Local flaps are a group of surgical procedures that can solve the thoracic closure of large defects after breast cancer surgery with low morbidity. Its use in skin necrosis complications after conservative surgery or skin sparing mastectomies facilitates the initiation of adjuvant treatments and reduces delays in this patient group. This article describes the anatomical basis for the planning of thoracic and abdominal local flaps. Also, the application of these local flaps for closing large defects in the chest and selective flaps for skin coverage by necrosis in breast conserving surgery.
Los colgajos por rotación constituyen un grupo de procedimientos quirúrgicos que permiten solventar el cierre de grandes defectos torácicos después de la cirugía oncológica de la mama con una menor morbilidad y dificultad técnica respecto a los colgajos a distancia. Su utilización en las complicaciones por necrosis cutánea después de una cirugía conservadora o mastectomías preservadoras de piel permite el inicio de los tratamientos adyuvantes y disminuye las demoras en este grupo de pacientes. Este artículo describe los fundamentos anatómicos para la planificación de colgajos por rotación torácicos y abdominales. Asimismo, se muestra la aplicación de estos colgajos para el cierre de grandes defectos en el tórax y de colgajos selectivos para la cobertura cutánea después de una necrosis durante la cirugía conservadora de mama.
Currently, oncologic surgery intends to preserve the body image of most women who are treated surgically for breast cancer, which is made possible by the preservation of anatomical structures in breast-conserving surgery or high-quality breast reconstruction.1,2 These techniques are possible thanks to the improvements made in the early diagnosis of the disease and the evolution of therapeutic measures that provide better locoregional control of the process.
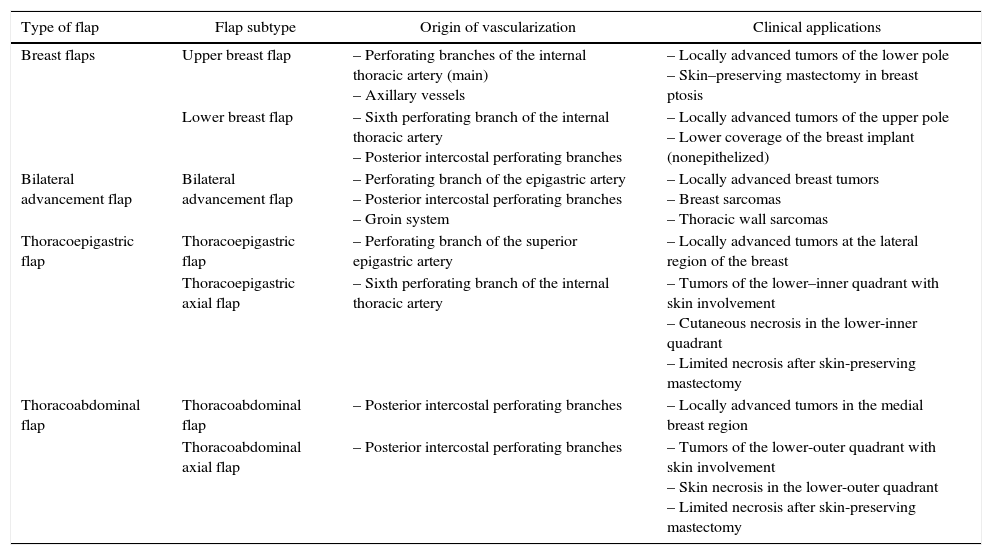
Nonetheless, there are certain clinical situations in which the excessive loss of local structures makes it necessary to use local anatomical resources for the closure of surgical wounds. These clinical situations are based on 2 different contexts. The first refers to those clinical presentations of breast cancer in locally advanced stages (T3 and T4 tumors) that require extensive excisions in which the closure of the surgical defect is encumbered by the elevated tension on the surgical edges and the high rate of necrosis. These situations involve locally advanced carcinomas that do not respond to neoadjuvant chemotherapy, such as breast sarcomas, whose only therapeutic option is extensive excision of the breast and adjacent structures.3,4 The second scenario entails complications due to ischemia that would cause a loss of skin coverage and complicate wound closure. This complication affects both conservative surgery, generally associated with oncoplastic procedures,5 as well as mastectomies, especially in skin preservations.6 In both situations, the absence of skin coverage would cause a delay in the start of adjuvant treatments (chemotherapy, radiotherapy) (Table 1).
