Robotic assisted transanal polipectomy may have advantages compared with the conventional transanal minimally invasive surgery technique. We evaluate the safety, feasibility and advantages of this technique.
MethodsBetween February 2014 and October 2015, 9 patients underwent robotic transanal polypectomy. We performed a retrospective study in which we analyze prospectively collected data regarding patient and tumor characteristics, perioperative outcomes, pathological report, morbidity and mortality.
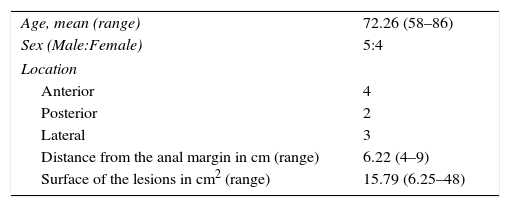
ResultsA total of 5 male and 4 female patients underwent robotic TAMIS. Lesions were 6.22cm from the anal verge. Mean size was 15.8cm2. All procedures were performed in the lithotomy position. Closure of the defect was performed in all cases. Mean blood loss was 39.8ml. Mean operative time was 71.9min. No severe postoperative complications or readmissions occurred. Median hospital stay was 2.5 days.
ConclusionsRobotic TAMIS is useful to treat complex rectal lesions. Our transanal platform allowed a wider range of movements of the robotic arms and to perform all procedures in the lithotomy position.
La polipectomía transanal asistida por robot puede tener ventajas respecto a la cirugía laparoscópica transanal convencional. Evaluamos la seguridad, factibilidad y ventajas potenciales de esta técnica.
MétodosEntre febrero de 2014 y octubre de 2015, se realizaron un total de 9 polipectomías transanales en nuestro centro. Realizamos un estudio retrospectivo de datos recogidos prospectivamente referentes a las características de los pacientes, tumores tratados, resultados perioperatorios, informe anatomopatológico y morbimortalidad.
ResultadosFueron tratados 5 hombres y 4 mujeres mediante polipectomía robótica transanal. Las lesiones se encontraban a una distancia media de 6,2cm respecto al margen anal. La superficie media de las lesiones fue de 15,8 cm2. Todos los procedimientos fueron realizados en posición de litotomía, independientemente de la localización de la lesión. Se realizó cierre del defecto en todos los casos. El sangrado intraoperatorio medio fue de 39,8mL. El tiempo quirúrgico medio fue de 71,9 min. No se objetivaron complicaciones graves postoperatorias ni reingresos y la estancia mediana fue de 2,5 días.
ConclusionesLa polipectomía transanal asistida por robot es útil para tratar lesiones rectales complejas o voluminosas. Nuestra plataforma de acceso transanal permitió un amplio rango de movimientos con los pacientes en litotomía.
Transanal endoscopic microsurgery (TEM), introduced by Dr. Gerhard Buess more than 30 years ago1 has demonstrated its superiority over conventional transanal excision for the resection of rectal tumors, mainly due to its capacity to carry out high-quality resections.2,3 Langer et al. showed better long-term oncological results when they compared 54 TEM resections with conventional transanal excisions. When the results were compared between TEM and conventional transanal excision from a 17-year period, de Graaf et al. found a lower rate of fragmentation, recurrence and morbidity in TEM, with higher rates of negative resection margins.4,5 In spite of all this, TEM has not been adopted in a generalized manner, due to its complex learning curve and the cost of the equipment necessary.6
Transanal minimally invasive surgery (TAMIS) is a novel procedure developed by Atallah et al.7 as a hybrid technique between TEM and single-incision laparoscopic surgery for the resection of rectal lesions. TAMIS was designed as an affordable platform for many hospitals to provide access to lower rectal lesions for all surgeons with advanced laparoscopic skills and familiar with rectal surgery.
