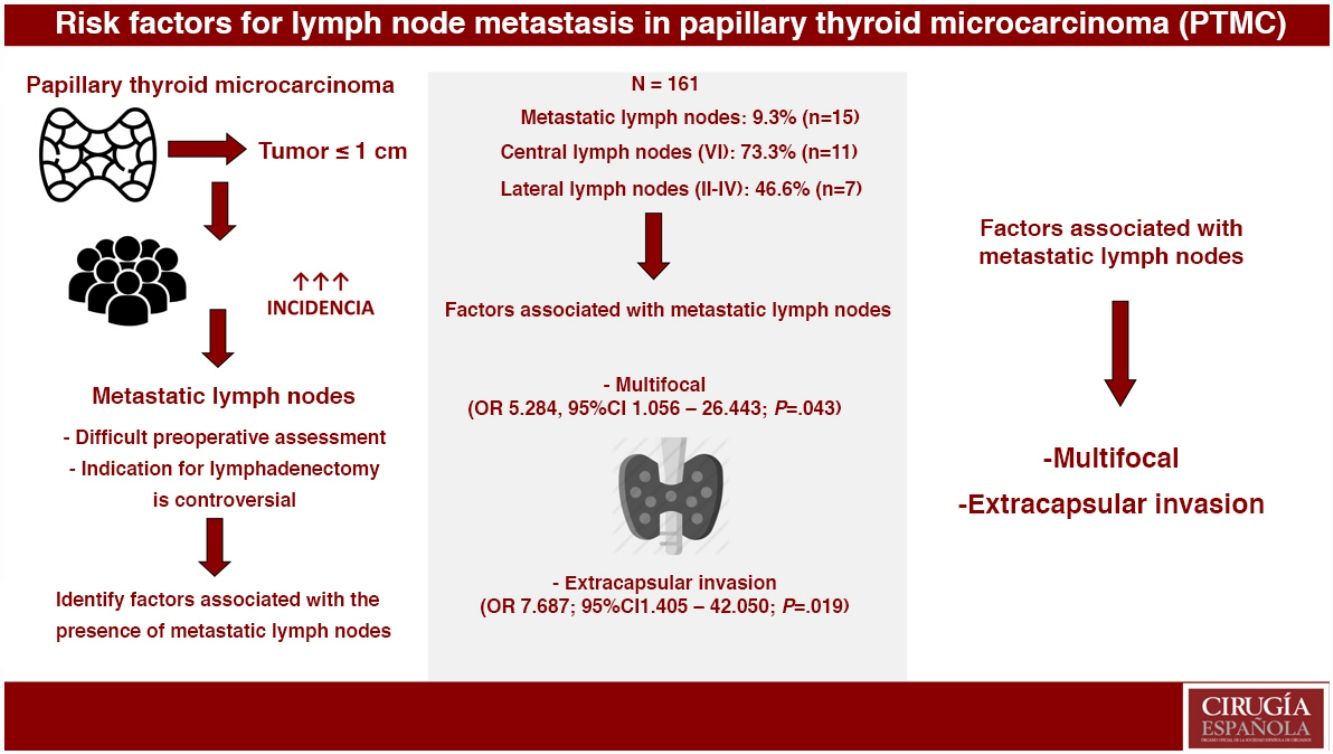
ResultsOut of the 161 selected patients, 9.3% (n=15) had metastatic lymph nodes. Multifocality (OR 5.284, 95%CI 1.056–26.443; P=.043) and extrathyroidal extension (OR 7.687, 95%CI 1.405–42.050; P=.019) were associated with the presence of metastatic lymph nodes. In PTMC with metastatic lymph nodes, more aggressive treatments were performed: lymphadenectomy (4.8% vs. 100%; P<.001) and radioactive iodine (24.7% vs. 100%; P<.001). During a mean follow-up of 119.8±65 months, one recurrence was detected in group 2 (0% vs. 6.7%; P=.093). No patients died due to the disease.