Anatomical Characteristics and Clinical Applications of the Rotational Flaps.
| Type of flap | Flap subtype | Origin of vascularization | Clinical applications |
|---|---|---|---|
| Breast flaps | Upper breast flap | – Perforating branches of the internal thoracic artery (main) – Axillary vessels | – Locally advanced tumors of the lower pole – Skin–preserving mastectomy in breast ptosis |
| Lower breast flap | – Sixth perforating branch of the internal thoracic artery – Posterior intercostal perforating branches | – Locally advanced tumors of the upper pole – Lower coverage of the breast implant (nonepithelized) | |
| Bilateral advancement flap | Bilateral advancement flap | – Perforating branch of the epigastric artery – Posterior intercostal perforating branches – Groin system | – Locally advanced breast tumors – Breast sarcomas – Thoracic wall sarcomas |
| Thoracoepigastric flap | Thoracoepigastric flap | – Perforating branch of the superior epigastric artery | – Locally advanced tumors at the lateral region of the breast |
| Thoracoepigastric axial flap | – Sixth perforating branch of the internal thoracic artery | – Tumors of the lower–inner quadrant with skin involvement – Cutaneous necrosis in the lower-inner quadrant – Limited necrosis after skin-preserving mastectomy | |
| Thoracoabdominal flap | Thoracoabdominal flap | – Posterior intercostal perforating branches | – Locally advanced tumors in the medial breast region |
| Thoracoabdominal axial flap | – Posterior intercostal perforating branches | – Tumors of the lower-outer quadrant with skin involvement – Skin necrosis in the lower-outer quadrant – Limited necrosis after skin-preserving mastectomy |
Rotational flaps are a group of surgical procedures that enable surgeons to close large defects with a lower morbidity compared to muscle flaps, be these either pedicled or free. Planning for this technique requires an adequate length-to-width ratio and knowledge of the vascular anatomy of the donor region.
This article describes the anatomical principles for planning thoracoabdominal flaps and the applicability of rotational (or local) flaps during oncologic breast surgery.
Vascular Anatomy of the Anterior Thoracic and Abdominal WallIrrigation of the anterior wall of the thorax and abdomen is provided by a complex assembly of interconnected vascular components that contribute to the blood supply of adjacent muscles and teguments. This capacity is based on 2 anatomical structures that guarantee the viability and use of any local flap: the perforating artery trunks and the subdermal plexus. The perforating arteries are responsible for the origin of the blood supply. Knowledge of their locations and distribution is the most important element for the success of a local flap.7 The subdermal plexus is comprised of an intricate network of microvessels dispersed throughout the skin of the abdomen and thorax, which distributes the blood supplied by the perforating arteries over an extensive skin surface.8 The subdermal plexus is not affected by the surgical technique. Thus, the most important aspect in designing a local flap is understanding the distribution of the perforating arteries, which allows for flap planning, manipulation during surgery and viability in the postoperative period.