The indications for TAMIS are similar to those of TEM or the conventional transanal approach.8 The TAMIS should be considered in patients with benign tumors or T1, properly selecting those with good prognostic factors in whom the risk of lymph node involvement is low.9
Although TAMIS is still under development, it has been explored extensively worldwide, with more than 30 retrospective studies to date covering more than 400 procedures.10 TAMIS has been shown to be feasible in benign lesions and well selected early stage malignant lesions in the middle and lower rectum, making it a promising alternative to TEM.11
There are certain limitations when carrying out the TAMIS. Conventional laparoscopic instruments should be used in a limited surgical field like the rectal lumen. In this field, work angles and triangulation can be affected significantly. It is sometimes necessary to change the camera access port to a work port, and vice versa. These space restrictions sometimes make it necessary to force the access angulation of the work port in the TAMIS or to constantly move the orientation of the work port in the TEM. These changes can oscillate the pneumorectum, making the procedure more tedious and technically complicated. In some cases, the work port itself can be expelled from the rectal lumen due to these oscillations in pressure. The closed work angles and the need to change instrument or camera positions often make it essential to have either an assistant who is experienced in this type of procedures or a second expert surgeon.12
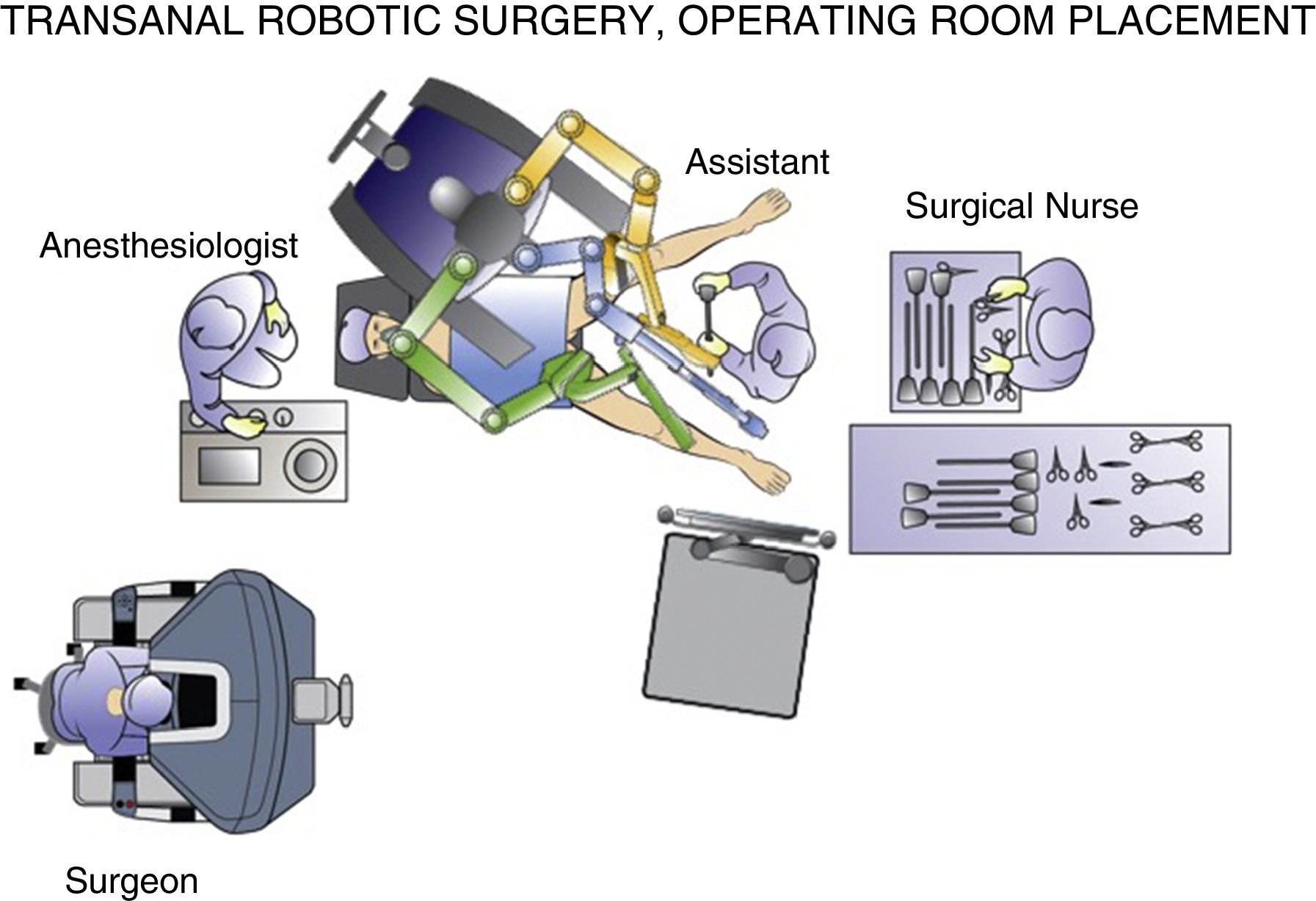
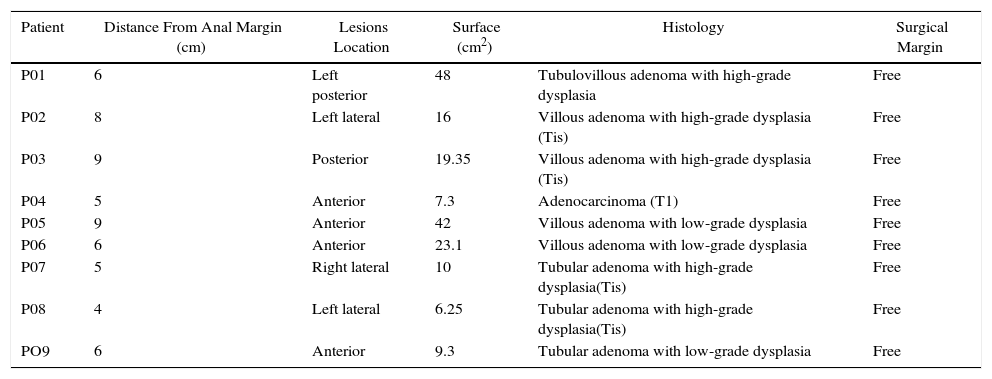
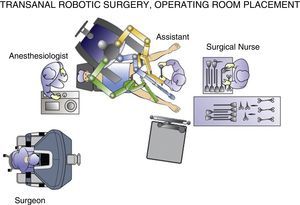
Perhaps the best way to express the advantage provided by the da Vinci surgical robot (Intuitive Surgical, Sunnyvale, CA, USA) is that it allows the surgeon to perform the intervention in a clear surgical field, with a magnified 3-D image and articulated instruments free of tremor transmission. These characteristics should minimize the inherent difficulties of endoluminal surgery.13 In robot-assisted procedures, the surgeon can be in an ergonomic position on the console while the assistant is at the patient's side (Fig. 1). The control of the camera by the assistant is no longer a problem, since it is done by the surgeon himself from the console. In the specific case of robot-assisted transanal surgery, this instrument facilitates dissection of the rectal wall at the desired angles and closure of the defect after polypectomy. Sutures and knotting are facilitated clearly with robotic assistance. All these reasons make transanal robotic surgery a field of great interest.