ConclusionsMultifocality and extrathyroidal extension of PTMC were associated with the presence of metastatic lymph nodes. Metastatic PTMC, with more aggressive treatments, presents an excellent long-term prognosis.
El microcarcinoma papilar de tiroides (MCPT) suele tener un curso indolente, pero algunos presentan factores de peor pronóstico, como la presencia de adenopatías metastásicas centrales (6,9-51,5%) y laterales (3-49,6%). El objetivo de este estudio es analizar los factores asociados al MCPT con adenopatías metastásicas y su pronóstico a largo plazo.
MétodosEstudio retrospectivo cuya población a estudio la constituyen los pacientes con MCPT (tamaño ≤1cm). Se excluyen los pacientes con cirugía tiroidea previa, otras patologías malignas sincrónicas, y localización ectópica del MCPT. Se comparan dos grupos: MCPT sin adenopatías metastásicas (grupo 1) y MCPT con adenopatías metastásicas (grupo 2). Se realizan un análisis multivariante mediante una regresión logística y un análisis de la supervivencia mediante el método de Kaplan-Meier y el test log-rank.
ResultadosDe los 161 pacientes seleccionados, el 9,3% (n=15) tuvo adenopatías metastásicas. La multifocalidad (OR 5,284, IC 95% 1,056-26,443; p=0,043) y la invasión extracapsular (OR 7,687, IC 95% 1,405-42,050; p=0,019) se asociaron con la presencia de adenopatías metastásicas. En el MCPT con adenopatías metastásicas, se realizaron tratamientos más agresivos: linfadenectomía cervical (4,8% vs. 100%; p<0,001) y radioyodo (24,7% vs. 100%; p<0,001). Durante un seguimiento medio de 119,8±65 meses se detectó una recidiva en el grupo 2 (0% vs. 6,7%; p=0,093). Ningún paciente falleció debido a la enfermedad.
ConclusionesLa multifocalidad y la invasión extracapsular del MCPT se asocian con la presencia de adenopatías metastásicas. El MCPT metastásico, con tratamientos más agresivos, presenta un excelente pronóstico a largo plazo.
Papillary thyroid microcarcinomas (PTMC) are papillary carcinomas measuring ≤1cm.1 The incidence of these lesions has increased considerably in recent years.2
The presence of metastatic lymph nodes in papillary thyroid carcinoma (PTC) is a factor for poor prognosis that has been widely documented in the literature, as it is associated with recurrence and mortality.3 In addition, the indications for lateral cervical lymph node dissection, especially in the central compartment, are controversial due to the low rate of lymph node involvement in PTC during preoperative evaluation. For these reasons, new techniques like selective sentinel lymph node biopsy are being applied to achieve better disease staging, although their usefulness is still not clear.4
Since there are no reliable and proven methods that provide adequate preoperative assessment of the presence or absence of metastatic lymph nodes, it is important to determine which factors of PTMC are associated with their presence in order to perform more aggressive treatments (cervical lymphadenectomy and/or thyroid remnant ablation with iodine-131 [I131]) in the appropriate patients to minimize persistence, recurrence and mortality associated with the disease.5
The objective of this study is to analyze and compare the differentiating factors and long-term prognosis of PTMC in patients with and without metastatic lymph nodes.
MethodsStudy PopulationWe conducted a retrospective study of a population of patients with a histopathological diagnosis of PTMC (papillary carcinoma ≤1cm1) treated surgically between 1995 and 2015. The patients included had a minimum follow-up of 2 years.
Patients with thyroid surgery prior to the diagnosis of PTMC were excluded, as were patients with other synchronous malignant thyroid or extrathyroid pathologies or ectopic location of PTMC (sublingual, thyroglossal cyst, struma ovarii, etc.).