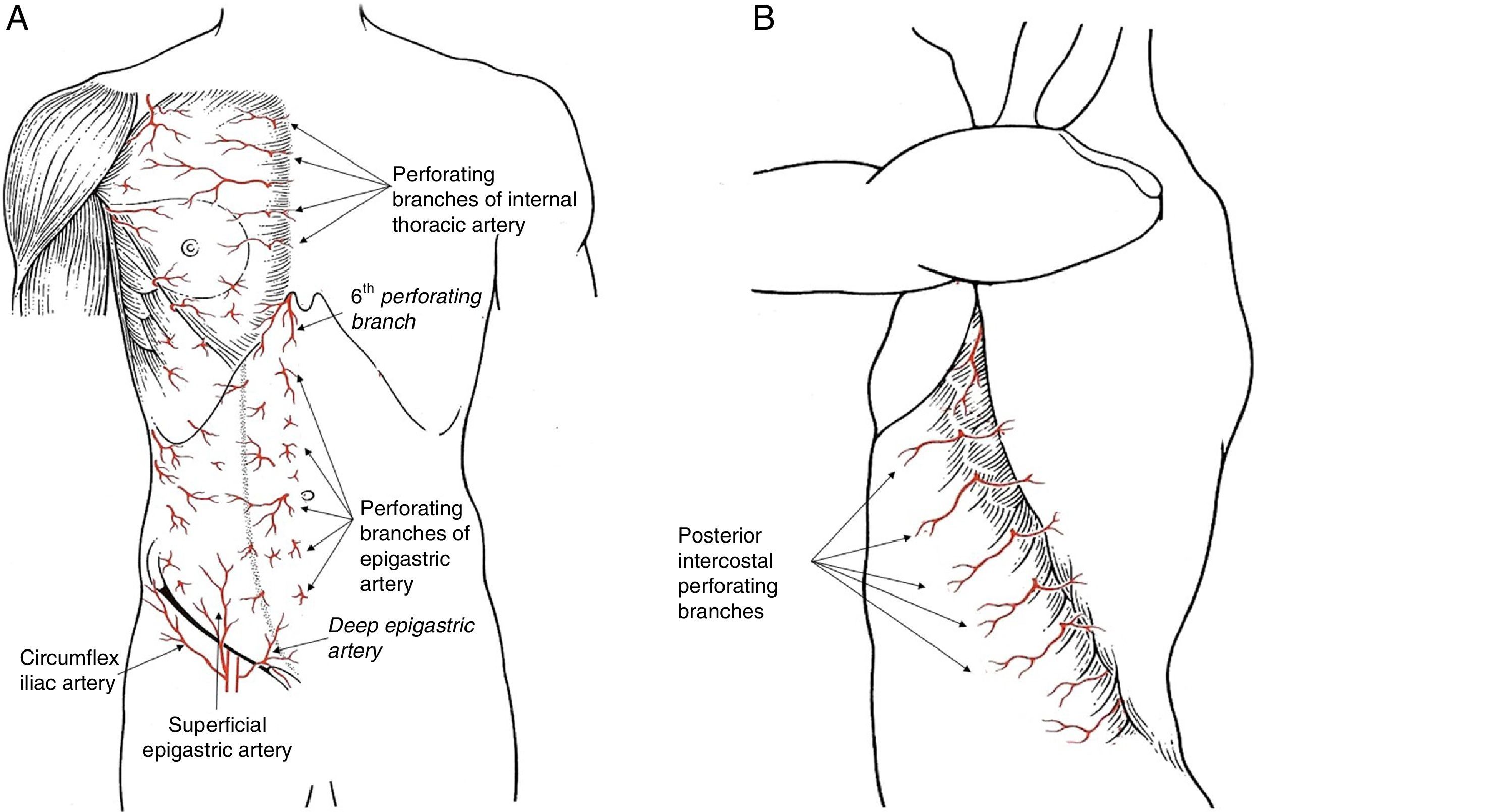
There are 3 perforating branch systems that guarantee the irrigation of the thoracic and abdominal wall: the epigastric system, the anterolateral intercostal system and the groin system (Fig. 1).
The blood supply in the anterior region of the trunk is based on the epigastric arcade, which connects the subclavian artery with the external iliac artery through the internal thoracic artery in the thorax and the rectus abdominis muscle. The artery that comprises this arcade receives different names during its evolution (internal thoracic, superior epigastric and inferior epigastric), but along its entire length it projects perforating branches to the subcutaneous tissue of the thorax and abdomen, reaching the subdermal plexus and significantly contributing to regional skin irrigation. There are 3 perforating branches of this system that present special clinical significance:
- -
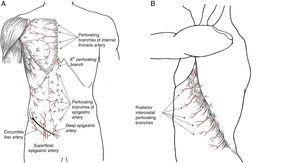
Perforating arteries of the internal thoracic artery. The first 5 perforating branches of the internal thoracic artery contribute to the formation of the subdermal plexus of the breast, together with the vessels originating in the axilla. These perforating branches of the epigastric system contribute to 60% of the flow in this vascular plexus which, situated above the superficial fascia, guarantees the viability of the skin flap in skin-preserving mastectomies. Once they pass through the pectoralis major muscle, these perforating branches emit communicating vessels toward the mammary gland tissue situated in the internal quadrants, which means that the medial pedicles are a good guarantee for irrigation to the nipple-areolar complex (NAC) during an oncoplastic procedure. There are habitually one or 2 dominant perforating branches on each side of the sternum, which should be preserved when carving out a dermal flap in a mastectomy, since the lesion of a dominant vessel increases the risk for cutaneous necrosis after mastectomy.
- -
Sixth perforating branch of the internal thoracic artery. This perforating branch is a constant in clinical practice as it is invariably situated outside the xiphoid process. Its role is decisive in epigastric flaps for the coverage of the internal part of the breast, as its viability depends on this vessel exclusively.
- -
Periumbilical epigastric perforating arteries. Most of the perforating branches of the epigastric artery are concentrated in the periumbilical region, in the area of the deep epigastric artery, and are essential anatomical reference points for the planning and execution of rectus abdominis muscle flaps, which can be either pedicled (TRAM, transverse rectus abdominis myocutaneous) or free (DIEP, deep inferior epigastric perforators).