The experience in transanal robotic surgery for the local excision of lesions is still very limited.14–20 There are very few publications and almost all of them with a small number of cases. At a time when the technique is not yet standardized, we present our initial experience after a series of cases.
The objective of this study is to define the postoperative and oncological results of our initial series of cases treated with transanal robotic polypectomy and to identify the potential benefits of this approach.
MethodsWe conducted a retrospective study of 9 patients with rectal lesions who underwent consecutive transanal robotic surgery between February 2014 and October 2015. All patients signed a specific informed consent form for TAMIS, and the guidelines for proper clinical practice of our institution were followed. The use of the transanal access port and transanal robotic surgery for the excision of rectal tumors was approved by the Ethics Committee of our region, as was the retrospective study of prospective database information.
At our center, the indication for TAMIS is routinely benign rectal lesions or T1 rectal neoplasms with criteria for a good prognosis in which the risk of lymph node metastasis is low. In cases of lesions with uncertain diagnoses between T1 and T2 with no suspected lymph node metastasis, TAMIS is used as an “excisional biopsy”, followed by the appropriate treatment determined by the definitive pathology report, if necessary. All patients are studied preoperatively by colonoscopy, thoracoabdominal-pelvic computed tomography and magnetic resonance imaging. Endorectal ultrasound is used when the lesions are accessible to rigid transanal ultrasound. All patients receive antibiotic and thromboembolic prophylaxis. The decision to perform or not mechanical bowel preparation of the intestine is made depending on the preferences of the surgeon and the possibility to penetrate the peritoneal cavity during transanal resection of the polyp.