Study GroupsThe following groups were analyzed and compared:
- •
Group 1: PTMC with no lymphadenopathies
- •
Group 2: PTMC with metastatic lymph nodes (central and lateral)
- ∘
Group 2.1: PTMC with central metastatic lymph nodes (compartment VI)
- ∘
Group 2.2: PTMC with lateral metastatic lymph nodes (compartment I–V)
In cases of PTMC with clinical diagnosis, total thyroidectomy is performed. In addition, central and/or lateral cervical lymph node dissection is conducted based on the presence of metastatic lymphadenopathy, when PTMC is diagnosed preoperatively by FNA of a metastatic lymph node, or intraoperatively by freezing when there is suspicion of metastatic lymphadenopathies. Whenever lateral cervical lymph node dissection is performed, ipsilateral central dissection is done. If there is lymph node involvement, multifocal presentation, aggressive histological variants (tall cells, solid, etc.), extracapsular invasion and/or involved resection margins, I131 is administered except when contraindicated.
In cases of PTMC with incidental diagnosis in patients who undergo surgery for presumably benign thyroid disease, the treatment is determined based on histopathological findings. Likewise, in cases of multifocality, aggressive histological variants (tall cells, solid, etc.), extracapsular invasion and/or affected resection margins, I131 is administered unless contraindicated.
Variables Under StudyFor the comparison of the study groups, the following variables are used:
Socio-PersonalAge and sex.
ClinicalFamily history of PTC, body mass index (BMI) measured in kg/m2, and thyroid function (hypothyroidism, euthyroidism, hyperthyroidism).
TherapeuticSurgical technique and ablation of thyroid remnant with I131.
HistopathologicalSize is defined by the maximum diameter of the lesion, measured in millimeters. In multifocal tumors, the size is defined by the focus with the largest maximum diameter. In cases where the size reaches statistical significance, the cut-off point is calculated after which good sensitivity and specificity is reached), focal type (the tumor is considered solitary if there is a single focus, or multifocal there is more than one focus; the maximum diameter of each tumor foci must measure ≤10mm, although the sum of all the maximum diameters of the foci totals more than 10mm), laterality (the tumor is considered unilateral if it only occupies one thyroid lobe and bilateral if it is multifocal and occupies both thyroid lobes), histopathological variants, capsular invasion, extracapsular invasion and involvement of the resection margins.
PrognosisPersistence is defined as the presence of clinical, biochemical or radiological evidence of disease (ultrasound, CT scan, full-body scan with I131 or PET/CT scan with 18F-FDG) in the first 6 months after surgery.
Recurrence is defined as the presence of clinical, biochemical or radiological evidence of disease (ultrasound, CT scan, I131 whole-body scintigraphy or PET/CT scan with 18F-FDG) 6 months or more after surgery.
Disease free interval (DFI)
Disease-related mortality
Statistical AnalysisThe collected data were analyzed in a database. For categorical variables, data were expressed using frequencies and percentages, then compared using Pearson's chi-squared test or Fisher's exact test when appropriate. For continuous quantitative variables, data are expressed as mean±standard deviation. The normal distribution of the variables was verified using the Kolmogorov–Smirnov test. The quantitative variables of the groups were compared using the Student's t test for independent data when they followed normal distribution. When the quantitative variables did not follow normal distribution, the non-parametric Mann–Whitney U test was used for comparison.
The multivariate analysis was conducted using binary logistic regression to evaluate the independent associations of all the factors that were statistically significant in the bivariate analysis. Results are presented as odds ratio (OR) with a 95% confidence interval (CI) and P-value.
The Kaplan–Meier method was used to analyze DFI, and the log-rank test was used to compare survival between groups.
A P value <.05 was considered statistically significant.
This study was approved by the Ethics Committee of our hospital.
ResultsThe diagnosis of PTMC was incidental in 54% (n=87) of patients, meaning that the diagnosis was made intraoperatively or during the histopathological analysis of thyroidectomy specimens of patients treated surgically for presumably benign thyroid pathology. In contrast, the diagnosis of PTMC was clinical in 46% (n=74) of patients, using fine-needle aspiration (FNA) cytology of a thyroid nodule (Bethesda classification categories III-VI) or FNA of a metastatic lymph node.
Study GroupsGroup 1. 90.7% (n=146) of patients did not present metastatic lymph nodes.