The intercostal arteries provide vascular communication between the aorta and the internal thoracic artery. During their trajectory in the upper section of the intercostal space, they produce a perforating artery on the lateral side of the thorax that passes through the intercostal muscles to reach the subcutaneous tissue. This branch is known as the lateral cutaneous branch of the posterior intercostal artery. At this point, it divides into 2 branches: anterior or mammary, and posterior.9,10 The location of these perforating branches is constant and predictable, as they originate in the anterior edge of the serratus anterior muscle, at the termination of its insertion onto the rib. Due to this anatomical relationship with the serratus anterior muscle, the emergence point of these perforating branches is displaced backwards as we go down the ribs, which should be considered when planning lower flaps. These perforating arteries are the anatomical grounds for the irrigation of thoracoabdominal flaps, especially those that are designed as axial flaps to one of these perforating branches for selective coverage at the lower pole of the breast.
Groin SystemThe skin and subcutaneous tissue of the lower abdominal region receive supplemental blood flow from the arterial branches originating in the groin. Thus, we can identify 2 independent branches that complement the irrigation of the epigastric perforating arteries and constitute the base for bilateral advancement flaps.
- -
Superficial epigastric artery. This artery is a direct cutaneous vessel that originates at the femoral artery, 2–3cm under the inguinal ligament, or at a common trunk with the superficial iliac circumflex artery. This artery crosses the inguinal ligament and continues superomedially toward the navel through the superficial subcutaneous tissue, where it anastomoses with the periumbilical epigastric perforating branches at the subdermal plexus.
- -
Superficial iliac circumflex artery. Similar to the anterior artery, this artery starts at the femoral artery but does not cross the inguinal ligament, as it runs parallel to it and only ascends the abdominal wall at the anterior superior iliac spine.