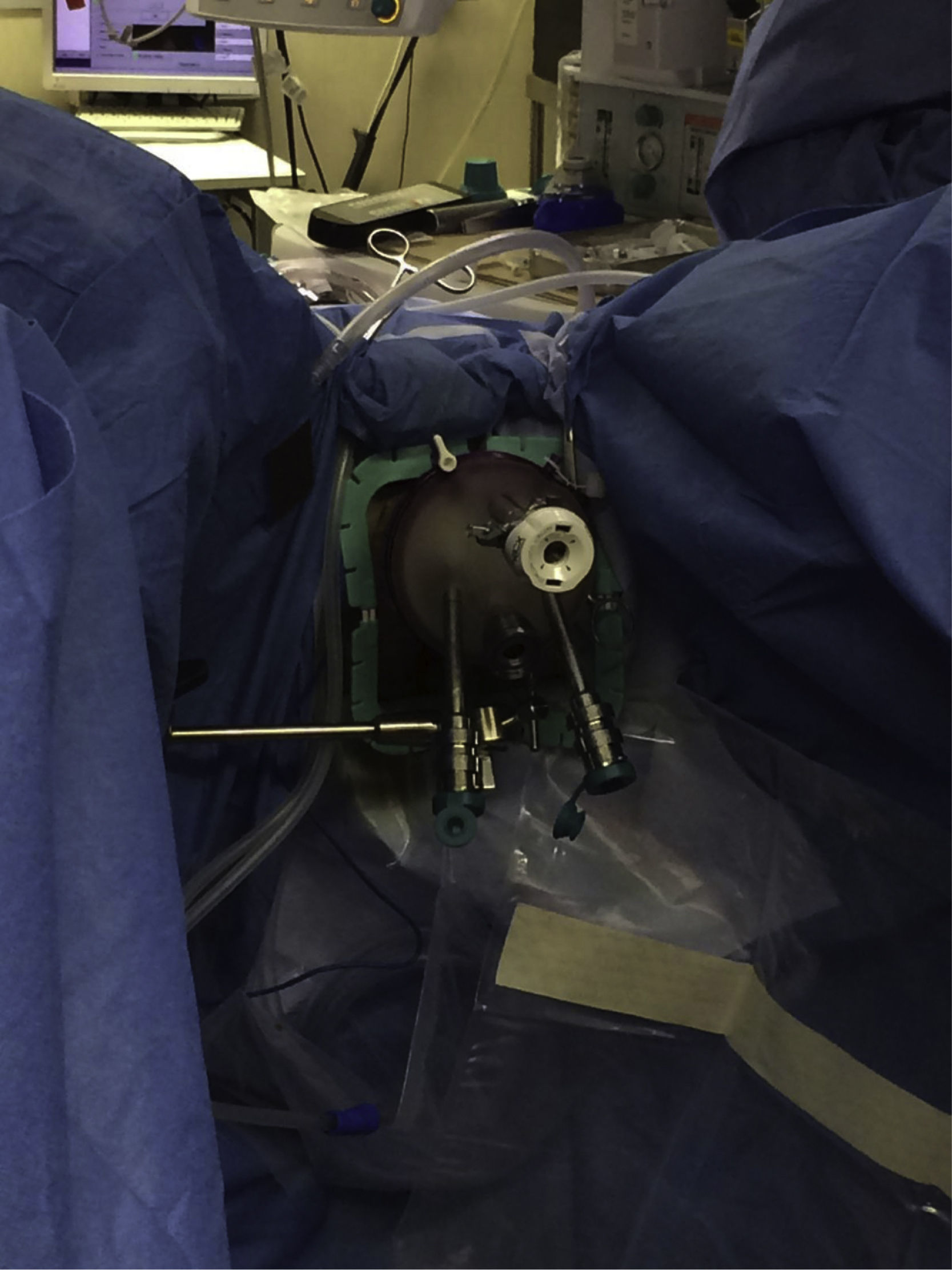
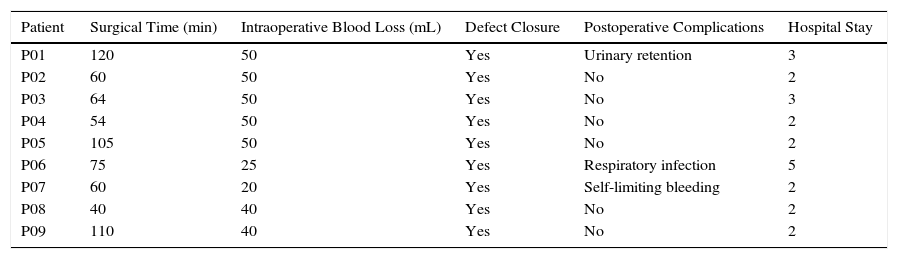
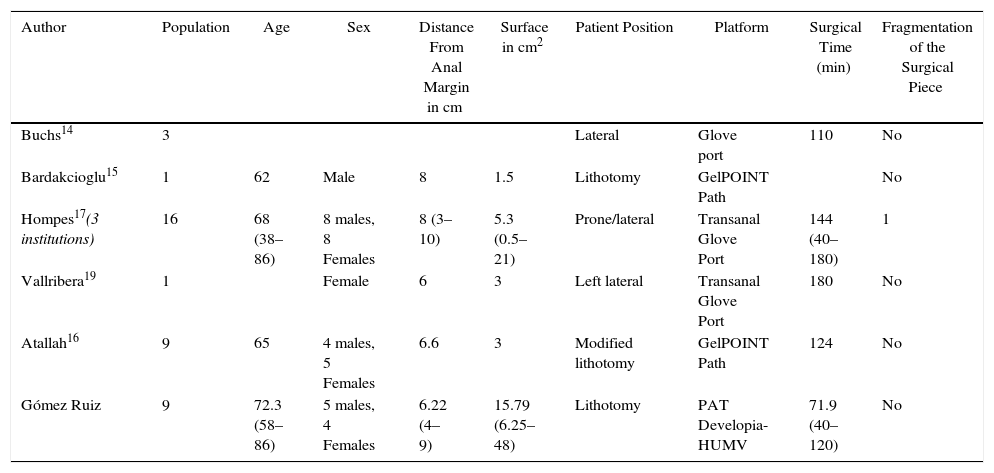
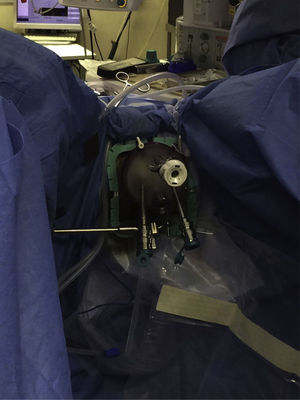
The procedures were performed under general anesthesia with deep muscle relaxation in order to avoid the “respiratory movements” of the rectal lumen. All the patients were operated on in a lithotomy position with low leg supports, regardless of the location of the rectal lesion. Occasionally, patients were placed with a slight Trendelenburg when necessary. The port of access used was the PAT transanal access port (Developia, Santander, Spain), affixed to the surgical table by means of an articulated fixation arch (Karl Storz, GmbH, Tuttlingen, Germany). The PAT was covered externally with a GelPOINT access platform (Applied Medical, Rancho Santa Margarita, CA, USA). A conventional 12-mm trocar was placed in the upper part of the GelPOINT for the optics of the robot. For the insertion of the robotic instruments through arms 1 and 2, two 8-mm trocars were placed on each side of the GelPOINT and an additional trocar was placed at the lower part of the GelPOINT for the assistant. The latter was preferably an 8-mm trocar for the AirSeal insufflation system (Conmed, Utica, NY, USA) (Fig. 2). In the robotic arm 2, a bipolar fenestrated clamp was used, and monopolar scissors were used in arm 1. Pneumorectum was maintained between 10 and 15mmHg, either with a conventional insufflation system or AirSeal (Conmed, Utica, NY, USA). The carriage of the da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was anchored to the patient from the left side and on the left leg (Fig. 3).