Group 2. Metastatic lymph nodes were evident in 9.3% (n=15), 46.7% of which (n=7) were micrometastases. Lymph node metastases were central (compartment VI) in 73.3% (n=11) and lateral (compartments II–IV) in 46.6% (n=7). Skip metastasis was observed in 57.1% (n=4) of patients with lateral lymphadenopathies.
Cervical lymphadenectomy was performed in 13.7% (n=22). Central emptying (compartment VI) was done in 100% (n=22), which was ipsilateral in 54.5% (n=12) and bilateral in 45.5% (n=10). Lateral lymph node dissection (compartments II–IV) was carried out in 40.9% (n=9), which was ipsilateral in 77.8% (n=7) and bilateral in 22.2% (n=2).
Differentiating Factors Between GroupsFactors Associated With the Presence of Metastatic Lymphadenopathy (Central+Lateral)The patients with PTMC and metastatic lymphadenopathies had a larger mean tumor size (5.1±2.8mm vs. 7±2.3mm; P=.015), higher frequency of tumor size ≥7mm, greater presence of multifocal lesions, more bilateral presentations, more extracapsular invasion and greater presence of involved resection margins (Tables 1 and 2).
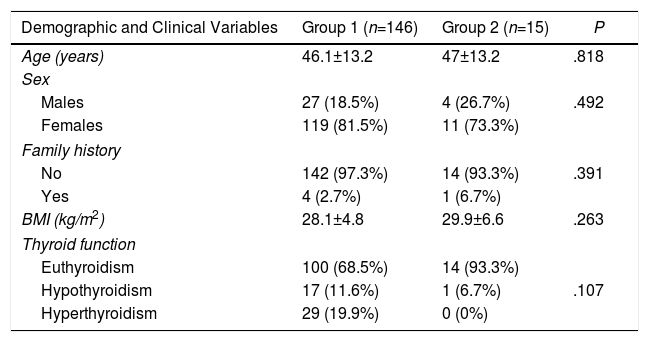
Comparison of Demographic and Clinical Variables According to the Presence or Absence of Metastatic Lymph Nodes (Central and Lateral) of the PTMC.
| Demographic and Clinical Variables | Group 1 (n=146) | Group 2 (n=15) | P |
|---|---|---|---|
| Age (years) | 46.1±13.2 | 47±13.2 | .818 |
| Sex | |||
| Males | 27 (18.5%) | 4 (26.7%) | .492 |
| Females | 119 (81.5%) | 11 (73.3%) | |
| Family history | |||
| No | 142 (97.3%) | 14 (93.3%) | .391 |
| Yes | 4 (2.7%) | 1 (6.7%) | |
| BMI (kg/m2) | 28.1±4.8 | 29.9±6.6 | .263 |
| Thyroid function | |||
| Euthyroidism | 100 (68.5%) | 14 (93.3%) | |
| Hypothyroidism | 17 (11.6%) | 1 (6.7%) | .107 |
| Hyperthyroidism | 29 (19.9%) | 0 (0%) | |
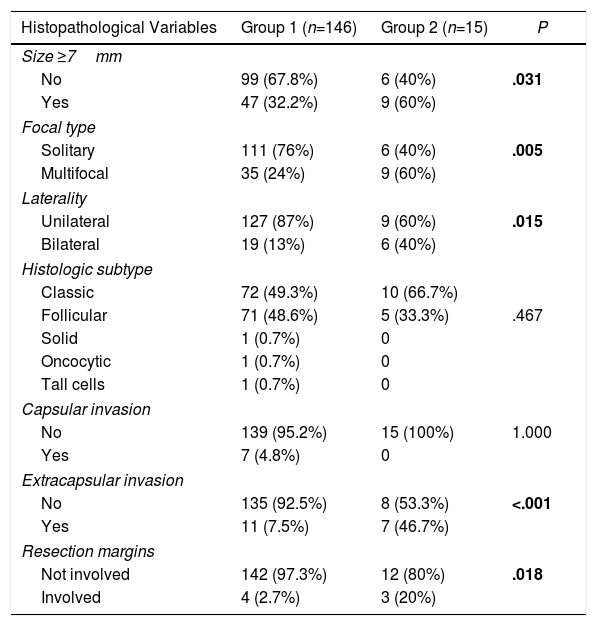
Comparison of Histopathological Variables According to the Presence or not of Metastatic Lymphadenopathies (Central and Lateral) of the PTMC.