The skin enveloping the breast can be used for the closure of large defects caused after extensive excision of the breast and adjacent elements. This group of procedures entails a low morbidity rate, whose main challenge is to identify the skin area that should be preserved and to visualize the origin of its blood supply. The viability of this flap is based on the subdermal plexus situated above the superficial fascia of the breast, and the origin of its vascularization should be found in the internal breast perforating branches and in the vessels originating at the axilla. Based on this anatomical layout, two types of local breast flaps can be designed.
- -
Upper breast flap. This is comprised of the skin at the superior pole of the breast to the upper edge of the nipple-areolar complex. The extension of this flap is directly proportional to the breast size and ptosis: the larger the breast volume and greater the distance of the NAC to the jugular notch, the greater the skin area available for this flap. This flap is useful in locally advanced tumors of the lower pole of the breast, which require extensive resection of the breast and of the tissue adjacent to the inframammary fold, or as a skin-preserving procedure for immediate breast reconstruction. In this latter case, the design of an upper breast flap leaves the scar-line at the lower pole of the reconstructed breast, thereby reducing its visibility. In both cases, carving a skin flap from the upper pole requires an oncological criterion for complete excision of the breast tissue at that level. It is a local flap with good irrigation, as the subdermal plexus of the breast is more developed in the superior pole; nonetheless, during its design we should not surpass the inframammary fold in order to avoid excessively long flaps that necrotize at their most distal point.
- -
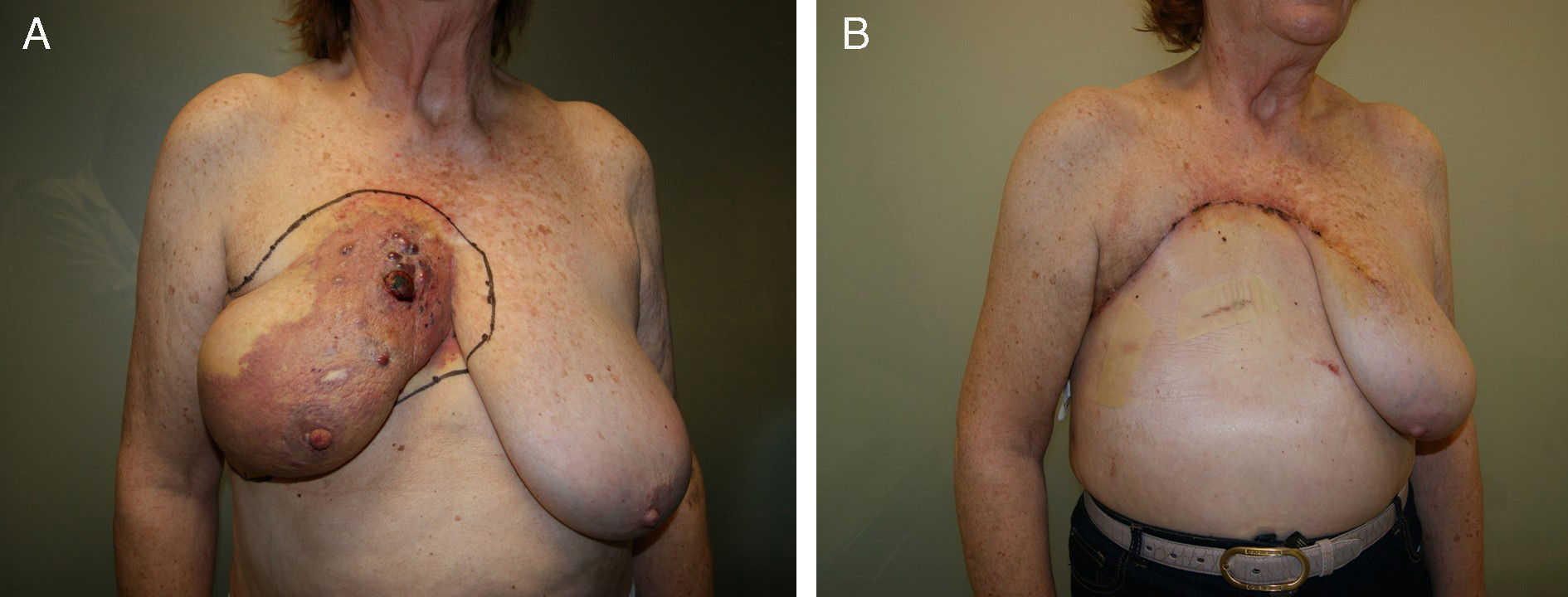
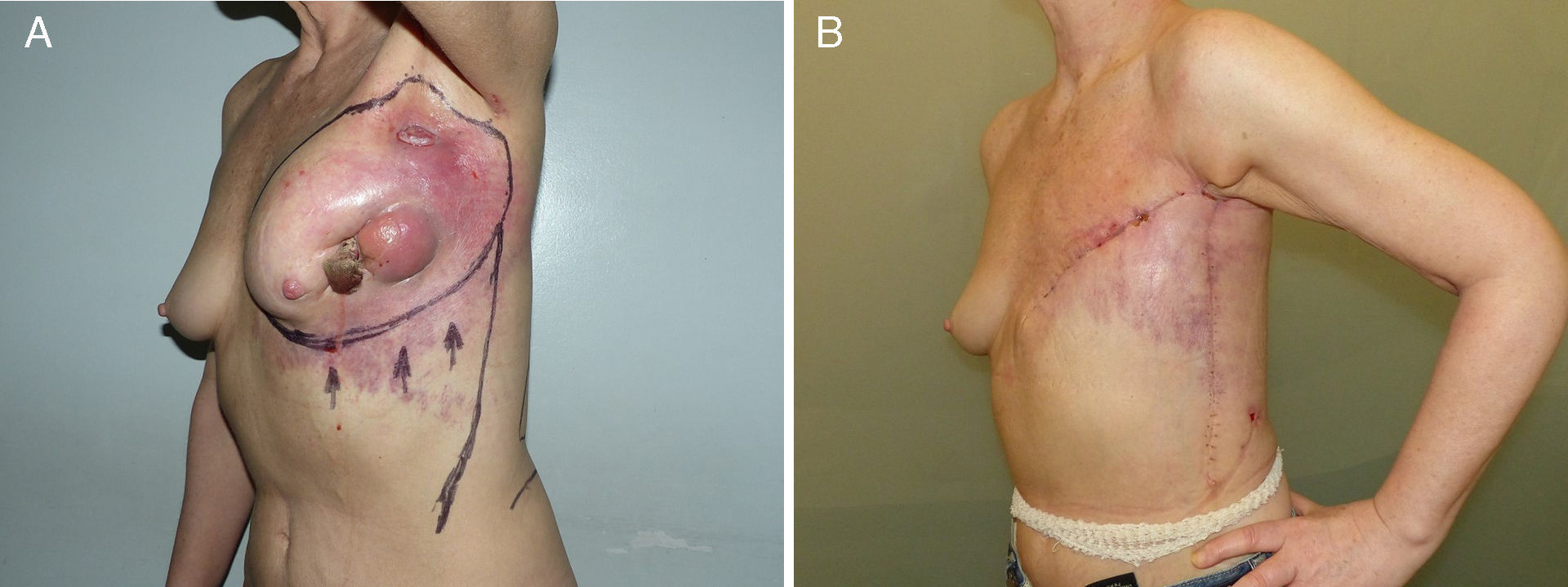
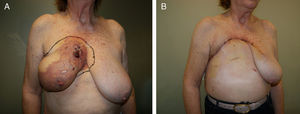
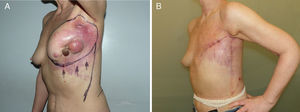
Lower breast flap. This is created from the skin at the lower pole of the breast to the lower edge of the NAC. Its vascularization is more precarious than the previously mentioned flap type because in this area there is no available dominant branch of the internal thoracic artery, which limits blood flow in this skin area. Therefore, its vascular support will be provided by the sixth perforating branch of the