The dissection of the rectal lesions was performed with monopolar scissors; bipolar clamps were used for proper hemostasis. Special care was taken to maintain the dissection perpendicular to the rectal wall in order to ensure a full-thickness resection away from the margin of the lesions. The resection defect was closed in all cases with one or several continuous sutures with 3–0 absorbable monofilament. A robotic holder on arm 1 and the bipolar fenestrated clamp on arm 2 were used to suture the rectal wall. The specimen was analyzed in our pathology laboratory. In the postoperative period, a multimodal recovery protocol was followed, in which oral intake was reinitiated the same day of the surgery and patients were discharged without complications on the second postoperative day.
Follow-up was conducted in or outpatient clinic from 3 weeks post-op and then consecutively every 3 months, using rigid rectoscope. In cases with suspected local recurrence, biopsies were taken with rigid rectoscope at the office visit. During follow-up in the outpatient setting, functional results were assessed using the Cleveland Clinic Incontinence Score.
The following items were prospectively recorded: age, sex, lesion size, lesion location, distance from the anal margin, intraoperative bleeding, closure of the defect, duration of the procedure, morbidity, median hospital stay, readmission and mortality.
As for the preoperative evaluation, data are presented for mean age, lesion size and distance from the anal margin, with ranges. Intraoperative results included average intraoperative blood loss and mean duration of the operation, with ranges. The mean and median postoperative hospital stays are also presented.
ResultsThe procedure was performed in 9 patients without the need for conversion to another technique. The demographic characteristics of the patients and the characteristics of the lesions are shown in Tables 1–4.
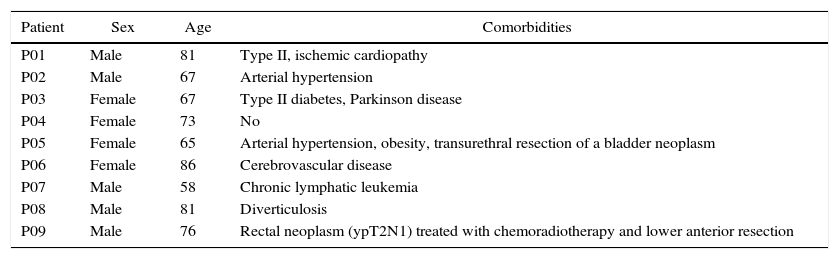
Demographic Characteristics And Preoperative Comorbidities.