| Histopathological Variables | Group 1 (n=146) | Group 2 (n=15) | P |
|---|---|---|---|
| Size ≥7mm | |||
| No | 99 (67.8%) | 6 (40%) | .031 |
| Yes | 47 (32.2%) | 9 (60%) | |
| Focal type | |||
| Solitary | 111 (76%) | 6 (40%) | .005 |
| Multifocal | 35 (24%) | 9 (60%) | |
| Laterality | |||
| Unilateral | 127 (87%) | 9 (60%) | .015 |
| Bilateral | 19 (13%) | 6 (40%) | |
| Histologic subtype | |||
| Classic | 72 (49.3%) | 10 (66.7%) | |
| Follicular | 71 (48.6%) | 5 (33.3%) | .467 |
| Solid | 1 (0.7%) | 0 | |
| Oncocytic | 1 (0.7%) | 0 | |
| Tall cells | 1 (0.7%) | 0 | |
| Capsular invasion | |||
| No | 139 (95.2%) | 15 (100%) | 1.000 |
| Yes | 7 (4.8%) | 0 | |
| Extracapsular invasion | |||
| No | 135 (92.5%) | 8 (53.3%) | <.001 |
| Yes | 11 (7.5%) | 7 (46.7%) | |
| Resection margins | |||
| Not involved | 142 (97.3%) | 12 (80%) | .018 |
| Involved | 4 (2.7%) | 3 (20%) | |
Significant differences are shown in bold.
There were no differences between groups 1 and 2 regarding the performance of total thyroidectomy (92.5% vs. 100%; P=.601). However, in patients with PTMC and metastatic lymphadenopathies, more cervical emptying was performed (4.8% vs. 100%; P<.001) and I131 was administered more frequently (24.7% vs. 100%; P<.001).
Factors Associated With the Presence of Central Metastatic Lymph Nodes (Compartment VI)Patients with PTMC and central metastatic lymph nodes had a larger mean tumor size (5.2±2.5mm vs. 7.4±2.5mm; P=.012), higher frequency of size ≥8mm (22.7% vs. 54.5%; P=.028), greater presence of extracapsular invasion (7.3% vs. 63.9%; P<.001), greater presence of involved resection margins (2.7% vs. 27.3%; P=.007) and higher frequency of lateral metastatic lymph nodes (2.7% vs. 27.3%; P=.007).
Factors Associated With the Presence of Lateral Metastatic Lymphadenopathy (Compartments II–IV)Patients with PTMC and lateral metastatic lymph nodes had a higher frequency of multifocal presentation (24.7% vs. 85.7%; P=.002), higher frequency of bilateral disease (13.6% vs. 57.1%; P=.012), higher frequency of resection margin involvement (3.2% vs. 28.6%; P=.031) and higher frequency of central metastatic lymph nodes (5.2% vs. 42.9%; P=.007).
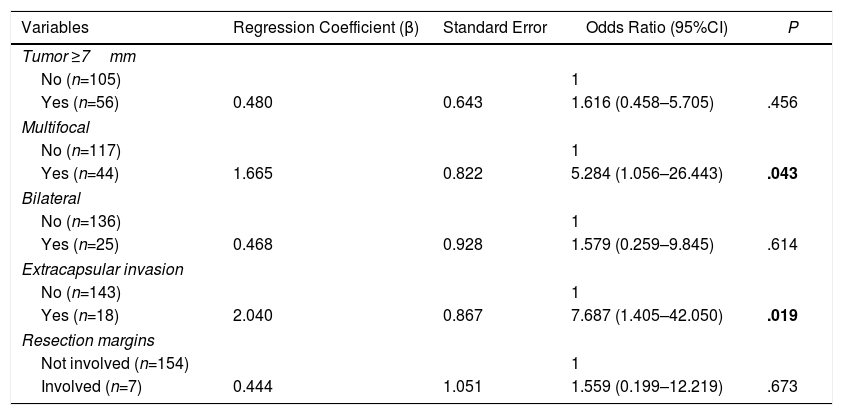
Multivariate AnalysisIn the multivariate analysis, the independent risk factors associated with the presence of metastatic lymph nodes (central+lateral) were: multifocal presentations and extracapsular invasion (Table 3).