internal thoracic artery and the perforator of the posterior intercostal artery situated beneath the inframammary fold. This local flap can be used for 2 different clinical situations. First of all, as skin coverage after breast resection at the polo superior, generally due to locally advanced tumors or ulcerated neoplasms at the upper edge of the breast. In these cases, the upper incision of the mastectomy is too high and requires skin preservation of the lower pole in order to reduce the tension of the closure. The consequence of this closure is a visible, elevated wound, although this does not impede immediate breast reconstruction (Fig. 2). The second clinical application of this inferior flap is for the creation of a nonepithelized dermal flap and its use for creating a dermal-muscular casing. This is the so-called Spira technique,11 in which this nonepithelized dermal flap and the pectoralis major form a sac to house the silicone implant during immediate breast reconstruction.
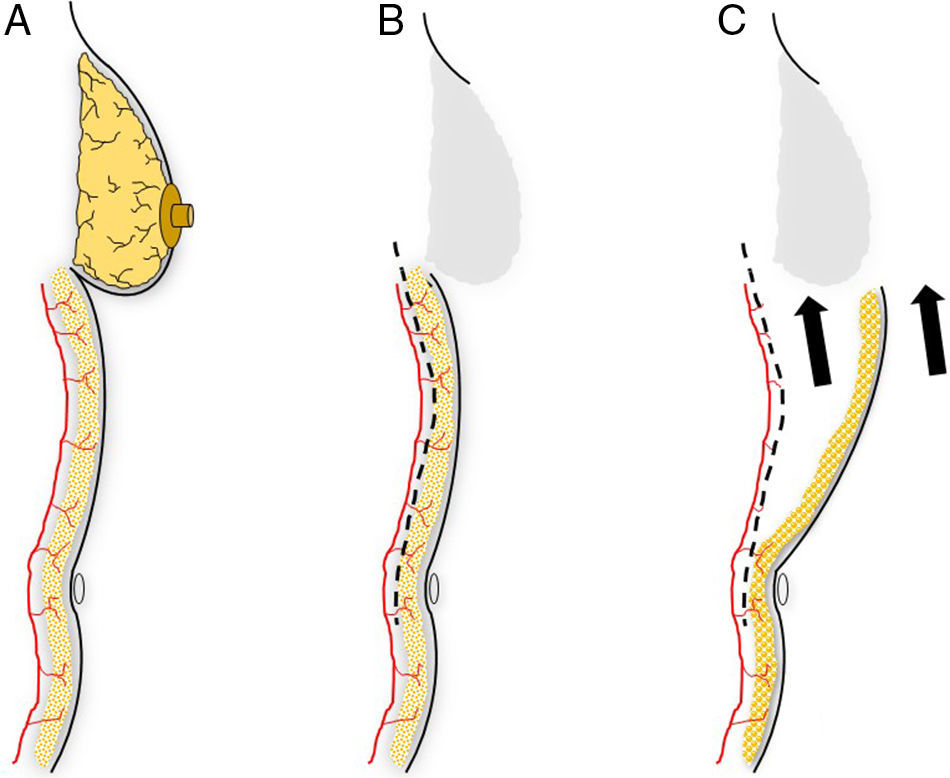
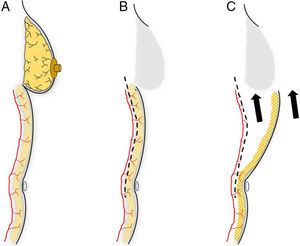
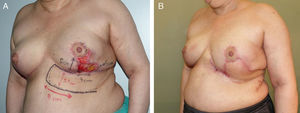
This flap is comprised of the skin of the upper abdominal region, the hypochondrium, which, after being released from the abdominal wall, is mobilized cephalically toward the thorax.12 Its use in oncological surgery provides coverage of large defects in the thorax after extensive mastectomies or exeresis of sarcomas in the thoracic wall (Fig. 2). Usually, it is possible to close defects measuring up to 15cm. In order to do so, however, it is necessary for the patient to have abundant abdominal tissue and, consequently, this procedure is not indicated in very thin women. During dissection, the surgeon releases the subcutaneous abdominal tissue at the prefascial plane while attempting to preserve as many posterior intercostal and epigastric perforators as possible. It is generally necessary to dissect up to the umbilical region in order to guarantee good mobilization of this flap (Fig. 3). The tension of the closure can be reduced by simultaneously detaching the upper surgical edge to the clavicle. It is a flap with low degree of complexity and good vascularization thanks to the anteromedial intercostal and epigastric perforating branches and the arterial system of the groin.