| Patient | Sex | Age | Comorbidities |
|---|---|---|---|
| P01 | Male | 81 | Type II, ischemic cardiopathy |
| P02 | Male | 67 | Arterial hypertension |
| P03 | Female | 67 | Type II diabetes, Parkinson disease |
| P04 | Female | 73 | No |
| P05 | Female | 65 | Arterial hypertension, obesity, transurethral resection of a bladder neoplasm |
| P06 | Female | 86 | Cerebrovascular disease |
| P07 | Male | 58 | Chronic lymphatic leukemia |
| P08 | Male | 81 | Diverticulosis |
| P09 | Male | 76 | Rectal neoplasm (ypT2N1) treated with chemoradiotherapy and lower anterior resection |
Pathology Results.
| Patient | Distance From Anal Margin (cm) | Lesions Location | Surface (cm2) | Histology | Surgical Margin |
|---|---|---|---|---|---|
| P01 | 6 | Left posterior | 48 | Tubulovillous adenoma with high-grade dysplasia | Free |
| P02 | 8 | Left lateral | 16 | Villous adenoma with high-grade dysplasia (Tis) | Free |
| P03 | 9 | Posterior | 19.35 | Villous adenoma with high-grade dysplasia (Tis) | Free |
| P04 | 5 | Anterior | 7.3 | Adenocarcinoma (T1) | Free |
| P05 | 9 | Anterior | 42 | Villous adenoma with low-grade dysplasia | Free |
| P06 | 6 | Anterior | 23.1 | Villous adenoma with low-grade dysplasia | Free |
| P07 | 5 | Right lateral | 10 | Tubular adenoma with high-grade dysplasia(Tis) | Free |
| P08 | 4 | Left lateral | 6.25 | Tubular adenoma with high-grade dysplasia(Tis) | Free |
| PO9 | 6 | Anterior | 9.3 | Tubular adenoma with low-grade dysplasia | Free |
Perioperative Results.
| Patient | Surgical Time (min) | Intraoperative Blood Loss (mL) | Defect Closure | Postoperative Complications | Hospital Stay |
|---|---|---|---|---|---|
| P01 | 120 | 50 | Yes | Urinary retention | 3 |
| P02 | 60 | 50 | Yes | No | 2 |
| P03 | 64 | 50 | Yes | No | 3 |
| P04 | 54 | 50 | Yes | No | 2 |
| P05 | 105 | 50 | Yes | No | 2 |
| P06 | 75 | 25 | Yes | Respiratory infection | 5 |
| P07 | 60 | 20 | Yes | Self-limiting bleeding | 2 |
| P08 | 40 | 40 | Yes | No | 2 |
| P09 | 110 | 40 | Yes | No | 2 |
The defect was closed in all cases in our series. Mean intraoperative blood loss was 39.8mL (20–50mL). The surgical time used for this type of procedure, including the robot anchoring time, was 71.9min (40–120). No intraoperative complications were observed. In the postoperative period, observed complications included urinary retention and respiratory infection (both Clavien II). Another patient presented self-limiting postoperative bleeding that did not require transfusion or surgical procedures (Clavien I). Re-hospitalizations were not necessary and no re-operations were performed. The average stay of the patients was 2 days (1–5 days) and the median was 2.5 days. No mortality was observed.
During the procedures, the following robotic instruments were used: monopolar scissors, bipolar fenestrated clamp and a robotic holder. The cost of the instruments per procedure was €1165.10. The PAT was developed in conjunction with Developia and is sterilizable, so there was no cost to the hospital. The GelPOINT and Airseal had a combined cost of €555. Therefore, the total expense in materials was €1720.1, without considering the initial investment in the surgical system.
In the pathology study, no lesion fragmentation was observed, and all cases presented free resection margins. The mean surface of the lesions was 15.79cm2. Eight patients had adenomatous polyps (4 of them with high-grade dysplasia [Tis]). In one case, an infiltrating adenocarcinoma was observed with criteria for a good prognosis that was considered correctly treated.
During a mean follow-up of 18 months (15–23 months), no functional alterations or local recurrences were observed. After 6 months of postoperative follow-up, the Cleveland Clinic Incontinence Score was 0 in the 9 patients.