Multivariate Analysis of Factors Associated With the Presence of Lymph Node Metastases (Central and Lateral) of the PTMC.
| Variables | Regression Coefficient (β) | Standard Error | Odds Ratio (95%CI) | P |
|---|---|---|---|---|
| Tumor ≥7mm | ||||
| No (n=105) | 1 | |||
| Yes (n=56) | 0.480 | 0.643 | 1.616 (0.458–5.705) | .456 |
| Multifocal | ||||
| No (n=117) | 1 | |||
| Yes (n=44) | 1.665 | 0.822 | 5.284 (1.056–26.443) | .043 |
| Bilateral | ||||
| No (n=136) | 1 | |||
| Yes (n=25) | 0.468 | 0.928 | 1.579 (0.259–9.845) | .614 |
| Extracapsular invasion | ||||
| No (n=143) | 1 | |||
| Yes (n=18) | 2.040 | 0.867 | 7.687 (1.405–42.050) | .019 |
| Resection margins | ||||
| Not involved (n=154) | 1 | |||
| Involved (n=7) | 0.444 | 1.051 | 1.559 (0.199–12.219) | .673 |
Significant differences are shown in bold.
The factors associated with the presence of central metastatic lymph nodes were: extracapsular invasion (odds ratio 16.276, 95%CI 2.505–105.770; P=.003) and the presence of lateral metastatic lymphadenopathies (odds ratio 20.451, 95% CI 2.526–165.568; P=.005).
The only risk factor associated with the presence of lateral metastatic lymph nodes was multifocality (odds ratio 14.789, 95%CI 1.135–192.634; P=.040).
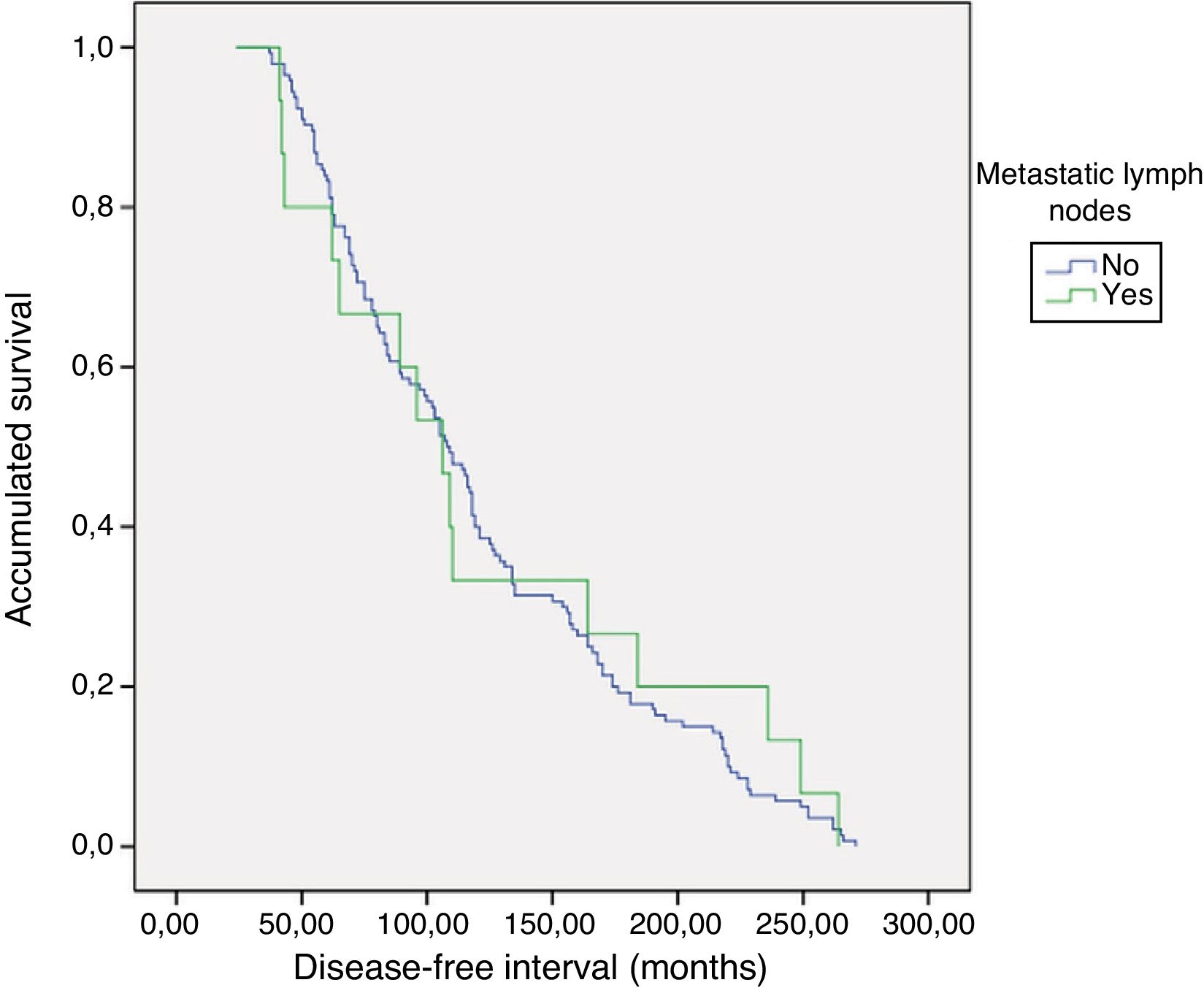
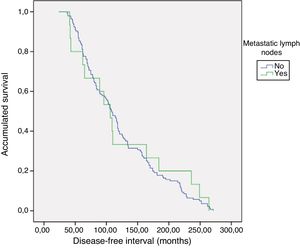
PrognosisWith a mean follow-up of 119.8±65 months (24–271), no persistence was detected, but recurrence of the disease was detected in one patient with metastatic lymphadenopathy (0% vs. 6.7%; P=.093). As for the DFI, there were no differences between groups 1 and 2 (121.5±5.3 vs. 124±19.9; P=.783) (Fig. 1). No patient died from the disease.