Planning an abdominal advancement flap: (A) the dissection of an abdominal advancement flap is based around the muscle perforators of the abdominal wall; (B) subcutaneous detachment at the muscle plane is able to free the covering skin to be mobilized toward the thoracic defect (C).
Thoracoepigastric flaps are a group of procedures that provide coverage of thoracic defects by pulling up and rotating part of the abdominal skin surface.12 The blood supply of these flaps is situated in the perforating branches of the superior epigastric artery and, depending on the skin surface mobilized, we can define 2 types of thoracoepigastric flaps:
- -
Thoracoepigastric flap. This is a rotational advancement flap that allows for the skin situated in the lateral regions of the abdomen to be raised up to the thorax. It is mainly indicated in the coverage of mastectomies in which the largest defect is situated laterally toward the axilla. The vascular support of this flap is provided by the perforating branches of the superior epigastric artery, although during dissection some posterior intercostal perforating branches may be preserved. The design involves making an incision that is begun at the lowest lateral portion of the defect and extends to the area of the anterior superior iliac spine (Fig. 4). Like bilateral advancement flaps, dissection is done at the fascial plane in a medial and downward direction, during which we preserve some perforating branches that will contribute to the viability of theflap.
- -
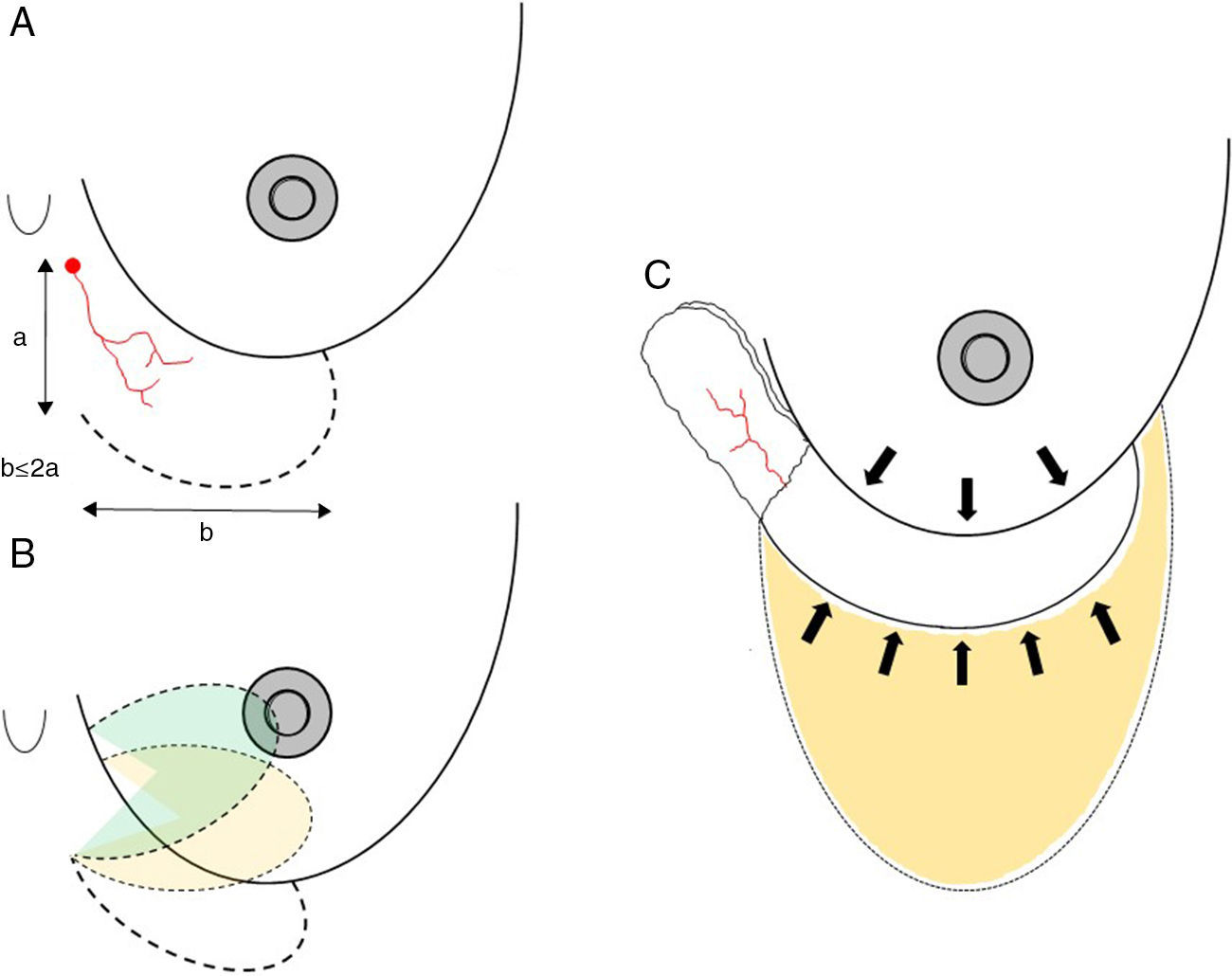
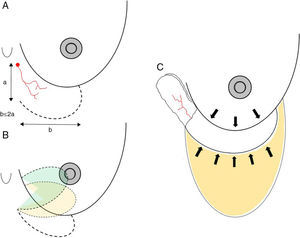
Axial thoracoepigastric flap. This is a limited variety of the thoracoepigastric flap that circumscribes the angiosome that depends on the sixth perforating branch of the internal thoracic artery, providing a skin surface for the coverage of the lower-inner quadrant of the breast (Fig. 5). As it is an axial flap, the length-to width ratio should not be greater than 2:1. Unlike the flaps described up to now, the main use of this flap is in conservative surgery, whether to reconstruct the lower-inner quadrant after excision of a tumor with cutaneous involvement or to resolve local complications due to skin necrosis. In selected cases, it can be used for skin coverage in areas of necrosis after skin-preserving mastectomy, especially when it occurs at the lower pole (Fig. 5).