DiscussionCurrently, the number of local resections for the treatment of early rectal cancer with good prognostic criteria is experiencing a significant increase, given the low rate of postoperative complications compared with anterior resection of the rectum,21 the lower number of functional alterations22 and the possibility of preserving not only the anal sphincter but the entire rectum.23 Most surgeons limit the use of conventional transanal resection to tumors smaller than 4cm in diameter and located 6–8cm from the anal margin.24 This approach is associated with access difficulties, less precision, difficult visualization, higher rates of local recurrence and lower rates of disease-free survival. It has been hypothesized that suboptimal visualization is the main cause of the increased risk of affected margins and tumor fragmentation.25 TEM has shown excellent long-term oncological results as a curative treatment for early rectal carcinomas without high-risk criteria when a full-thickness resection is performed. In high-risk carcinomas, treatment by TEM alone should be assessed only as palliative treatment.26
Currently, there are two transanal access ports approved by the Food and Drug Administration in the USA for the TAMIS: the SILS port (Medtronics, Minneapolis, USA) and the GelPOINT system (Applied Medical, Rancho Santa Margarita, USA).27 Both ports are easily insertable transanally and provide insufflation for the pneumorectum through its own channel. The instruments necessary for the intervention are conventional laparoscopic instruments that can usually be found in the operating room.
TAMIS is now indicated for the resection of benign or early malignant rectal lesions with good prognostic criteria. Locally advanced lesions or those with poor prognostic criteria in the middle or lower rectal third require total mesorectal excision, either anterior (lower anterior resection) or transanal (transanal total mesorectal excision). In cases with sphincter involvement, abdominoperineal resection is considered the technique of choice for these patients, unless comorbidities contraindicate major surgery.2 There are also other possible indications for TAMIS, such as the resection of residual scars after chemoradiotherapy in the treatment of locally advanced rectal neoplasms in order to confirm complete clinical-pathological response (ypT0).28–30
Although TAMIS has been used to resect rectal lesions along the entire rectum, its best indication is probably for the resection of lesions in the middle and lower third. The versatility of TAMIS also allows for treatment of rectovaginal/rectourethral fistulae, hemostasis of low gastrointestinal bleeding or extraction of foreign bodies. Probably the most interesting and promising indication today is transanal total mesorectal excision in patients with complex pelvis (narrow pelvis, large prostate, obesity, etc.).31
The ports currently available for the TAMIS and approved by the Food and Drug Administration do not provide good access for the procedure in all patients.32 Furthermore, there are certain cases in which TAMIS remains a technically complicated procedure due to the volume or location of the lesion, requiring the patient to be placed in positions that complicate the procedure from an anesthetic standpoint.
The potential advantages of transanal robotic surgery include excellent ergonomics, 3D magnification of the image, elimination of tremor, filtering for precise movements and articulated instruments with multiple degrees of freedom of movement. All these characteristics make the robotic instruments superior to the conventional ones for working in limited spaces where conflicts between the instruments and the camera are frequent and interrupt the flow of the procedure, as well as its accuracy.31 Atallah et al. published their initial experience in transanal robotic surgery in a cadaver model12 and concluded that it is a feasible technique that enables for simple, quick closure of defects. Hompes et al. also published the feasibility of this technique using the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA) and a glove port.33
From our point of view, and after our initial experience, this technique also has at least 2 other potential advantages, such as having an extra transanal instrument handled by the transanal assistant, as well as facilitating learning when a double console is available. These advantages and those previously mentioned make transanal robotic surgery a very promising technique.
As a main drawback, we have observed that the expense in surgical materials is higher than usual. The total expenditure of €1720.10 per procedure includes both the port and the insufflation system, common to any TAMIS procedure, although it is true that the instrument cost is clearly higher than that of this technique performed with a conventional laparoscopic instrument.
To date, there have been few publications about robot-assisted transanal polypectomy. Many of them are isolated clinical cases or series of less than 5 cases. There are different access ports that have been used to date; the most widely used is the glove port.17 Hompes et al. have published the experience of 3 institutions with a total of 16 cases. In this publication, they describe the technical difficulties that have been encountered with the glove port, mainly related with pneumorectum leaks, which required relocation of the port on several occasions. Buchs et al. also report having used the glove port. Bardakcioglu et al. published their experience in a patient with a flat polyp measuring 1.5cm in diameter, 8cm from the anal margin.15 Atallah has published an experience similar to ours in 9 patients.20 The position of the patient on the surgical table has varied in the different publications and this could be seen as one of the disadvantages of the technique, having to adapt the position of the patient to the da Vinci Si surgical system. In the experience of Atallah and Bardakcioglu, the position chosen was modified lithotomy. Others, however, have used lateral decubitus to overcome the aforementioned difficulties of anchoring the robot to the patient.14,17,19
In our series, the port used was the PAT, developed in our institution through collaboration with engineers at Developia. As we have described in this scientific publication and in others,34–36 this port has also allowed us to perform transanal total mesorectal excision with robotic assistance in patients with locally advanced rectal cancer. One of the advantages we see in the use of this technique with this port is that we have been able to operate on all patients in the lithotomy position, with the resulting benefits for patients from an anesthetic standpoint. In Tables 5 and 6, we compared our series with that of other groups. Our surgical time was shorter than that of the rest of the groups and our specimen resections were complete, with no fractionation, unlike the experience presented by Hompes et al.17 Additionally, the defects were closed in all cases, unlike other series.17,20 Moreover, our resection margins were free in all cases, unlike the multicenter series by Hompes, who presented involvement of the margin in 3 cases,17 or that of Atallah, who reported affected margins in 3 out of 9 cases.20 Our series presents a lesion size significantly greater than that of other series, as shown in the comparative table, with a low complication rate and mild complications (Clavien I–II).