DiscussionWithout differentiating between central and lateral nodes,6 the scientific literature that has analyzed risk factors for metastatic lymph nodes in patients with PTMC concludes that patients with PTMC who have a larger tumor size, a higher frequency of multifocality, higher frequency of capsular invasion and/or a higher percentage of lymphovascular invasion have a significantly higher risk of presenting metastatic lymph nodes. Likewise, in this study, when central and lateral metastatic lymph nodes were not differentiated, multifocality was a risk factor associated with the presence of metastatic lymph nodes. In contrast, size and capsular invasion were not associated factors. In addition, this paper also highlights extracapsular invasion as a risk factor for lymph node involvement.
Regarding the presence of central metastatic lymph nodes, published series have reported figures that vary between 6.9 and 51.5%.7–11 This figure depends on whether or not cervical lymphadenectomy of the central compartment is performed, because if not the presence of subclinical lymph nodes with micrometastasis may go unnoticed. In this study, 6.8% of the patients had metastatic central lymph nodes, which is a lower figure compared to other studies.7–19 However, as previously mentioned, we must consider the fact that cervical lymph node dissection was not performed in most patients.
As for extracapsular invasion, this was associated with the presence of metastatic lymph nodes of the central compartment, as shown by several published studies.8,12–14,18 This finding could be explained by the invasion of the perithyroid fatty tissue, a circumstance that would favor spread to the regional nodes of the central compartment.