Fig. 5.Planning axial thoracoepigastric flap: (A) the design of an axial thoracoepigastric or thoracoabdominal flap is based on the planning of a skin island with a length-to-width ratio no greater than 2:1; (B) the rotation of this flap provides skin coverage in the lower-inner breast quadrant; if an axial thoracoabdominal flap is used, the skin coverage will be done on the lower-outer breast quadrant; (C) the defect caused by the skin “island” should be covered by an abdominal advancement flap once the subcutaneous fatty layer is detached from the muscle plane.
(0.09MB).
Thoracoabdominal flaps mirror thoracoepigastric flaps, meaning that they provide for the abdominal skin surface to be rotated and pulled up to the medial thoracic region.12–15 As in the previous section, 2 types of thoracoabdominal flaps are defined according to the extension of the abdominal surface mobilized:
- -
Thoracoabdominal flap. This flap is indicated when most of the defect affects the medial portion of the thorax after mastectomy. In this case, an incision is designed to initiate at the medial edge of the defect and descend along the abdominal midline to the height of the navel. The irrigation of the flap is maintained by the anteromedial intercostal perforators. Once dissected from the abdominal wall, it can be mobilized to the thorax by being pulled up and rotated.
- -
Axial thoracoabdominal flap. This is a variation of the previous flap type to be used in a limited area of abdominal skin for the coverage of a breast defect. Its clinical application is focused on complications due to skin necrosis at the lower pole of the breast after breast-conserving surgery and its axial vascularization, which is exclusively dependent on the anteromedial intercostal perforating branch (Fig. 6).
In conclusion, local rotational flaps for breast-conserving therapy are a group of surgical procedures that are able to resolve the closure of large thoracic defects after breast cancer surgery, with lower morbidity and technical difficulties than distant flaps. Their use in skin necrosis complications after breast-conserving surgery or skin-preserving mastectomies allow for adjuvant treatments to be initiated, thereby reducing delays in this group of patients.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Acea Nebril B, Builes Ramírez S, García Novoa A, Varela Lamas C. Colgajos por rotación en la cirugía oncológica de la mama. Fundamentos anatómicos y técnicos para su planificación quirúrgica. Cir Esp. 2016;94:372–378.