Review of the Literature and Comparative Demographic Data.
| Author | Population | Age | Sex | Distance From Anal Margin in cm | Surface in cm2 | Patient Position | Platform | Surgical Time (min) | Fragmentation of the Surgical Piece |
|---|---|---|---|---|---|---|---|---|---|
| Buchs14 | 3 | Lateral | Glove port | 110 | No | ||||
| Bardakcioglu15 | 1 | 62 | Male | 8 | 1.5 | Lithotomy | GelPOINT Path | No | |
| Hompes17(3 institutions) | 16 | 68 (38–86) | 8 males, 8 Females | 8 (3–10) | 5.3 (0.5–21) | Prone/lateral | Transanal Glove Port | 144 (40–180) | 1 |
| Vallribera19 | 1 | Female | 6 | 3 | Left lateral | Transanal Glove Port | 180 | No | |
| Atallah16 | 9 | 65 | 4 males, 5 Females | 6.6 | 3 | Modified lithotomy | GelPOINT Path | 124 | No |
| Gómez Ruiz | 9 | 72.3 (58–86) | 5 males, 4 Females | 6.22 (4–9) | 15.79 (6.25–48) | Lithotomy | PAT Developia-HUMV | 71.9 (40–120) | No |
Review of the Literature; Comparative Perioperative and Histological Results.
| Author | Defect Closure | Histology | Resection Margin | Complications | Technical Problems |
|---|---|---|---|---|---|
| Buchs14 | 3/3 | Pneumoperitoneum | |||
| Bardakcioglu15 | 1 adenoma | Free | No | ||
| Hompes17 (3 institutions) | 13/16 | 6 adenomas, 4 adenocarcinomas, 6 scars from previous resections | 2 affected | 2 urinary retention | Glove port dysfunction |
| Atallah16 | 5/9 | 6 adenomas, 3 invasive carcinomas | 1 affected | Self-limiting bleeding, pneumoperitoneum, atrial fibrillation | |
| Gómez Ruiz | 9/9 | 8 adenomas, 1 adenocarcinoma | Free | Urinary retention, respiratory infection, self-limiting bleeding | None |
In view of these data, we believe that robot-assisted transanal polypectomies using our PAT port are feasible, safe and have the following advantages: they allow for resections of large polyps in lithotomy regardless of location, with adequate surgical resection, no severe postoperative complications and within an adequate surgical time. The mid-term functional results are equivalent to other reports in the literature using TAMIS and other ports.
Patients with indications for the transanal robotic approach are those with voluminous polyps or anterior polyps in whom prone position should be avoided for anesthesia reasons or comorbidities. It is possible that these technically more demanding cases justify the increased expense involved given the observed results. At our hospital, some 12 transanal resections are performed per year, about half of which had the aforementioned indication. The development of advanced endoscopic excision techniques means that fewer and fewer patients with small polyps are referred by endoscopists for resection via TAMIS, therefore the percentage of cases that are technically demanding is increasing.
The optimization and lowering of the costs of this technique, including the improvement of the access port, the design of new instruments and a flexible camera, will increase the reproducibility and standardization of this technique. We believe that prospective and randomized studies are necessary to evaluate the cost-effectiveness of this approach for this indication.
Conflict of InterestsDr. M. Gómez Ruiz collaborates as a proctor and consultant in robotic surgery with Intuitive Surgical, Abex and Medtronic.
The authors would like to thank the professionals at the Hospital Universitario Marqués de Valdecilla and the Cantabrian Healthcare Service for their permanent support of the development of colorectal robotic surgery, as well as the support shown for different research projects in this area.
The authors would also like to acknowledge the support of our families.
Please cite this article as: Gómez Ruiz M, Cagigas Fernández C, Alonso Martín J, Cristobal Poch L, Manuel Palazuelos C, Barredo Cañibano FJ, et al. Polipectomía transanal asistida por robot: ¿tiene alguna indicación? Cir Esp. 2017;95:601–609.