Studies have been published16 where the presence of lateral metastatic lymph nodes was associated with the presence of central metastatic lymph nodes. Similarly, our study has also demonstrated this association. This could be explained by the fact that 27.3% of the patients with central lymph node dissection had lateral metastatic lymph nodes, and only 2.7% of the patients without central metastatic lymph nodes had lateral metastatic lymph nodes.
Likewise, the medical literature has identified the following risk factors associated with the presence of central metastatic lymph nodes: male sex, age <45 years, location in the lower pole of the thyroid gland, multifocal, bilateral, capsular invasion and the classic histopathological variant.7–18
Therefore, in patients with extracapsular invasion and/or metastatic lymph nodes in the lateral compartment (levels II–IV), central lymph node dissection is recommended. Furthermore, it might not be a priority to consider performing a central lymph node dissection solely based on the size or multifocality of the PTMC.
In this study, 4.3% of patients had lateral metastatic lymph nodes, which is a low figure but it is consistent with other published studies (3%–49.6%).19–24 However, as previously mentioned, we must again take into account the low number of cervical lymph node dissections of the lateral compartment performed.
Furthermore, in this study, only multifocality was a risk factor associated with the presence of lateral metastatic lymph nodes, a finding that has also been documented in several published studies.9,10,18,20 However, bilateral presentation was not associated in the multivariate analysis with the presence of metastatic lymph nodes, contrary to what other studies show.18 Therefore, in patients with multifocal PTMC, it may be advisable to thoroughly evaluate lateral lymph node chains (levels II–IV) by imaging tests, either preoperatively if multifocality is detected on ultrasound or during follow-up after surgery.
In contrast with most studies, where the presence of central lymphadenopathies is associated with the presence of lateral metastatic lymph nodes in the multivariate analysis,7,10,15,16,18,22–25 this study has not shown this correlation. This finding could be explained by the fact that 57% of the patients with lateral metastatic lymph nodes had skip metastasis, which is lymph node metastases in the lateral compartment, with no involvement of the central compartment. In the literature, the percentage of skip metastasis is 0.6%–37.5%,26 a lower figure than in this study. This figure may be a coincidental finding, since only 7 patients presented metastatic lymph nodes in the lateral compartment, in contrast with other studies where the percentage of patients with metastatic lymph nodes is much higher.26 Even so, in the presence of lateral metastatic lymph nodes, central lymph node emptying is still recommended today.27
Finally, in the different studies published, other independent risk factors associated with the presence of lateral lymph node dissection have been described, such as: the location at the upper pole of the thyroid gland,15,16,19,21,23,25 the subcapsular location,19 male sex,9,10,19,20,24 age <459 or 5019 years, size >5mm,10,15,20 classical variant,9 loss of polarity and cell cohesion,7 capsular invasion,21 contact >25% of the tumor perimeter with the thyroid capsule,25 extracapsular invasion,9–11,18,20,22 the presence of calcifications,19,23,25 poorly defined margins23 and the presence of Hashimoto thyroiditis.22,23
Although the presence of metastatic lymph nodes has been a risk factor associated with the recurrence of PTMC in several studies,28–30 in this study it was not an associated factor. When we compared DFI between patients with and without metastatic lymph nodes, we observed no differences between the two groups. However, we must also consider that patients who present metastatic lymph nodes have a higher frequency of more aggressive treatments, such as cervical lymph node dissection and I131 thyroid remnant ablation. Therefore, we could state that PTMC has an excellent long-term prognosis in the presence of metastatic lymph nodes, although it requires more aggressive treatments.
This study has the limitations of being retrospective and the small sample sizes for the groups of patients with PTMC and central and lateral metastatic lymph nodes (n=15), only central nodes (n=11) and only lateral nodes (n=7). Therefore, the statistical power is lower, and the results must be interpreted with caution.
In conclusion, multifocality and extracapsular invasion of PTMC are associated with the presence of metastatic lymph nodes. Furthermore, with more aggressive treatments, these patients have an excellent long-term prognosis.
Conflict of InterestsThe authors declare that there are no conflicts of interest.